Back to Journals » Drug Design, Development and Therapy » Volume 16
Chikungunya Vaccine Candidates: Current Landscape and Future Prospects
Authors Schmidt C, Schnierle BS
Received 8 July 2022
Accepted for publication 15 October 2022
Published 20 October 2022 Volume 2022:16 Pages 3663—3673
DOI https://doi.org/10.2147/DDDT.S366112
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Anastasios Lymperopoulos
Christin Schmidt, Barbara S Schnierle
Paul-Ehrlich-Institut, Department of Virology, Section AIDS and Newly Emerging Pathogens, Langen, Germany
Correspondence: Barbara S Schnierle, Paul-Ehrlich-Institut, Department of Virology, Section AIDS and newly emerging pathogens, Paul-Ehrlich-Strasse 51.59, Langen, 63225, Germany, Tel/Fax +49 6103 77 5504, Email [email protected]
Abstract: Chikungunya virus (CHIKV) is an alphavirus that has spread globally in the last twenty years. Although mortality is rather low, infection can result in debilitating arthralgia that can persist for years. Unfortunately, no treatments or preventive vaccines are currently licensed against CHIKV infections. However, a large range of promising preclinical and clinical vaccine candidates have been developed during recent years. This review will give an introduction into the biology of CHIKV and the immune responses that are induced by infection, and will summarize CHIKV vaccine development.
Keywords: alphavirus, vector vaccines, inactivated virus, nucleic acid vaccines, immune response, arthralgia
Introduction
“Chikungunya” is a Kimakonde word of the Makonde tribe in Africa describing the disease symptoms of a virus infection, and can be translated as “disease that bends up the joints”. The causative chikungunya virus (CHIKV) belongs to the genus Alphavirus of the Togavirdae family. Traditionally, the members of the Alphavirus genus were categorized according to their global distribution into Old World and New World viruses.1 The Old World alphaviruses like CHIKV, Ross River virus, Mayaro virus, Sindbis virus and Semliki Forest virus mainly cause arthritogenic symptoms, whereas the New World viruses like Eastern, Western and Venezuelan equine encephalitis virus cause encephalitic symptoms.
CHIKV was first isolated from a patient in Tanzania in 1952.2 During the following 50 years, CHIKV circulated between vertebrate hosts and mosquito vectors of the species Aedes aegypti and caused multiple outbreaks in Africa and Asia. During an outbreak on La Reunion island in 2006, a mutation in the E1 gene enabled CHIKV to be spread by Aedes albopictus.3 In contrast to Aedes aegypti, which is only common in the tropics and sub-tropics, Aedes albopictus is endemic almost globally.4 This allowed a rapid global spread of CHIKV and it has now been reported in over 100 countries worldwide.5,6 Further spread and epidemics are likely and thus the WHO lists CHIKV in the Research and Development Blueprint for preparedness for priority diseases/pathogens.7
Based on phylogeny, CHIKV has been divided into three main genotypes: West African (WA), East Central South African (ECSA) and Asian (likely derived from ECSA).8 The 2006 La Reunion genotype is now referred to as a fourth genotype, the Indian Ocean Lineage (IOL). However, antibodies raised against one genotype have been described to be cross-reactive against the others, indicating that there is only a single serotype.9–11
Chikungunya Virus
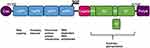
Like all alphaviruses, CHIKV is an enveloped, single-stranded, positive-sensed RNA virus with a genome of 11.8 kb (Figure 1). The genome consists of two open-reading frames (ORFs). The first encodes the four non-structural proteins nsP1–nsP4, which assemble the alphavirus replicase complex. The second encodes the structural proteins Capsid-E3-E2-6K-E1. This ORF contains a slippery codon motif in the 6K gene that mediates ribosomal frameshifting resulting in the production of the transframe (TF) protein.12 For review on alphavirus biology see: Strauss and Strauss, 1994.13
CHIKV enters cells by receptor-mediated endocytosis in a pH-dependent fusion step. It has two surface envelope proteins: the transmembrane glycoproteins E2 and E1. E1 is a class II viral fusion protein and E2 mediates receptor binding and cell attachment.14 The E2 protein is subdivided into three immunoglobulin domains called A, B and C. Domains A and B are involved in receptor binding.15–17 Epitope mapping of antibodies induced by CHIKV infections in humans has indicated that the E2 protein is the main target of CHIKV-neutralizing antibodies.18–21 Therefore, vaccine candidates directed against CHIKV contain the structural genes as antigens.
The Disease: Chikungunya Fever (CHIKF)
CHIKV has a broad cellular and tissue tropism and can replicate in most cell types apart from B and T cells.22,23 After a mosquito bite, CHIKV first infects and replicates in fibroblasts in the skin.24 Subsequently, the virus disseminates via the blood stream and infects cells in the liver, muscle, joints, lymphoid tissue and brain. During this phase, viral titers in the blood are very high and have been reported as mean values of 3.4 × 103 pfu/mL and 5.6 × 105 pfu/mL in asymptomatic and symptomatic patients, respectively.25 The incubation time until disease onset is between 2 and 7 days. Only about 15% of patients remain asymptomatic.26 The typical symptoms of an acute CHIKV infection are fever, headache, rigors, photophobia, rash and severe joint pain.27 Neurological symptoms are rare, but have been described in children and include seizures, compromised consciousness, blindness due to retrobulbar neuritis and acute flaccid paralysis.28 The virus is usually cleared by innate and adaptive immune responses and disease symptoms resolve after 7 to 14 days.29 However, in around 40% of patients, the disease symptoms can persist for several months up to years.30 In this chronic phase, patients show severe joint pain and arthritis. The exact mechanisms are still not fully understood, but likely involve tissue damage by proinflammatory responses or autoimmune reactivity and/or a chronic virus infection.31–33 The fatality rate of CHIKV infection is low and is reported to be 0.1% on La Reunion; however, other publications have reported up to 5%.34,35 Fatality mainly affects the elderly, neonates and young children, pregnant women and patients with co-morbidities.36,37 Treatment of patients is limited to symptomatic therapy using analgesics and/or nonsteroidal anti-inflammatory drugs for pain and fever relief.38
Animal Models for CHIKV Infections
Small animal models can be used to assess immunogenicity and protective efficacy of CHIKV vaccine candidates. Either immunocompetent (eg C57BL/6, BALB/c) or immunodeficient mice (eg IFNAR-/- mice) are used.39 In adult immunocompetent mice, peripheral inoculation with CHIKV strains causes no clinical signs, so efficacy endpoints are typically viremia assessed by viral load determination and/or footpad swelling.40–42 Immunodeficient mice are susceptible to CHIKV infections due to the lack of innate immune responses and are a lethal endpoint model. However, they are less suitable for studying the immunogenicity of vaccines due to their impaired innate immunity.39
Models using nonhuman primates (NHP) like cynomolgus or rhesus macaques much more closely resemble the disease observed in humans. Infection of NHP with CHIKV results in viremia, fever, rash, changes in circulating inflammatory cytokines and, after infection with high doses, can be associated with joint pathology.43 Although vaccine candidates have been assessed in mice and NHP, exact correlates of protection like the minimal protective antibody titer are still in its infancies. These titers needed to directly translate titers induced in humans into protection.
Immune Response to CHIKV Infection
Protection from an infection with a pathogen relies on multiple immune responses, including innate and adaptive immunity. Early in infection, alphaviruses induce type-I IFN responses due to virus recognition by pattern-recognition receptors (PRRs). Elevated IFN-α levels have been found in the plasma of CHIKV-infected patients during acute infections.44 Antigen-presenting cells like Langerhans cells, macrophages and dendritic cells are the main inducers of innate immune responses and CHIKV has been shown to be able to replicate in monocytes that produce high levels of IFN-α.22
Antibodies directed against the envelope proteins are central for protection against incoming viruses and cellular responses usually modulate disease progression. CHIKV infections potently induce adaptive humoral and cellular immune responses. CHIKV-specific IgM and IgG appear early after symptom onset and IgG persists at high levels for years.18 Both anti-CHIKV IgM and IgG antibodies are able to neutralize CHIKV.45 Analyses of human serum antibodies have indicated that the E2 protein is the dominant antigen.18,21
Several groups have demonstrated a clear correlation between neutralizing antibodies and protection against CHIKV infection in mice.31 The role of antibodies was initially studied by CHIKV infection of B cell-deficient muMT mice. Wild type mice cleared the viremia, but CHIKV-infected muMT mice showed persisting viremia and more severe joint disease, indicating a role for B cells in viremia and disease.46 In addition, passive transfer of IgG from convalescent patients prevented and reduced CHIKV infection in mice.47 In humans, the presence of CHIKV-neutralizing antibodies prior to infection prevented disease.48 However, it is difficult to define exact correlates of protection based on these results.49 Furthermore, in contrast to dengue virus,50 suboptimal levels of pathogen-specific neutralizing antibodies that enhance infectivity and disease severity through antibody-dependent enhancement (ADE), seem not to be a concern for CHIKV infections. However, in vitro infection of macrophages via ADE has been described for the alphavirus Ross River virus.51 Real world data concerning ADE in alphavirus infection is missing, but the lack of viremia and lifelong protection in previously CHIKV-infected people do not argue for an involvement of ADE.48 Nevertheless, effective vaccines will need to elicit a strong, durable, neutralizing antibody response.
CHIKV infection also triggers cell-mediated immunity and CD8+ and CD4+ T cells reactive to CHIKV antigens have been detected in animal models52,53 and in humans.54 T cells contribute to limiting the spread of virus; however, the exact role of T cells in viral clearance or pathogenicity is not well understood.55 At early stages of the disease, in the acute phase, CD8+ T cells predominate, and CD4+ T cells mediate the adaptive response at later time points of infection.44 CD4+ T cells seem to be activated during the chronic phase of CHIKV infection, inducing inflammation by proinflammatory cytokine release, which results in joint swelling.56 However, an association of regulatory T cells (Tregs), which are essential for the induction and maintenance of peripheral tolerance, and IL-10 with recovery from CHIKV infection has been described. The levels of Tregs and IL-10 were lower in acutely and chronically infected CHIKV arthritis patients than in CHIKV-recovered patients, indicating that a reduction in Tregs is associated with an essential role in establishing the pathogenesis of CHIKV.57,58
Chikungunya Vaccine Candidates
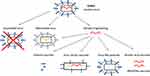
Several experimental vaccines and vaccine candidates are currently under development, involving a large array of technology platforms (Figure 2).
Inactivated Viruses and Subunit Vaccines
Inactivated viruses, protein subunits and live-attenuated viruses are considered classical vaccine platforms.
For inactivated viruses, the virus is first cultured in high biosafety level facilities and is subsequently inactivated with formaldehyde or by irradiation. In addition, chemically inactivated virus requires purification. Inactivated CHIKV was first tested as a vaccine candidate in 1970 and later in 2009, and showed efficacy in animal models.59,60 However, further development of inactivated CHIKV vaccines was not pursued.
Recombinant proteins are an alternative and their production does not require high biosafety containment. Some candidate CHIKV vaccines have been developed using recombinant proteins and have shown efficacy in animal models, including adjuvanted E1 and E2 envelope proteins, or subfragments of E2.61–63 However, protein vaccines require multiple doses and normally generate only a short-lived immune response. Further developments will be necessary to prove the applicability of protein vaccines against CHIKV.
Live-Attenuated Viruses
Live-attenuated viruses are attenuated viruses that exhibit limited replication in humans but are still able to induce a good immune response without signs of disease. However, they frequently have suboptimal safety profiles and always retain their potential to revert to a pathogenic virus.
The first CHIKV vaccine candidate, CHIK 181/clone 25, elicited neutralizing antibodies and protected mice and rhesus monkeys against a challenge infection. It was derived from a clinical CHIKV strain by attenuation through in vitro passage on human MRC-5 cells.64 In clinical trials, CHIK 181/clone 25 was highly immunogenic, but 10% of the study participants experienced mild arthralgia.65
Careful modification of the CHIKV genome to alter viral growth characteristics has resulted in more advanced live-attenuated viruses. Several deletions in CHIKV genes for the generation of live-attenuated viruses have been described, such as a large deletion in nsP3 (∆5nsP3) or the 6K open reading frame66 or in the capsid.67,68
The ∆5nsP3 virus showed a very good safety profile and efficacy in mice and cynomolgus macaques69 and has undergone clinical testing by Valneva.70 The company recently successfully completed a Phase III trial (NCT04546724) (Table 1). The trial analysis showed that 98.9% of participants achieved protective levels of CHIKV-neutralizing antibodies one month after receiving a single vaccination, and 96.3% of participants had protective CHIKV-neutralizing antibody titers six months after receiving a single vaccination (Valneva, press release). Protective titers were determined by passive transfer of sera from vaccinated humans into NHP, followed by a challenge infection. At a dilution of >1:150, serum protected animals from CHIKV infection and the associated clinical symptoms, as well as CHIKV persistence in tissue.71
 |
Table 1 The Following Vaccine Candidates are in Clinical Development |
Another rational attenuation was accomplished by replacing the viral subgenomic promoter, which is needed for the expression of the structural genes, with an internal ribosomal entry site (IRES), thereby reducing their protein synthesis.72,73 The attenuated virus CHIK/IRES grows slowly in vitro and does not replicate in mosquito cells, which blocks the spread between hosts. In mice and NHP, CHIK/IRES was highly immunogenic and protected against a CHIKV infection with a good safety profile.11,72,74 In addition, CHIK/IRES cross-protected mice against the related alphavirus o’nyong’nyong virus (ONNV).75
Chimeric alphaviruses are another approach to obtaining attenuated CHIKV. This method makes use of the fact that the insect virus Eilat virus (EILV) does not replicate in mammalian cells. Replacing the EILV structural genes with those of CHIKV produced a chimeric virus that can be amplified in insect cells and contains the CHIKV immunogens. Due to the lack of replication in human cells, reversion to replication-competent virus is virtually excluded. This virus has been shown to be highly immunogenic and protect mice and NHP against a CHIKV challenge infection.41,76
Virus-Like Particles
One of the first CHIKV vaccine candidates consisted of virus-like particles (VLPs). VLPs are self-assembling viral structural proteins that resemble wild-type virions in their natural conformation but do not contain genomic nucleic acids. Their safety profile is therefore superior to live-attenuated viruses since the generation of replicating virus is impossible. In addition, production does not require high biosafety levels. Expression of the viral structural proteins (C-E3-E2-6K-E1) in cell lines leads to the secretion of particles that are similar in structure and protein configuration to the native virus and VLPs can be purified by buoyant density gradient sedimentation.77,78
Immunization with VLPs synthesized in mammalian or insect cells elicited protective antibody responses in wild-type and IFN-deficient mouse models as well as in NHP.78 Phase I and Phase II human trials showed that the vaccine candidate was well tolerated and produced neutralizing antibodies after the first boost.79,80 Neutralization was cross-protective against nine CHIKV strains, comprising the three clades.81 Another phase II study was performed with adjuvant-formulated VLPs. PXVX0317, an aluminium hydroxide-adjuvanted VLP vaccine, was well tolerated and induced a robust and durable serum neutralizing antibody response against CHIKV for up to 2 years.82 The adjuvanted PXVX0317 is now being further investigated as a single injection in phase III clinical trials that are currently recruiting participants (NCT05072080 and NCT05349617) (Table 1).
Viral Vector Vaccines
Vector vaccines are chimeric viruses that consist of a non-pathogenic virus vector backbone equipped with foreign gene products. Viral vectors use the host-cell protein-processing machinery that leads to antigen presentation via the MHC I complex and consequently cytotoxic T cell stimulation in addition to humoral immune responses. These types of vaccines produce high levels of humoral and cellular immunity and do not rely on the use of adjuvants. Measles virus and simian adenovirus vectors are currently in clinical development as CHIKV vaccine candidates.
Vesicular Stomatitis Virus
An experimental vaccine was created by replacing the glycoprotein G of vesicular stomatitis virus (VSV) with the CHIKV structural genes E3–E1 (VSVΔG-CHIKV). VSV is a negative-strand RNA virus of the Rhabdoviridae family. VSVΔG-CHIKV incorporated the CHIKV glycoproteins efficiently into virus particles and could be propagated without VSV G complementation. It generated robust neutralizing antibody and cellular immune responses to CHIKV in C57BL/6 mice after a single dose and protected mice against CHIKV infection.83
Modified Vaccinia Virus Ankara
The modified vaccinia virus Ankara (MVA) is a highly attenuated DNA virus generated by 570 passages of a vaccinia virus smallpox vaccine on avian cells. During this passage, MVA acquired large genomic deletions that restricted its replication in mammalian cells, including human cells.84 However, it is still capable of high expression rates of foreign gene products and elicits strong humoral and cellular immune responses.85 MVA is licensed as a smallpox vaccine under the name “Imvanex”.
Several recombinant MVA expressing CHIKV genes have been constructed. Expression of a short fragment containing the receptor-binding domains of E2 by MVA resulted in only a slightly reduced titer after challenge infection of vaccinated mice.63 The monomeric molecule may not have been sufficiently immunogenic in the MVA system. A recombinant MVA-CHIKV expressing only the E3 and E2 structural proteins generated low levels of neutralizing antibodies but protected animals against a lethal challenge infection.69,86 However, MVA-CHIKV expressing the E3, E2, 6K and E1 structural proteins was superior in eliciting immune responses and protected IFNR-knockout A129 mice from a lethal CHIKV challenge infection.87 Lastly, MVA containing all of the structural genes (C-E3-E2-6K-E1) generated neutralizing antibodies in vaccinated mice and protected animals against a lethal challenge infection.42 A single dose of this vector protected mice from a high-dose challenge with CHIKV and induced strong, broad, highly polyfunctional and long-lasting CHIKV-specific CD8+ T cell responses, together with neutralizing antibodies against CHIKV.66
Recombinant Adenoviruses
The chimpanzee adenoviral vector platform ChAdOx1 was developed to evade preexisting antibodies against the frequently circulating human adenoviruses, which could limit their use as vector. This vector was used to generate the COVID-19 vaccine with the brand name Jcovden. Two ChAdOx1 CHIKV vaccine candidates have been constructed that express either all of the structural genes C-E3-E2-6K-E1 (ChAdOx1 Chik) or only the E3–E1 genes (ChAdOx1 Chik Δcap). Both vaccine candidates, without significant differences, triggered a protective immune response and neutralizing antibodies in A129 mice.88 Immunogenicity against all CHIKV lineages and the safety of a single dose ChAdOx1 Chik vaccine was recently evaluated in a phase I clinical trial at the Jenner Institute, University of Oxford (NCT03590392), (Table 1).89 ChAdOx1 Chik showed excellent safety, tolerability and 100% seroconversion after a single dose.89
Measles Virus
Another clinically advanced vector platform is the attenuated measles virus (MV) Schwarz strain. MV is a negative-strand RNA virus and can be engineered to express foreign gene products.90 The MV Schwarz strain is safe and efficacious in inducing a lifelong protective immune response against measles virus.91 However, the small animal model for CHIKV infection had to be adapted as mice cannot be infected with MV. Therefore, IFNAR-/-, huCD46 transgenic mice were established which allow MV replication and these now serve as a preclinical model.91
A recombinant MV expressing all CHIKV structural genes induced high antibody-mediated protective immunity against CHIKV in IFN-deficient mice and in cynomolgus macaques.92 This vaccine candidate was then further evaluated in phase I/II clinical trials, which demonstrated safety, tolerability and immunogenicity (Table 1).93,94 No vaccination-related serious adverse events were observed. A booster vaccination on day 28 was required for full seroconversion of all study participants and preexisting immunity to MV did not affect the reactivity of the vaccine candidate.95 The MV-CHIKV vector is currently being commercially developed by Themis Bioscience.
Nucleic Acid-Based Vaccines
Delivery by in vivo electroporation of a DNA vaccine based on codon-optimized consensus envelope protein sequences (E3, E2 and E1) has been described to induce robust antigen-specific cellular and humoral immune responses that provide protection against CHIKV challenge in mice.96
The mRNA vaccines against COVID-19 have demonstrated the enormous success of this technology. For CHIKV, an mRNA-lipid nanoparticle (mRNA-LNP) vaccine expressing CHIKV E2-E1 antigen has been developed that induced potent humoral and cellular responses in C57BL/6 mice.97 A phase I trial revealed that the vaccine was well tolerated at dose levels between 25 and 100 μg and resulted in 100% seroconversion after a boost in subjects immunized with 100 μg (Table 1).98
Another approach using RNA is a trans-amplifying RNA based on CHIKV. The vaccine candidate consists of two RNAs: a non-replicating mRNA encoding for the CHIKV nonstructural proteins, forming the replicase complex, and a trans-replicon (TR) RNA encoding the CHIKV envelope proteins. The TR-RNA is amplified by the replicase in trans, and small RNA amounts can induce a potent immune response. First proof of principle has been shown in mice, which developed CHIKV-specific humoral and cellular immune responses and were protected against a CHIKV infection.99
A different approach was implemented by August et al.100 They used mRNA (mRNA-1944) to deliver a monoclonal, CHIKV-specific, neutralizing antibody as a passive immunization approach. A first phase I clinical trial has been conducted to determine safety and pharmacology of mRNA-1944. Adverse effects were mild to moderate in severity and did not worsen with a second dose. In this clinical trial, in vivo expression and detectable ex vivo neutralizing activity of the mRNA-encoded monoclonal antibody could be shown (Table 1).100
Regulatory Aspects for CHIKV Vaccine Licensure
Vaccines can only be approved and used if they comply with all the requirements of quality, safety and efficacy set out in the pharmaceutical legislations. Vaccine safety has to be studied in phase I and II clinical trials. Efficacy of a vaccine candidate has to be demonstrated in clinical efficacy phase III trials demonstrating a direct benefit in well controlled clinical disease endpoint studies. However, these clinical trials for candidate CHIKV vaccines will be very challenging because outbreaks and their duration are unpredictable. Consequently, field efficacy trials may delay clinical development or will be entirely unrealistic to perform, and alternative methods using correlates of protection in humans or using animal data have to be pursued.49,101 Adoptive antibody transfer studies have demonstrated that protection is primarily mediated by anti-CHIKV neutralizing antibodies.20,46,101 First insights into surrogates of protection have been gained for the phase III study of VLA1553 (NCT04546724). Passive transfer of human sera from the phase I study was used to establish a surrogate of protection that could be applied to predict clinical benefit during the phase III study. The passive transfer study suggested that a 50% plaque reduction neutralization titer of ≥150 was sufficient for protection. This titer was also supported by analysis of samples from a sero-epidemiological study.71 The establishment of an international serological standard will also allow the comparison of data between different laboratories and vaccine developers.49
Conclusion and Outlook
Several promising vaccine candidates are in clinical development and will soon enter the marketing authorization process. However, due to the spontaneous appearance of CHIKV infections during outbreaks, vaccine efficacy studies are difficult. To simplify marketing authorization, the establishment of serological correlates of protection would be a suitable method. Yet, this is challenging as good animal models, an international serological standard and correlates of protection are still lacking.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Powers AM, Logue CH. Changing patterns of chikungunya virus: re-emergence of a zoonotic arbovirus. J Gen Virol. 2007;88(Pt 9):2363–2377. doi:10.1099/vir.0.82858-0
2. Ross RW. A laboratory technique for studying the insect transmission of animal viruses, employing a bat-wing membrane, demonstrated with two African viruses. J Hyg. 1956;54(2):192–200. doi:10.1017/s0022172400044454
3. Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007;3(12):e201. doi:10.1371/journal.ppat.0030201
4. Townson H, Nathan MB. Resurgence of chikungunya. Trans R Soc Trop Med Hyg. 2008;102(4):308–309. doi:10.1016/j.trstmh.2007.11.013
5. Nsoesie EO, Kraemer MU, Golding N, et al. Global distribution and environmental suitability for chikungunya virus, 1952 to 2015. Euro Surveill. 2016;21(20). doi:10.2807/1560-7917.ES.2016.21.20.30234
6. Wahid B, Ali A, Rafique S, Idrees M. Global expansion of chikungunya virus: mapping the 64-year history. Int J Infect Dis. 2017;58:69–76. doi:10.1016/j.ijid.2017.03.006
7. WHO Research and Development Blueprint. Annual review of diseases prioritized under the Research and Development Blueprint [2017]; 2017. Available from: http://www.who.int/blueprint/what/research-development/2017-Prioritization-Long-Report.pdf?ua=1.
8. Petersen LR, Powers AM. Chikungunya: epidemiology. F1000Res. 2016;5:82. doi:10.12688/f1000research.7171.1
9. Volk SM, Chen R, Tsetsarkin KA, et al. Genome-scale phylogenetic analyses of chikungunya virus reveal independent emergences of recent epidemics and various evolutionary rates. J Virol. 2010;84(13):6497–6504. doi:10.1128/JVI.01603-09
10. Chua C-L, Sam I-C, Merits A, Chan Y-F. Antigenic variation of East/Central/South African and Asian chikungunya virus genotypes in neutralization by immune sera. PLoS Negl Trop Dis. 2016;10(8):e0004960. doi:10.1371/journal.pntd.0004960
11. Langsjoen RM, Haller SL, Roy CJ, et al. Chikungunya virus strains show lineage-specific variations in virulence and cross-protective ability in murine and nonhuman primate models. MBio. 2018;9(2). doi:10.1128/mBio.02449-17
12. Firth AE, Chung BY, Fleeton MN, Atkins JF. Discovery of frameshifting in alphavirus 6K resolves a 20-year enigma. Virol J. 2008;5:108. doi:10.1186/1743-422X-5-108
13. Strauss JH, Strauss EG. The alphaviruses: gene expression, replication, and evolution. Microbiol Rev. 1994;58(3):491–562. doi:10.1128/mr.58.3.491-562.1994
14. Voss JE, Vaney M-C, Duquerroy S, et al. Glycoprotein organization of chikungunya virus particles revealed by X-ray crystallography. Nature. 2010;468(7324):709–712. doi:10.1038/nature09555
15. Zhang R, Kim AS, Fox JM, et al. Mxra8 is a receptor for multiple arthritogenic alphaviruses. Nature. 2018;557(7706):570–574. doi:10.1038/s41586-018-0121-3
16. Basore K, Kim AS, Nelson CA, et al. Cryo-EM structure of chikungunya virus in complex with the Mxra8 receptor. Cell. 2019;177(7):1725–1737. doi:10.1016/j.cell.2019.04.006
17. Song H, Zhao Z, Chai Y, et al. Molecular basis of arthritogenic alphavirus receptor MXRA8 binding to chikungunya virus envelope protein. Cell. 2019;177(7):1714–1724. doi:10.1016/j.cell.2019.04.008
18. Kam Y-W, Lum F-M, Teo T-H, et al. Early neutralizing IgG response to chikungunya virus in infected patients targets a dominant linear epitope on the E2 glycoprotein. EMBO Mol Med. 2012;4(4):330–343. doi:10.1002/emmm.201200213
19. Kam Y-W, Pok K-Y, Eng KE, et al. Sero-prevalence and cross-reactivity of chikungunya virus specific anti-E2EP3 antibodies in arbovirus-infected patients. PLoS Negl Trop Dis. 2015;9(1):e3445. doi:10.1371/journal.pntd.0003445
20. Fox JM, Long F, Edeling MA, et al. Broadly neutralizing alphavirus antibodies bind an epitope on E2 and inhibit entry and egress. Cell. 2015;163(5):1095–1107. doi:10.1016/j.cell.2015.10.050
21. Henss L, Yue C, von Rhein C, et al. Analysis of humoral immune responses in Chikungunya Virus (CHIKV)-infected patients and individuals vaccinated with a candidate CHIKV vaccine. J Infect Dis. 2020;221(10):1713–1723. doi:10.1093/infdis/jiz658
22. Her Z, Malleret B, Chan M, et al. Active infection of human blood monocytes by chikungunya virus triggers an innate immune response. J Immunol. 2010;184(10):5903–5913. doi:10.4049/jimmunol.0904181
23. Wikan N, Sakoonwatanyoo P, Ubol S, Yoksan S, Smith DR. Chikungunya virus infection of cell lines: analysis of the East, Central and South African lineage. PLoS One. 2012;7(1):e31102. doi:10.1371/journal.pone.0031102
24. Appassakij H, Khuntikij P, Kemapunmanus M, Wutthanarungsan R, Silpapojakul K. Viremic profiles in asymptomatic and symptomatic chikungunya fever: a blood transfusion threat? Transfusion. 2013;53(10 Pt 2):2567–2574. doi:10.1111/j.1537-2995.2012.03960.x
25. Lemant J, Boisson V, Winer A, et al. Serious acute chikungunya virus infection requiring intensive care during the Reunion Island outbreak in 2005–2006. Crit Care Med. 2008;36(9):2536–2541. doi:10.1097/CCM.0b013e318183f2d2
26. Thiberville S-D, Moyen N, Dupuis-Maguiraga L, et al. Chikungunya fever: epidemiology, clinical syndrome, pathogenesis and therapy. Antiviral Res. 2013;99(3):345–370. doi:10.1016/j.antiviral.2013.06.009
27. Sebastian MR, Lodha R, Kabra SK. Chikungunya infection in children. Indian J Pediatr. 2009;76(2):185–189. doi:10.1007/s12098-009-0049-6
28. Da Cunha RV, Trinta KS. Chikungunya virus: clinical aspects and treatment - A Review. Mem Inst Oswaldo Cruz. 2017;112(8):523–531. doi:10.1590/0074-02760170044
29. Rodríguez-Morales AJ, Cardona-Ospina JA, Fernanda Urbano-Garzón S, Sebastian Hurtado-Zapata J. Prevalence of post-chikungunya infection chronic inflammatory arthritis: a systematic review and meta-analysis. Arthritis Care Res. 2016;68(12):1849–1858. doi:10.1002/acr.22900
30. Hawman DW, Stoermer KA, Montgomery SA, et al. Chronic joint disease caused by persistent chikungunya virus infection is controlled by the adaptive immune response. J Virol. 2013;87(24):13878–13888. doi:10.1128/JVI.02666-13
31. Pott F, Postmus D, Brown RJP, et al. Single-cell analysis of arthritogenic alphavirus-infected human synovial fibroblasts links low abundance of viral RNA to induction of innate immunity and arthralgia-associated gene expression. Emerg Microbes Infect. 2021;10(1):2151–2168. doi:10.1080/22221751.2021.2000891
32. Chow A, Her Z, Ong EKS, et al. Persistent arthralgia induced by chikungunya virus infection is associated with interleukin-6 and granulocyte macrophage colony-stimulating factor. J Infect Dis. 2011;203(2):149–157. doi:10.1093/infdis/jiq042
33. Renault P, Solet J-L, Sissoko D, et al. A major epidemic of chikungunya virus infection on Reunion Island, France, 2005–2006. Am J Trop Med Hyg. 2007;77(4):727–731. doi:10.4269/ajtmh.2007.77.727
34. Mavalankar D, Shastri P, Bandyopadhyay T, Parmar J, Ramani KV. Increased mortality rate associated with chikungunya epidemic, Ahmedabad, India. Emerg Infect Dis. 2008;14(3):412–415. doi:10.3201/eid1403.070720
35. Vidal ERN, Frutuoso LCV, Duarte EC, Peixoto HM. Epidemiological burden of chikungunya fever in Brazil, 2016 and 2017. Trop Med Int Health. 2022;27(2):174–184. doi:10.1111/tmi.13711
36. Gogia A, Kakar A, Kakar A. CHIKUNGUNYA: a Mortality Report. Open Forum Infect Dis. 2017;4(suppl_1):S518–S518. doi:10.1093/ofid/ofx163.1348
37. Burt FJ, Chen W, Miner JJ, et al. Chikungunya virus: an update on the biology and pathogenesis of this emerging pathogen. Lancet Infect Dis. 2017;17(4):e107–e117. doi:10.1016/S1473-3099(16)30385-1
38. Haese NN, Broeckel RM, Hawman DW, Heise MT, Morrison TE, Streblow DN. Animal models of chikungunya virus infection and disease. J Infect Dis. 2016;214(suppl 5):S482–S487. doi:10.1093/infdis/jiw284
39. Nguyen W, Nakayama E, Yan K, et al. Arthritogenic alphavirus vaccines: serogrouping versus cross-protection in mouse models. Vaccines. 2020;8(2):209. doi:10.3390/vaccines8020209
40. Erasmus JH, Auguste AJ, Kaelber JT, et al. A chikungunya fever vaccine utilizing an insect-specific virus platform. Nat Med. 2017;23(2):192–199. doi:10.1038/nm.4253
41. Garcia-Arriaza J, Cepeda V, Hallengard D, et al. A novel poxvirus-based vaccine, MVA-CHIKV, is highly immunogenic and protects mice against chikungunya infection. J Virol. 2014;88(6):3527–3547. doi:10.1128/JVI.03418-13
42. Broeckel R, Haese N, Messaoudi I, Streblow DN. Nonhuman primate models of chikungunya virus infection and disease (CHIKV NHP model). Pathogens. 2015;4(3):662–681. doi:10.3390/pathogens4030662
43. Wauquier N, Becquart P, Nkoghe D, Padilla C, Ndjoyi-Mbiguino A, Leroy EM. The acute phase of chikungunya virus infection in humans is associated with strong innate immunity and T CD8 cell activation. J Infect Dis. 2011;204(1):115–123. doi:10.1093/infdis/jiq006
44. Chua C-L, Sam I-C, Chiam C-W, Chan Y-F. The neutralizing role of IgM during early chikungunya virus infection. PLoS One. 2017;12(2):e0171989. doi:10.1371/journal.pone.0171989
45. Lum F-M, Teo T-H, Lee W, Kam Y-W, Renia L, Ng LFP. An essential role of antibodies in the control of chikungunya virus infection. J Immunol. 2013;190(12):6295–6302. doi:10.4049/jimmunol.1300304
46. Couderc T, Lecuit M. Focus on chikungunya pathophysiology in human and animal models. Microbes Infect. 2009;11(14–15):1197–1205. doi:10.1016/j.micinf.2009.09.002
47. Yoon I-K, Alera MT, Lago CB, et al. High rate of subclinical chikungunya virus infection and association of neutralizing antibody with protection in a prospective cohort in the Philippines. PLoS Negl Trop Dis. 2015;9(5):e0003764. doi:10.1371/journal.pntd.0003764
48. Milligan GN, Schnierle BS, McAuley AJ, Beasley DWC. Defining a correlate of protection for chikungunya virus vaccines. Vaccine. 2018. doi:10.1016/j.vaccine.2018.10.033
49. Huang C-H, Tsai Y-T, Wang S-F, Wang W-H, Chen Y-H. Dengue vaccine: an update. Expert Rev Anti Infect Ther. 2021;19(12):1495–1502. doi:10.1080/14787210.2021.1949983
50. Linn ML, Aaskov JG, Suhrbier A. Antibody-dependent enhancement and persistence in macrophages of an arbovirus associated with arthritis. J Gen Virol. 1996;77(Pt 3):407–411. doi:10.1099/0022-1317-77-3-407
51. Teo TH, Lum FM, Claser C, et al. A pathogenic role for CD4+ T cells during chikungunya virus infection in mice. J Immunol. 2013;190(1):259–269. doi:10.4049/jimmunol.1202177
52. Morrison TE, Oko L, Montgomery SA, et al. A mouse model of chikungunya virus-induced musculoskeletal inflammatory disease: evidence of arthritis, tenosynovitis, myositis, and persistence. Am J Pathol. 2011;178(1):32–40. doi:10.1016/j.ajpath.2010.11.018
53. Hoarau -J-J, Gay F, Pellé O, et al. Identical strength of the T cell responses against E2, nsP1 and capsid CHIKV proteins in recovered and chronic patients after the epidemics of 2005–2006 in La Reunion Island. PLoS One. 2013;8(12):e84695. doi:10.1371/journal.pone.0084695
54. Mapalagamage M, Weiskopf D, Sette A, de Silva AD. Current understanding of the role of T cells in chikungunya, dengue and zika infections. Viruses. 2022;14(2):242. doi:10.3390/v14020242
55. Petitdemange C, Wauquier N, Vieillard V. Control of immunopathology during chikungunya virus infection. J Allergy Clin Immunol. 2015;135(4):846–855. doi:10.1016/j.jaci.2015.01.039
56. Kulkarni SP, Ganu M, Jayawant P, Thanapati S, Ganu A, Tripathy AS. Regulatory T cells and IL-10 as modulators of chikungunya disease outcome: a preliminary study. Eur J Clin Microbiol Infect Dis. 2017;36(12):2475–2481. doi:10.1007/s10096-017-3087-4
57. Gois BM, Peixoto RF, Guerra-Gomes IC, et al. Regulatory T cells in acute and chronic human chikungunya infection. Microbes Infect. 2022;24(3):104927. doi:10.1016/j.micinf.2021.104927
58. Harrison VR, Eckels KH, Bartelloni PJ, Hampton C. Production and evaluation of a formalin-killed chikungunya vaccine. J Immunol. 1971;107(3):643–647.
59. Tiwari M, Parida M, Santhosh SR, Khan M, Dash PK, Rao PVL. Assessment of immunogenic potential of Vero adapted formalin inactivated vaccine derived from novel ECSA genotype of chikungunya virus. Vaccine. 2009;27(18):2513–2522. doi:10.1016/j.vaccine.2009.02.062
60. Khan M, Dhanwani R, Rao PV, Parida M. Subunit vaccine formulations based on recombinant envelope proteins of Chikungunya virus elicit balanced Th1/Th2 response and virus-neutralizing antibodies in mice. Virus Res. 2012;167(2):236–246. doi:10.1016/j.virusres.2012.05.004
61. Kumar M, Sudeep AB, Arankalle VA. Evaluation of recombinant E2 protein-based and whole-virus inactivated candidate vaccines against chikungunya virus. Vaccine. 2012;30(43):6142–6149. doi:10.1016/j.vaccine.2012.07.072
62. Weber C, Büchner SM, Schnierle BS. A small antigenic determinant of the chikungunya virus E2 protein is sufficient to induce neutralizing antibodies which are partially protective in mice. PLoS Negl Trop Dis. 2015;9(4):e0003684. doi:10.1371/journal.pntd.0003684
63. Levitt NH, Ramsburg HH, Hasty SE, Repik PM, Cole FE, Lupton HW. Development of an attenuated strain of chikungunya virus for use in vaccine production. Vaccine. 1986;4(3):157–162. doi:10.1016/0264-410X(86)90003-4
64. Edelman R, Tacket CO, Wasserman SS, Bodison SA, Perry JG, Mangiafico JA. Phase II safety and immunogenicity study of live chikungunya virus vaccine TSI-GSD-218. Am J Trop Med Hyg. 2000;62(6):681–685. doi:10.4269/ajtmh.2000.62.681
65. Hallengärd D, Lum F-M, Kümmerer BM, et al. Prime-boost immunization strategies against chikungunya virus. J Virol. 2014;88(22):13333–13343. doi:10.1128/JVI.01926-14
66. Zhang Y-N, Deng C-L, Li J-Q, et al. Infectious Chikungunya Virus (CHIKV) with a complete capsid deletion: a new approach for a CHIKV vaccine. J Virol. 2019;93(15). doi:10.1128/JVI.00504-19
67. Zhang Y-N, Zhang Z-R, Li N, et al. High-titer self-propagating capsidless chikungunya virus generated in vero cells as a strategy for alphavirus vaccine development. J Virol. 2022;96(6):e0148021. doi:10.1128/JVI.01480-21
68. Roques P, Ljungberg K, Kümmerer BM, et al. Attenuated and vectored vaccines protect nonhuman primates against chikungunya virus. JCI Insight. 2017;2(6):e83527. doi:10.1172/jci.insight.83527
69. Wressnigg N, Hochreiter R, Zoihsl O, et al. Single-shot live-attenuated chikungunya vaccine in healthy adults: a phase 1, randomised controlled trial. Lancet Infect Dis. 2020;20(10):1193–1203. doi:10.1016/S1473-3099(20)30238-3
70. Roques P, Fritzer A, Dereuddre-Bosquet N, et al. Effectiveness of CHIKV vaccine VLA1553 demonstrated by passive transfer of human sera. JCI Insight. 2022;7. doi:10.1172/jci.insight.160173
71. Chu H, Das SC, Fuchs JF, et al. Deciphering the protective role of adaptive immunity to CHIKV/IRES a novel candidate vaccine against Chikungunya in the A129 mouse model. Vaccine. 2013;31(33):3353–3360. doi:10.1016/j.vaccine.2013.05.059
72. Plante K, Wang E, Partidos CD, et al. Novel chikungunya vaccine candidate with an IRES-based attenuation and host range alteration mechanism. PLoS Pathog. 2011;7(7):e1002142. doi:10.1371/journal.ppat.1002142
73. Roy CJ, Adams AP, Wang E, et al. Chikungunya vaccine candidate is highly attenuated and protects nonhuman primates against telemetrically monitored disease following a single dose. J Infect Dis. 2014;209(12):1891–1899. doi:10.1093/infdis/jiu014
74. Partidos CD, Paykel J, Weger J, et al. Cross-protective immunity against o’nyong-nyong virus afforded by a novel recombinant chikungunya vaccine. Vaccine. 2012;30(31):4638–4643. doi:10.1016/j.vaccine.2012.04.099
75. Erasmus JH, Seymour RL, Kaelber JT, et al. Novel insect-specific eilat virus-based chimeric vaccine candidates provide durable, mono- and multivalent, single-dose protection against lethal alphavirus challenge. J Virol. 2018;92(4). doi:10.1128/JVI.01274-17
76. Sun S, Xiang Y, Akahata W, et al. Structural analyses at pseudo atomic resolution of chikungunya virus and antibodies show mechanisms of neutralization. Elife. 2013;2:e00435. doi:10.7554/eLife.00435
77. Akahata W, Yang Z-Y, Andersen H, et al. A virus-like particle vaccine for epidemic chikungunya virus protects nonhuman primates against infection. Nat Med. 2010;16(3):334–338. doi:10.1038/nm.2105
78. Chang L-J, Dowd KA, Mendoza FH, et al. Safety and tolerability of chikungunya virus-like particle vaccine in healthy adults: a phase 1 dose-escalation trial. Lancet. 2014;384(9959):2046–2052. doi:10.1016/S0140-6736(14)61185-5
79. Chen GL, Coates EE, Plummer SH, et al. Effect of a chikungunya virus-like particle vaccine on safety and tolerability outcomes: a randomized clinical trial. JAMA. 2020;323(14):1369–1377. doi:10.1001/jama.2020.2477
80. Goo L, Dowd KA, Lin T-Y, et al. A virus-like particle vaccine elicits broad neutralizing antibody responses in humans to all chikungunya virus genotypes. J Infect Dis. 2016;214(10):1487–1491. doi:10.1093/infdis/jiw431
81. Bennett SR, McCarty JM, Ramanathan R, et al. Safety and immunogenicity of PXVX0317, an aluminium hydroxide-adjuvanted chikungunya virus-like particle vaccine: a randomised, double-blind, parallel-group, Phase 2 trial. Lancet Infect Dis. 2022;22:1343–1355. doi:10.1016/S1473-3099(22)00226-2
82. Chattopadhyay A, Wang E, Seymour R, Weaver SC, Rose JK. A chimeric vesiculo/alphavirus is an effective alphavirus vaccine. J Virol. 2013;87(1):395–402. doi:10.1128/JVI.01860-12
83. Sutter G, Moss B. Novel vaccinia vector derived from the host range restricted and highly attenuated MVA strain of vaccinia virus. Dev Biol Stand. 1995;84:195–200.
84. Volz A, Sutter G. Modified vaccinia virus ankara: history, value in basic research, and current perspectives for vaccine development. Adv Virus Res. 2017;97:187–243. doi:10.1016/bs.aivir.2016.07.001
85. Weger-Lucarelli J, Chu H, Aliota MT, Partidos CD, Osorio JE. A novel MVA vectored Chikungunya virus vaccine elicits protective immunity in mice. PLoS Negl Trop Dis. 2014;8(7):e2970. doi:10.1371/journal.pntd.0002970
86. van den Doel P, Volz A, Roose JM, et al. Recombinant modified vaccinia virus Ankara expressing glycoprotein E2 of Chikungunya virus protects AG129 mice against lethal challenge. PLoS Negl Trop Dis. 2014;8(9):e3101. doi:10.1371/journal.pntd.0003101
87. Campos RK, Preciado-Llanes L, Azar SR, et al. Un-adjuvanted dose of a chimpanzee adenovirus-vectored vaccine against chikungunya virus fully protects mice from lethal disease. Pathogens. 2019;8(4). doi:10.3390/pathogens8040231
88. Folegatti PM, Harrison K, Preciado-Llanes L, et al. A single dose of ChAdOx1 Chik vaccine induces neutralizing antibodies against four chikungunya virus lineages in a phase 1 clinical trial. Nat Commun. 2021;12(1):4636. doi:10.1038/s41467-021-24906-y
89. Mühlebach MD. Vaccine platform recombinant measles virus. Virus Genes. 2017;53(5):733–740. doi:10.1007/s11262-017-1486-3
90. Combredet C, Labrousse V, Mollet L, et al. A molecularly cloned Schwarz strain of measles virus vaccine induces strong immune responses in macaques and transgenic mice. J Virol. 2003;77(21):11546–11554. doi:10.1128/jvi.77.21.11546-11554.2003
91. Rossi SL, Comer JE, Wang E, et al. Immunogenicity and efficacy of a measles virus-vectored chikungunya vaccine in nonhuman primates. J Infect Dis. 2019;jiz202. doi:10.1093/infdis/jiz202
92. Reisinger EC, Tschismarov R, Beubler E, et al. Immunogenicity, safety, and tolerability of the measles-vectored chikungunya virus vaccine MV-CHIK: a double-blind, randomised, placebo-controlled and active-controlled phase 2 trial. Lancet. 2019;392(10165):2718–2727. doi:10.1016/S0140-6736(18)32488-7
93. Ramsauer K, Schwameis M, Firbas C, et al. Immunogenicity, safety, and tolerability of a recombinant measles-virus-based chikungunya vaccine: a randomised, double-blind, placebo-controlled, active-comparator, first-in-man trial. Lancet Infect Dis. 2015;15(5):519–527. doi:10.1016/S1473-3099(15)70043-5
94. Tschismarov R, Zellweger RM, Koh MJ, et al. Antibody effector analysis of prime versus prime-boost immunizations with a recombinant measles-vectored chikungunya virus vaccine. JCI Insight. 2021;6(21). doi:10.1172/jci.insight.151095
95. Mallilankaraman K, Shedlock DJ, Bao H, et al. A DNA vaccine against chikungunya virus is protective in mice and induces neutralizing antibodies in mice and nonhuman primates. PLoS Negl Trop Dis. 2011;5(1):e928. doi:10.1371/journal.pntd.0000928
96. Ge N, Sun J, Liu Z, et al. An mRNA vaccine encoding chikungunya virus E2-E1 protein elicits robust neutralizing antibody responses and CTL immune responses. Virol Sin. 2022;37(2):266–276. doi:10.1016/j.virs.2022.01.032
97. Shaw C, Panther L, August A, et al. Safety and immunogenicity of a mRNA-based chikungunya vaccine in a phase 1 dose-ranging trial. Int J Infect Dis. 2019;79:17. doi:10.1016/j.ijid.2018.11.058
98. Schmidt C, Haefner E, Gerbeth J, et al. A taRNA vaccine candidate induces a specific immune response that protects mice against chikungunya virus infections. Mol Ther Nucleic Acids. 2022;28:743–754. doi:10.1016/j.omtn.2022.04.036
99. August A, Attarwala HZ, Himansu S, et al. A phase 1 trial of lipid-encapsulated mRNA encoding a monoclonal antibody with neutralizing activity against Chikungunya virus. Nat Med. 2021;27(12):2224–2233. doi:10.1038/s41591-021-01573-6
100. Yang S, Fink D, Hulse A, Pratt RD. Regulatory considerations in development of vaccines to prevent disease caused by chikungunya virus. Vaccine. 2017;35(37):4851–4858. doi:10.1016/j.vaccine.2017.07.065
101. Couderc T, Khandoudi N, Grandadam M, et al. Prophylaxis and therapy for chikungunya virus infection. J Infect Dis. 2009;200(4):516–523. doi:10.1086/600381
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.