Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 8
Chemotherapy-associated paronychia treated with a dilute povidone-iodine/dimethylsulfoxide preparation
Authors Capriotti K, Capriotti J
Received 15 June 2015
Accepted for publication 20 August 2015
Published 21 September 2015 Volume 2015:8 Pages 489—491
DOI https://doi.org/10.2147/CCID.S90542
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Kara Capriotti,1,2 Joseph A Capriotti1,3
1ALC Therapeutics, LLC, Springhouse, PA, 2Bryn Mawr Skin and Cancer Institute, Rosemont, PA, 3Plessen Ophthalmology Consultants, Christiansted, VI, USA
Background: Nail changes associated with chemotherapy in general, and particularly with taxane and epidermal growth factor receptor inhibitor-based regimens, are common presentations in our clinical population. Currently, there are no consensuses about therapies supported by clinical trials nor are there any US Food and Drug Administration-approved treatments for this indication.
Findings: A 42-year-old woman with stage 2A breast cancer presented to our clinic with chemotherapy-induced paronychia. Symptoms were severe enough that cessation of chemotherapy was being considered. The patient's chemotherapy regimen included doxorubicin, cyclophosphamide, and docetaxel.
Conclusion: The topical povidone-iodine/dimethylsulfoxide system is very effective in alleviating the signs and symptoms of severe paronychia associated with chemotherapy. This novel combination warrants further investigation in randomized, controlled trials to further elucidate its clinical utility.
Keywords: paronychia, chemotherapy, breast cancer, povidone-iodine, dimethylsulfoxide
Introduction
Nail changes are a well-documented side effect of chemotherapy, most commonly arising from the taxanes (docetaxel, paclitaxel, and nab-paclitaxel) and epidermal growth factor receptor inhibitors (EGFRIs). Minor nail changes include hyperpigmentation, orange discoloration, splinter hemorrhages, subungual hematomas and hyperkeratosis, dystrophy, secondary infections, and Beau’s lines; none of which interfere with daily functioning.1 Periungual erythema, edema, exudation, and painful onycholysis with secondary infection represent the commoner and often limiting changes.2,3 The latter changes can significantly impair the patients’ health-related quality of life, impact activities of daily living, and sometimes necessitate modification or discontinuation of anticancer treatment.4
Case report
A 42-year-old woman with stage 2A breast cancer presented complaining of a 2-week history of painful swelling of the proximal and lateral nail folds, as well as discharge and tissue overgrowth from the proximal nail fold. The patients’ primary complaint was the limitation of her daily activities secondary to pain after the onset of nail changes, corresponding to grade 3 in the common terminology criteria for adverse events grading scale.5 The nail unit abnormalities began during the patient’s second cycle of chemotherapy, which included doxorubicin, cyclophosphamide, and docetaxel. There was no requirement for Institutional Review Board approval or patient consent, because no human experimentation or clinical trial was completed.
Physical examination revealed mild periungual swelling and erythema of eight fingernails. Serosanginous crusting/exudate and granulation tissue also involved the proximal nail folds (Figure 1). Toenails were not involved. A diagnosis of chemotherapy-associated paronychia was made.
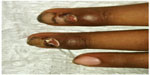  | Figure 1 Baseline treatment. |
The patient was given a topical solution of 1% povidone-iodine (PVP-I) in a dimethylsulfoxide (DMSO) vehicle that was prescribed from a licensed compounding pharmacy. The patient applied the solution twice daily. After 2 days of use, the pain limiting her daily activities had completely resolved. At the 4-week follow-up visit, all periungual swelling and erythema had resolved, and four of the eight nails with crusting and granulation tissue showed complete clearance. At the 8-week follow-up, all nail units had returned to baseline (Figure 2). The patient was able to complete the full course of chemotherapy.
  | Figure 2 Eight weeks of treatment. |
Discussion
Nail changes occur in 34.9% and 17.2% of patients receiving taxanes and EGFRIs, respectively.6,7 Anecdotal data have demonstrated topical application of antibiotics or steroids in mild cases, oral antibiotics or corticosteroids in moderate cases, with severe cases requiring surgical intervention in the form of complete or partial nail avulsions.8 There is a lack of standardized and effective treatment for nail toxicities. PVP-I is used primarily in dermatology as a surgical prep as it has been recognized as a broad spectrum, resistance-free biocidal agent for many years. Although incompletely understood, it is likely that free iodine poisons electron transport inhibits cellular respiration, destabilizes membranes, inhibits protein synthesis, and denatures nucleic acids. Although PVP-I kills microorganisms, including bacteria, viruses, yeasts, molds, fungi, and protozoa, it has scarcely been used for purposes outside of skin asepsis in dermatology.9
DMSO is a very effective pharmaceutical vehicle, greatly enhancing percutaneous penetration when used in combination with other substances. DMSO facilitates diffusion through the stratum corneum, triggers the formation of drug deposition in the dermis, and promotes transport into local blood vessels.10 Compounded PVP-I in a DMSO vehicle is commonly used in our practice for a variety of indications such as Verruca vulgaris, Molluscum contagiosum, and common paronychia.
Conclusion
We have had remarkable success, particularly in paronychia and nail disease, where all other agents, both topical and systemic, have failed. The lack of US Food and Drug Administration-approved therapies present a clinical dilemma for both the practitioner and the patient. The topical povidone-iodine/DMSO system which we have pioneered has been very effective in alleviating the signs and symptoms of severe paronychia associated with chemotherapy. This novel combination warrants further investigation in randomized, controlled trials to further elucidate its clinical utility with the aim of enabling an eventual US Food and Drug Administration approval for this currently unmet need.
Disclosure
The authors report no conflicts of interest in this work.
References
Vassallo C, Brazzelli B, Ardigo M, Borroni G. The irreplaceable image: nail changes in onco-hematologic patients. Haematologica. 2001;86:334–336. | |
Minisini AM, Tosti A, Sobrero AF, et al. Taxane-induced nail changes: incidence, clinical presentation and outcome. Ann Oncol. 2003;14:333–337. | |
Robert C, Sibaud V, Mateus C, et al. Nail toxicities induced by systemic chemotherapy treatments. Lancet Oncol. 2015;16(4):e181–e190. | |
Rosen AC, Case EC, Dusza SW, et al. Impact of dermatologic adverse events on quality of life in 283 cancer patients: a questionnaire study in a dermatology referral clinic. Am J Clin Dermatol. 2013;14:327–333. | |
Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events, Version 4.0. Available from: http://ctep.cancer.gov. Accessed July 30, 2015. | |
Capriotti K, Capriotti JA, Lessin S, et al. The risk of nail changes with taxane chemotherapy: a systematic review of the literature and meta-analysis. Br J Dermatol. Epub February 21, 2015. | |
Garden BC, Wu S, Lacouture ME. The risk of nail changes with epidermal growth factor receptor inhibitors. J Am Acad Dermatol. 2012;67(3):400–408. | |
Paraccini BM, Iorizzo M, Starace M, Tosti A. Drug-induced nail diseases. Dermatol Clin. 2006;24:387–391. | |
Capriotti K, Capriotti JA. Topical iodophor preparations: chemistry, microbiology, and clinical utility. Dermatol Online J. 2012;18(11):1. | |
Capriotti K, Capriotti JA. Dimethyl sulfoxide: history, chemistry, and clinical utility in dermatology. J Clin Aesthet Dermatol. 2102;5(9):24–26. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
