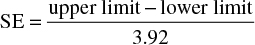Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 10
Checkpoint inhibitors for malignant melanoma: a systematic review and meta-analysis
Authors Karlsson AK, Saleh SN
Received 28 August 2016
Accepted for publication 13 January 2017
Published 24 August 2017 Volume 2017:10 Pages 325—339
DOI https://doi.org/10.2147/CCID.S120877
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Adam K Karlsson,1 Sohag N Saleh2
1Faculty of Medicine, Imperial College London, 2Faculty of Medicine, Hammersmith Hospital, Imperial College London, London, UK
Background and objectives: Rates of malignant melanoma are continuing to increase, and until recently effective treatments were lacking. However, since 2011 three immunotherapeutic agents, known as checkpoint inhibitors, have been approved. This review aims to establish whether these three drugs – ipilimumab, nivolumab, and pembrolizumab – offer greater efficacy and tolerability compared to control interventions (placebo, immunotherapy, or chemotherapy) in patients with stage III or IV unresectable cutaneous melanoma.
Materials and methods: A search on four major medical and scientific databases yielded 7,553 records, of which seven met the inclusion criteria, with a total study population of 3,628. Only prospective Phase II or III randomized controlled trials on checkpoint inhibitors for patients with unresectable cutaneous melanoma that reported data on survival (overall or progression-free), tumor response, or adverse events were included. Three meta-analyses were carried out.
Results: The hazard ratio for progression or death was 0.54 (95% confidence interval [CI]: 0.44–0.67), and the odds ratio for best overall response rate was 4.48 (95% CI: 2.77–7.24), both in favor of checkpoint inhibitors. However, control treatments were associated with an insignificantly lower rate of discontinuation of treatment due to adverse effects or treatment-related adverse events (odds ratio =1.63 [95% CI: 0.55–4.88]).
Conclusion: This study finds that checkpoint inhibitors are more effective than control interventions, both in terms of survival and tumor response, and yet no less tolerable. PD1 therapies (nivolumab and pembrolizumab) appear to offer greater efficacy than CTLA4 therapy (ipilimumab). The combination of nivolumab and ipilimumab was, however, the most effective, but significantly less tolerable than monotherapy. The lack of published clinical data does, however, limit this study. Further research is needed in two areas in particular: 1) to determine the optimal use of checkpoint inhibitors, specifically in terms of combination therapy, and 2) to identify reliable biomarkers to predictive responders and guide treatment assignment.
Keywords: checkpoint inhibitors, immunotherapy, melanoma, ipilimumab, nivolumab, pembrolizumab
Introduction
Melanoma
Melanoma is a malignant neoplasm arising from melanocytes, the melanin-producing cells of the body. Over the last half century, the incidence of melanoma in most developed countries has risen more than any other form of cancer, with rates increasing by 360% in the UK since the late 1970s.1–3 The current World Health Organization (WHO) estimates are that 132,000 melanomas occur each year around the world, resulting in 65,000 deaths annually.4,5 While genetic and phenotypic factors, such as lightly pigmented skin, increases one’s risk, the main cause is thought to be environmental exposure to the sun’s ultraviolet radiation.6 Early diagnosis and resection will cure nine of ten cases of stage I melanoma.7 The prognosis for regional and distant metastatic melanoma (stages III and IV, respectively) is variable but generally poor, with 5-year survival rates for stage III of 13%–69% and as low as 6% in stage IV.8,9
The poor prognosis of advanced melanoma is in part due to the limited therapeutic options available. Surgery and radiotherapy provide mainly palliation, and chemotherapy, most commonly with dacarbazine, has failed to show any consistent survival benefit.10–12 Novel pharmacological agents have, however, been developed, such as BRAF13 and MEK inhibitors,14 as well as several immunotherapeutic agents, most notably the class of drugs known as checkpoint inhibitors.15
Checkpoint inhibitors
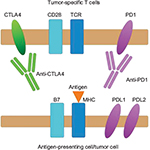
Cellular immune defense against neoplasms begins with the recognition of a tumor antigen by a tumor-specific T-cell receptor. The interaction of costimulatory and coinhibitory molecules with their respective receptors on T cells, as illustrated in Figure 1, determines the balance between T-cell activation and inhibition. CTLA4 is a coinhibitory receptor present on the cell surface of CD4+ and CD8+ T cells that acts to dampen down the immune response. CTLA4 expression is upregulated by increased T-cell activation and an inflammatory environment, suggesting that it acts as a physiological brake on immune response. Through higher affinity for CD80 and CD86 ligands present on antigen-presenting cells and tumor cells, CTLA4 is able to outcompete the costimulatory receptor CD28 for binding, and thus negatively regulates T-cell activation.16,17 Similarly, PD1 receptors expressed on T cells and other immune cells generate a coinhibitory signal upon binding to their ligands, PDL1 and PDL2, resulting in direct inhibition of tumor apoptosis, T-cell exhaustion, and conversion of effector T cells to regulatory T cells.18
Melanoma cells are able to hijack this system, for example, by expressing coinhibitory molecules within the tumor microenvironment. This dampens down the immune response, and thus hampers effective tumor clearance.19,20 Ipilimumab, a fully human IgG1 monoclonal antibody that targets CTLA4, along with nivolumab and pembrolizumab, humanized IgG4 monoclonal antibodies that target PD1, prevent the interaction between coinhibitory molecules and their receptors, thereby releasing the brake on the body’s natural defense to tumors.21,22
Ipilimumab received US Food and Drug Administration (FDA) approval in 2011 and pembrolizumab and nivolumab in 2014 for the treatment of unresectable or metastatic melanoma. Nivolumab is also licensed for combination therapy with ipilimumab, as well as for non-small-cell lung cancer (as is pembrolizumab) and renal cell cancer.23–25 All three drugs are also recommended for use by the National Institute for Health and Care Excellence in the UK.26
Rationale
Given the increasing rates of melanoma and the poor prognosis of advanced disease, checkpoint inhibitors have the potential to improve patient outcomes greatly. Therefore, a comprehensive overview of the evidence on the efficacy and tolerability of this drug class is needed to ascertain its value and identify any gaps in knowledge requiring further research and investigation.
Objectives
For this reason, this systematic review and meta-analysis aims to answer the question: Do the three currently approved checkpoint inhibitors – ipilimumab, nivolumab, and pembrolizumab – offer greater efficacy and tolerability compared to control interventions, consisting of a placebo, another immunotherapeutic agent, and/or chemotherapy (Table 1), in patients with stage III or IV unresectable cutaneous melanoma, in terms of progression-free and overall survival, tumor response, and discontinuation rates? The specific objectives were thus to identify all relevant studies and to use quantitative methods to compile their results. Any heterogeneity in the results between individual drugs and studies are also explored, in order to assess between-drug differences in efficacy and tolerability. Finally, the findings of this study are placed in their context, and areas requiring further research explored. The authors hypothesize that checkpoint inhibitors would be found to be both more effective and tolerable than control treatments.
Materials and methods
Study identification
An electronic search was carried out on four databases:
- Embase Classic and Embase: 1947–March 26, 2016
- Medline and Medline In-Process and Other Non-Indexed Citations: 1946–March 27, 2016
- Web of Science Core Collection: 1970–March 27, 2016
- Cochrane library: all years–March 27, 2016
A similar search strategy was conducted for all databases, consisting of various iterations of the drug names (for example, ipilimumab, MDX-010, MDX-101, Yervoy, and BMS-734016) and of melanoma (Supplementary materials Appendix A for full list). No limits in terms of date ranges or “NOT” search terms were used. The reference lists of other articles identified as relevant were manually screened for any missing studies.
Study selection
Search results were exported to Microsoft Excel and duplicates removed, before a first screening of the title and abstract of the remaining reports was conducted, wherein those that did not pertain to cutaneous malignant melanoma and/or checkpoint inhibitors, were not original research, were not available in English, or had a clearly inappropriate study design according to the inclusion and exclusion criteria were removed. The remaining articles were reviewed in their entirety and assessed according to the inclusion and exclusion criteria, as listed in Table 2. Two investigators, working independently, carried out the study selection, and came to a combined decision on the eligibility of studies when there were any differences of opinion.
Assessment of study quality
Using the 2010 CONSORT (Consolidated Standards of Reporting Trials) checklist,27 compromising 25 items related to the design, analysis, and interpretation of randomized controlled trials, the quality of all studies included was assessed. A test of the strength of correlation between study quality and primary efficacy outcome was carried out, in order to assess whether lower quality studies may have biased the results of the meta-analysis (Supplementary materials online [Section A] for full details).
A risk-of-bias assessment at the study level was carried out, using the criteria provided in Review Manager (version 5.3). The risk of bias across studies was also assessed by funnel plots to test for the presence of publication bias (Supplementary materials [Section D]), and by examining the source of funding for all included studies.
Data collection
Baseline participant-demographic data and outcome data were extracted into separate spreadsheets. No data were extrapolated or directly extracted from graphs. When multiple sets of data were reported, the data judged as the most robust and unbiased were extracted, for example, independent review committee’s data over investigator-assessed data.
Outcomes
The outcomes of this study relate to the efficacy and tolerability of checkpoint inhibitors compared to control interventions:
- Primary outcome
- Survival – hazard ratio (HR) for progression or death
- Secondary outcome
- Tumor response – odds ratio (OR) for best overall response rate (BORR)
- Tolerability – OR for rates of discontinuation due to adverse effects or treatment-related adverse events
The primary outcome used HRs for progression or HRs for death, based on progression-free survival (PFS), and overall survival (OS), respectively. While OS is defined as the time from randomization to death from any cause, PFS is the time from randomization to first disease progression or death from any cause, whichever comes first. Due to the disparity in the reporting of end points in the literature and to ensure an adequate sample size, these end points were combined for the primary outcome meta-analysis. Importantly, a meta-analysis has shown that for melanoma, PFS is a reliable surrogate for OS, with correlation coefficients of 0.55–0.96.28 Where both end points were reported,21,29 the HR for progression was used, meaning that for only one study15 was the HR for death used (Table 3 for an overview of outcomes reported in each study).
The secondary outcome on tumor response used BORR, defined as the proportion of patients with a partial or a complete response as assessed by the revised RECIST (Response Evaluation Criteria In Solid Tumors, version 1.1) criteria30 for five studies or the modified WHO criteria31 for two studies. The secondary outcome on tolerability was rate of discontinuation due to adverse events, or specifically treatment-related adverse events. The latter was used when available, meaning that for only one study29 were data on rates due to adverse events used.
Statistical analysis
Statistical analysis was carried out using the Cochrane Collaboration’s Review Manager (version 5.3) software. For dichotomous outcomes (tumor response and tolerability) an OR was calculated based on the Mantel–Haenszel statistical method. For primary outcome analysis with data in the form of HRs, generic inverse-variance analysis was used. Standard error (SE) was required for this analysis, and was manually calculated from the 95% confidence intervals (CIs) according to the following equation32:
|
|
The weight of each study was automatically calculated as the inverse variance of the effect estimate, meaning studies with narrower CIs were more heavily weighted.
Due to the inherent heterogeneity from combining three different drugs, the intervention treatments could not be said to be functionally equivalent, meaning a random rather than a fixed effects model was used. Tests of heterogeneity were performed on Review Manager. I2 was the measure used, as it emphasizes the effect of heterogeneity, rather than merely reporting its presence.33
Missing data
Attempts were made to contact four corresponding authors to request missing or unreported data, all without success.
Results
Included studies
Study selection
A total of 7,553 records across four databases were identified, with seven studies ultimately meeting the inclusion criteria, as seen in Figure 2, where the number of studies identified, reviewed, and excluded at each stage of the study selection is listed. After duplicates were removed, 4,947 records were screened and 295 full-text articles assessed for eligibility.
Study design
All seven studies that met the inclusion criteria were randomized, controlled Phase II or III trials, five of which were double-blinded, one completely open-labeled,22 and another partially open-labeled.34 Two studies included only ipilimumab, two only nivolumab, one only pembrolizumab, and two both ipilimumab and nivolumab. The control arms consisted of a placebo and checkpoint inhibitor in two studies, Gp100 (peptide cancer vaccine) and placebo in one study, dacarbazine alone or with a placebo in two studies, and investigator-choice chemotherapy in two studies, as illustrated in Table 1.
Three studies had three treatment arms, meaning a choice was made by the investigators as to which arms to compare.15,34,35 For the Hodi et al study,15 ipilimumab + Gp100 was compared to Gp100 alone, in order to isolate the effects of ipilimumab. For the Larkin et al study,35 the combination arm (nivolumab + ipilimumab) was compared with ipilimumab to isolate the effects of nivolumab, as comparison with nivolumab would fail to isolate the effects of ipilimumab, given the different nivolumab doses used in the two arms. Lastly, for the Ribas et al study,34 the approved dose of pembrolizumab (2 mg/kg) was compared to investigator-choice chemotherapy, rather than pembrolizumab 10 mg/kg.
In total, data from 3,628 patients were included. The mean age across the seven studies was 56.2–61.7 years, and the mean proportion of female participants was 38%, as shown in Supplementary materials, Table 1, Appendix B. The TNM (tumor, node, metastasis) system for melanoma by the American Joint Committee on Cancer was used in all included studies, with 2,383 patients classified as M1c and 1,143 patients classified as M0, M1a, or M1b.
All seven studies were included in the primary outcome analysis on survival and the secondary outcome analysis on tumor response, with one study reporting OS and the rest PFS. For the secondary outcome on tolerability, the Hodi et al15 study did not report data on discontinuations due to adverse events or treatment-related adverse events, as illustrated in Table 3, and was thus not included in the secondary outcome analysis on tolerability.
Study quality
The mean score across the seven studies for the 2010 CONSORT checklist was 64.4%, with only one study scoring <60%. The three parameters of the CONSORT checklist that were consistently done poorly, however, were providing a hypothesis or objective, describing the randomization procedure, and identifying any weaknesses or limitations in the study. There was a positive correlation (Pearson’s r=0.57) between the CONSORT checklist score and the HR for the primary efficacy outcome, wherein the lower quality studies reported more significant HRs (ie, closer to 0).
Heterogeneity
As seen in Table 4, there was a significant heterogeneity in all meta-analyses. Removing the lowest quality study as assessed by the CONSORT checklist or the two open-label studies had no significant effect on the I2 score.
  | Table 4 Heterogeneity scores for meta-analyses Note: The I2 heterogeneity score for each meta-analysis is listed, for three cases: when all studies were included; when the lowest quality study, as assessed by the CONSORT checklist, was excluded; and when the two open-label studies22,34 were excluded. Abbreviation: CONSORT, Consolidated Standards of Reporting Trials. |
Meta-analysis results
Primary outcome – hazard ratio for progression or death
This study found that median OS and PFS were consistently greater in the checkpoint inhibitor arms than in the control arms, with an overall HR of 0.54 (95% CI: 0.44–0.67) in favor of checkpoint inhibitors, as seen in Figure 3. The greatest advantage for checkpoint inhibitors was seen in the two studies comparing the combination of nivolumab + ipilimumab to ipilimumab monotherapy, which, if assuming an additive effect as opposed to a synergistic effect, isolates the effects of nivolumab.35,36 In one of these studies, the PFS in the combination arm, 11.5 (95% CI: 8.9–16.7) months, was only significantly superior when compared to the ipilimumab and placebo arm (2.9 [95% CI: 2.8–3.4] months), but not the nivolumab and placebo arm, (6.9 [95% CI: 4.3–9.5] months).35 The third greatest benefit for checkpoint inhibitors was seen for the comparison of nivolumab with dacarbazine.29
No statistically significant difference was found for PFS in the one study comparing two different doses of a checkpoint inhibitor (pembrolizumab).34 The only study to cross the line of no effect was the fully open-labeled study,22 which reported data for only a portion of its study population (182 of 405), and thus had a markedly wider CI. The I2 score was 91%, reflecting the poor alignment of CIs among the studies.
Secondary outcome – tumor response
Similar to the primary outcome on survival, all studies reporting BORR found that checkpoint inhibitors were superior to control interventions. The meta-analysis showed an overall effect estimate of OR =4.48 (95% CI: 2.77–7.24) favoring checkpoint inhibitors, as seen in Figure 4. Only two studies, both of which had ipilimumab as the checkpoint inhibitor, failed to show a statistically significant advantage, one compared to Gp100 vaccine,15 and the other to dacarbazine.21
The greatest tumor response was seen in the two studies combining nivolumab and ipilimumab (57.6% and 58.9%),35,36 but the BORR with nivolumab alone was more than twice as great as with ipilimumab alone (43.7% vs 19.0%) in the one study reporting both.35 The BORR in the chemotherapy arms was 4.5%–13.9%), but dacarbazine-specific arms had a narrower spread (10.3%–13.9%).21,22,29,34
Four studies had appreciably larger CIs, all of which had smaller control arms with fewer objective responses.15,22,34,36 The I2 score was 72%, suggesting moderate heterogeneity, but this was reduced to 0 after removing the two studies assessing the tumor response of ipilimumab.
Secondary outcome – tolerability
Unlike the previous two meta-analyses, the overall effect estimate for discontinuation due to adverse effects and treatment-related adverse events was OR =1.63 (95% CI: 0.55–4.88), insignificantly in favor of the control intervention compared to checkpoint inhibitors, as seen in Figure 5. Three studies favored control treatment, and three favored checkpoint inhibitors, but all of the latter crossed the line of no effect. These three studies all compared either nivolumab or pembrolizumab monotherapy to chemotherapy. Both studies comparing a combination of ipilimumab and nivolumab to ipilimumab alone found that more patients discontinued in the combination arms. The CIs were poorly aligned, with a high heterogeneity score – I2=93%.
In the one study reporting both tolerability end points, the order for discontinuations due to specifically treatment-related adverse events was pembrolizumab 10 mg/kg > chemotherapy > pembrolizumab 2 mg/kg. However, for discontinuation due to all adverse events, chemotherapy rather than pembrolizumab 2 mg/kg caused the lowest rate of discontinuation.
Bias
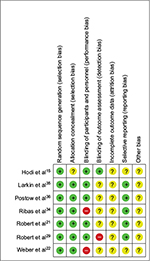
The risk of bias was assessed at both study and outcome levels, and in addition to this the presence of publication bias was assessed. As seen in Figure 6, most bias domains (selection bias, performance bias, attrition bias, reporting bias, and other bias) were marked as low or unclear risk, but three studies had one domain each marked as high risk. An unclear risk of bias was defined as a risk that was greater than low, but not sufficient to be considered high. At the study level, the Larkin et al and Postow et al studies35,36 had the lowest risk of bias, while the Weber et al study22 had the highest (Supplementary materials [Section C] for full risk-of-bias tables).
  | Figure 6 Risk of bias assessment at study level. Note: Low, unclear, and high scores given for the seven parameters assessed represented by green, yellow, and red circles, respectively. |
At the domain level, random sequence generation and allocation concealment were done well, while blinding of participants and personnel and blinding of outcome assessment were done poorly, as shown in Figure 7. Incomplete outcome data were marked as “unclear risk of bias” for all seven studies, for not adequately explaining why some patients were not evaluated or included in the analysis. All studies were funded by and designed in collaboration with the pharmaceutical company that developed or marketed the checkpoint inhibitor, which was noted under the “other bias” domain. The risk-of-bias or CONSORT quality scores were not used in the weighting of the meta-analyses, and are discussed only as part of the qualitative assessment of the studies included.
  | Figure 7 Risk-of-bias assessment at domain level. |
Publication bias
In assessing the presence of publication bias, the funnel plot for the primary outcome meta-analysis shows an even spread of studies on either side of the overall effect estimate line, as seen in Figure 8. There was a lack of low-quality studies with widespread effect estimates, reflecting the scarcity of published data. There did not, however, appear to be any significant publication bias. The funnel plots for the secondary outcome analysis on tumor response and tolerability (Appendices A and B, Supplementary materials [Section D]) were also spread evenly around their respective overall effect-estimate lines, but less closely clustered together, due to the greater disparity in the standard error of the logOR. There were too few studies in all of the meta-analyses carried out for any formal tests of funnel plot asymmetry to be performed.
Discussion
Checkpoint inhibitors used in the treatment of unresectable stage III and IV melanoma have been found to be more effective, as determined by prolonged survival times and improved tumor responses, and yet no less tolerable than control treatments in meta-analyses of seven randomized controlled trials. The discussion is divided into three sections: one exploring the results of the meta-analyses; another exploring the limitations of this study arising due to bias in the included studies, the outcomes used, and limitations at the review level; and one placing the findings of this study in their wider context and exploring the future directions of checkpoint inhibitors in the treatment of melanoma.
Meta-analyses
Efficacy
The HR for progression or death was 0.54 (95% CI: 0.44–0.67), and the OR for BORR was 4.48 (95% CI: 2.77–7.24), both in favor of checkpoint inhibitors. The two studies finding the greatest benefit in terms of survival and tumor response both compared the combination of nivolumab and ipilimumab to ipilimumab alone,35,36 suggesting that combination therapy is superior to ipilimumab monotherapy. However, the rates of discontinuation were significantly greater in the combination arms in both studies (ORs: 3.3 and 4.18, respectively).
Given that the greatest benefit was seen in the combination studies isolating the effects of nivolumab, followed by the two studies comparing nivolumab to dacarbazine29 and pembrolizumab to investigator-choice chemotherapy,34 one could suggest that anti-PD1 monoclonal antibodies are more effective than anti-CTLA4 monoclonal antibodies. However, comparing combination therapy A + B to drug A in order to isolate the effects of drug B assumes that the drugs have an additive rather than a synergistic effect. Additionally, the superiority of pembrolizumab over ipilimumab was not statistically significant, as the CIs overlapped.
Nonetheless, the Larkin et al study35 found that combination therapy vs ipilimumab and vs nivolumab yielded an HR of 0.42 (95% CI: 0.31–0.57) and an HR of 0.74 (95% CI: 0.6–0.92), respectively, indicating that while combination therapy was undoubtedly the most effective, those on nivolumab compared more favorably than those on ipilimumab. Moreover, the direct comparison of nivolumab and ipilimumab monotherapies gave a significant advantage to nivolumab, with an HR for progression of 0.57 (95% CI: 0.43–0.76).35 Taken together with the weakest benefit for checkpoint inhibitors coming from studies with ipilimumab in the experimental arm (with exception of the Weber et al study,22 due to its markedly wider CIs), PD1 therapy does in fact appear to be superior to CTLA4 therapy.
Data from the secondary meta-analysis on tumor response showing that both studies on ipilimumab failed to find statistically significant advantages over control treatments further suggests that ipilimumab lacks efficacy compared to the PD1-targeted therapies.15,21 This is despite the clear benefit of ipilimumab on PFS and OS, which raises the question of whether the two commonly used criteria for evaluating tumor response are suitable for checkpoint inhibitors. Unlike traditional cytotoxic agents, immunotherapies may mediate cytostatic rather than cytotoxic effects, or cause delayed tumor shrinkage due to the time lag between the disinhibition of the immune response and subsequent antitumor effects, meaning the traditional criteria may miss the positive effects of immunotherapies.37–39 New immunorelated response criteria (irRECIST) has been developed to capture better the atypical tumor responses seen with immunotherapeutic agents.40
None of the remaining studies failed to find statistically significant differences in BORR, although these studies used the RECIST criteria, while the ipilimumab studies used the modified WHO criteria. If, however, one assumes that this disparity was not due to the different criteria being used, given the data in (Table 4 Supplementary materials [Section B]), showing that the BORR for ipilimumab monotherapy was virtually the same in two studies, one using the modified WHO criteria15 and the other the RECIST criteria36 (10.9% and 10.6%, respectively), a reasonable interpretation is that PD1 therapy was again shown to be superior to CTLA4 therapy. A third plausible explanation is that differences in response kinetics are such that nivolumab and pembrolizumab are simply more suitable for evaluation with traditional criteria than ipilimumab is, and thus that no inference can be made about their relative efficacy based on this particular parameter.41
Tolerability
For the secondary outcome analysis on tolerability, checkpoint inhibitors were shown to be insignificantly inferior to control interventions for rates of discontinuation due to adverse events or treatment-related adverse events (OR =1.63, [95% CI: 0.55–4.88]). Importantly though, all three studies favoring control interventions compared combination therapy to monotherapy, which would naturally have made the monotherapy control arm appear more tolerable.21,35,36
The three studies favoring checkpoint inhibitors compared either pembrolizumab34 or nivolumab22,29 to chemotherapy, and although this may suggest superior tolerability compared to ipilimumab, differences in trial design prohibit such a conclusion. The three studies on PD1 therapy all compared monotherapy to chemotherapy, while the study on ipilimumab compared the combination of chemotherapy and ipilimumab to chemotherapy alone, where the monotherapy arm would naturally be expected to be more tolerable. Nonetheless, data in Table S5 (Supplementary materials [Section B]) show that 14.8%–17.4% discontinued ipilimumab monotherapy due to treatment-related adverse events across two studies35,36 compared to only 2.2% for pembrolizumab34 and 2.6%–7.7% for nivolumab.22,35 While comparing data directly across studies is confounded by differences in study design, the Larkin et al study did in fact have both a nivolumab and an ipilimumab-monotherapy arm, and yet almost twice as many patients on ipilimumab discontinued due to treatment-related adverse events (46 of 311), as did patients on nivolumab (24 of 313).35 There is thus reason to suspect that ipilimumab may be less tolerable than the two PD1-targeted therapies, although checkpoint inhibitors as a class were not shown to be significantly less tolerable than control treatments.
Heterogeneity
As was highlighted in the Results section, the heterogeneity of the meta-analyses was significant. For the primary outcome analysis, heterogeneity was I2=91%. However, when the three drugs are considered separately, the studies on each drug are well aligned, despite the different control interventions and the use of HRs for death rather than progression in one study.15 Similarly, the substantial heterogeneity for the secondary meta-analysis on tumor response (I2=72%) was reduced to 0 upon excluding the two ipilimumab studies.15,21 This suggests firstly that the heterogeneity stems from the combination of different checkpoint inhibitors into one arm rather than inconsistent effect estimates from individual studies, and secondly that the two end points for the primary meta-analysis (OS and PFS) were sufficiently similar to combine.
Limitations
Bias
The studies included in this systematic review and meta-analysis were of good quality, scientifically rigorous, and at low risk of bias. A further exploration of the bias assessment does however show that certain bias domains were more relevant than others, and that the most relevant domain varied between the different outcomes.
The primary outcome on survival and the secondary outcome on tumor response were objective outcomes where the impartiality of assessment of progression of disease and tumor response were vital, meaning the most relevant bias domain was blinding of outcome assessment. While most studies had an independent central review (ICR), five of seven studies were marked as unclear risk of bias, mostly due to the failure to specify who conducted the ICR. One study used only investigator-assessed tumor response, and did not specify whether they remained blinded during assessment, and was thus marked as high risk of bias.29 The impact on the results was, however, believed to minor, given that firstly the one study marked as high risk produced results on par with other studies, and secondly the failure to specify who sat on the ICR does not necessarily mean that they were either unqualified or biased.
The secondary outcome on tolerability was more subjective, meaning blinding of participants and personnel was the most important bias domain. Five studies were marked as low risk of bias, but two studies were completely or partially open-label and were thus marked as high risk of bias.22,34 Patients in the chemotherapy arms of these two trials may have been more likely to report adverse events, given that chemotherapy is commonly known to cause side effects. As these two studies were among the only three studies favoring checkpoint inhibitors, the results of this meta-analysis may have been biased in favor of checkpoint inhibitors. The third study favoring checkpoint inhibitors was, however, double-blinded, and the common denominator identified previously was that these were the only studies that compared checkpoint inhibitor monotherapy to control treatment.
All studies were marked as unclear risk under the “other bias” domain, due to the funding being provided by the patent-holding pharmaceutical company, who in collaboration with the authors was responsible for the study design, data collection, and analysis of results. On a study level, it is not possible to determine whether this potential bias was relevant or not, although it is noteworthy that all high-quality data on checkpoint inhibitors for melanoma were funded by the pharmaceutical industry.
Outcomes
At the outcome level, the use of PFS and thus HRs for progression as a surrogate for the gold standard end point, OS and HRs for death, was necessary, given the lack of published data on OS, but nonetheless a limitation. While a meta-analysis has shown that PFS is a reliable surrogate marker and that the correlation is stronger for melanoma than for any other cancer, only studies with dacarbazine in the control arm and only one study assessing a checkpoint inhibitor (ipilimumab) were included.28 The atypical tumor responses seen with immunotherapy make it possible for patients to be prematurely marked as progressing, even though a positive late response may still occur. This uncertainty is compounded by the use of different checkpoint inhibitors with different kinetics in both the experimental and control arms. The same applies for the secondary outcome on tumor response, wherein the use of the RECIST or modified WHO criteria may fail to capture the delayed response of checkpoint inhibitors.40,42
However, the direction of bias is such that if anything, the efficacy of checkpoint inhibitors would be underestimated, given that patients would have shorter PFS and a lower BORR if they were prematurely evaluated as having progressive disease. In fact, the HRs for progression were less substantial, that is, closer to 1, than the HRs for death in the studies that reported both end points, meaning this potential limitation did not impact the overall findings of this study.15,21,29
Review
At the review level, weaknesses include high heterogeneity, which may reduce the credibility of a meta-analysis and suggest that the studies are too dissimilar to pool. However, the studies on each individual drug produced similar results, suggesting that the studies were not producing randomly spurious results, and that the heterogeneity was a reflection of genuine differences among the three drugs. This study has compensated for the inherent heterogeneity from combining three drugs by firstly reviewing the results of each drug separately and comparing against one another, and secondly by not assuming a common effect estimate, and thus choosing a random effects model.
A second potential limitation is that in four of seven studies, the combination of a checkpoint inhibitor and control treatment was compared to the control treatment alone, in order to isolate the effect of the checkpoint inhibitor. This, however, assumes that the drugs do not act synergistically, which would exaggerate the effect of the checkpoint inhibitor. There is limited evidence on whether checkpoint inhibitors act in an additive or synergistic way when combined with chemotherapy or another immunotherapeutic agent. In a mouse model with a peritoneal ID8 tumor, a α-PD1 monoclonal antibody was shown to produce synergistic effects when combined with trabectedin, and separate to this, the combination of inefficacious doses of anti-PD1 and anti-CTLA4 antibodies were able to reduce tumor volume in a mouse significantly.43–45 This is, however, weak evidence, and in this review only two studies allowed for an evaluation of synergism, with neither providing especially convincing evidence. The Larkin et al study35 showed that in the combination arm, PFS was slightly greater (11.5 vs 9.8 months) but BORR slightly lower (57.6% vs 62.7%) than the combined sum of the monotherapy arms, while the Hodi et al study15 found that both were lower. The assumption of an additive effect is thus unlikely to have significantly biased the results of this study.
The relatively low number of studies (seven) and total participants (3,628) and the inclusion of only one study assessing the tolerability of ipilimumab is another limitation. Lastly, the presence of reporting bias, specifically in the form of time-lag bias, is also relevant, as median OS data are yet to be released for several studies. Based on the funnel plot, as shown in the Supplementary materials (Section D), there was, however, no significant publication bias.
Context and future directions
The results of this study support FDA and European Medicines Agency (EMA) approvals and National Institute for Health and Care Excellence recommendations for ipilimumab, nivolumab, and pembrolizumab.26 Previous systematic reviews and meta-analyses looking at only CTLA4- or PD1-targeted therapies separately, have (like this study) shown that ipilimumab,46 nivolumab, and pembrolizumab47,48 improve survival and tumor response. Furthermore, a recent systematic review and meta-analysis by Yun et al49 came to similar conclusions, and also found evidence to suggest that anti-PD1 treatment is of greater clinical benefit than anti-CTLA4 treatments. However, it did not include the recent clinical trials conducted by Larkin et al35 and Postow et al36 in its quantitative analyses, which were included in this study. However, it did include one study on the unapproved drug tremelimumab, another monoclonal antibody to CTLA4, which did not meet our inclusion criteria, as it is not FDA or EMA approved. Tremelimumab failed to show efficacy in its Phase III clinical trial, and is thus no longer being pursued as a treatment for melanoma.50 Additional evidence in favor of PD1-targeted therapy has come from the recent KEYNOTE 006 trial, which showed greater PFS, OS, and ORRs with two different dosing regimens of pembrolizumab compared to ipilimumab.51
Similar to this study though, the safety of ipilimumab especially has been of concern, while nivolumab has in fact been shown to cause an insignificant decrease in adverse events.47,48 Immunorelated adverse events have however been reported more frequently for the combination of nivolumab and ipilimumab than for ipilimumab alone, which is consistent with the poorer tolerability of combination therapy reported in this study.47,52 These immunorelated adverse events range from mild and self-limiting to life-threatening organ inflammation, and although they respond well to steroids and in severe cases infliximab, prophylactic budesonide failed to reduce the rate of grade ≥2 diarrhea in ipilimumab-treated patients.53
Combination therapies with multiple checkpoint inhibitors and/or with other treatments, such as signal transduction inhibitors (BRAF/MEK), remain an important avenue to explore, in order to obtain the maximum survival benefit of checkpoint inhibitors in advanced melanoma. In this study, even with a combination of ipilimumab and nivolumab, some 40% of patients nevertheless failed to respond to treatment, while still remaining at risk of toxicity. Predictive biomarkers capable of giving a pretreatment indication of the risk:benefit ratio in an individual patient may thus improve the use of checkpoint inhibitors, especially as several new checkpoint inhibitors with novel targets are coming close to market release. This means that choosing the best combination of drugs to prescribe may become difficult, which is especially problematic in advanced melanoma, where the poor prognosis makes a trial-and-error approach to treatment inappropriate.
Based on the findings of this study, the focus of future research should thus be on two areas: 1) determining the optimal use of checkpoint inhibitors, specifically in terms of combination therapy and the optimal duration, constellation, and sequence of such treatment; and 2) identifying reliable biomarker algorithms to predict responders and guide treatment assignments. Identifying reliable biomarkers to guide the use of checkpoint inhibitors may not only spare nonresponders from adverse effects and maximize benefit in responders, but also suggests novel drug targets. Moreover, carefully designed dose-ranging studies may also be helpful in determining the duration of treatment required to achieve optimal effect without causing undue side effects, given the results in this study showing prolonged survival with combination treatment despite increased rates of discontinuation.35,36
Studies on combination treatments, such as the Phase I/II KEYNOTE 022 study, which combines pembrolizumab with the MEK inhibitor trametinib and the BRAF inhibitor dabrafenib are under way,54 as are studies looking at potential biomarkers, such as tumor genomics, with a recent study on pembrolizumab for colorectal carcinoma showing that mismatch-repair status predicted clinical benefit.55 While BRAF mutation status has been shown not to affect the efficacy of checkpoint inhibitors,56 further studies are needed to clarify the usefulness of PDL1 status as a predictive marker. The Larkin et al study35 found a nominally greater tumor response in PDL1-positive patients treated with nivolumab alone or combined with ipilimumab compared to ipilimumab alone, while the Postow et al study36 found that tumor response was independent of PDL1 status. Finally, validating the new immunorelated response criteria, and incorporating them into clinical trials, along with the development of clearer guidelines on the management of checkpoint inhibitor-induced toxicities may improve the study and safe use of checkpoint inhibitors.
Conclusion
This meta-analysis has found that checkpoint inhibitors provide a statistically significant advantage over control interventions for PFS, OS, and BORR in patients with unresectable stage III or IV melanoma, without significantly worsening tolerability. The combination of ipilimumab and nivolumab was the most effective, but not surprisingly was less tolerable than monotherapy. Reliable and predictive biomarkers, along with clear guidelines for the optimal use of checkpoint inhibitors, holds the potential of improving the prognosis of patients with advanced melanoma, and moving immunotherapy toward becoming the fourth generation of cancer treatment, along with surgery, chemotherapy, and radiotherapy.
Disclosure
The authors report no conflicts of interest in this work.
References
Cancer Research UK. Skin cancer incidence statistics. Available from: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/skin-cancer/incidence#ref-2. Accessed March 11, 2017. | ||
Thompson JF, Scolyer RA, Kefford RF. Cutaneous melanoma. Lancet. 2005;365(9460):687–701. | ||
de Vries E, Bray FI, Coebergh JW, Parkin DM. Changing epidemiology of malignant cutaneous melanoma in Europe 1953–1997: rising trends in incidence and mortality but recent stabilizations in Western Europe and decreases in Scandinavia. Int J Cancer. 2003;107(1):119–126. | ||
Lucas R, McMichael T, Smith W, Armstrong B. Solar Ultraviolet Radiation: Global Burden of Disease from Solar Ultraviolet Radiation. Geneva: World Health Organization; 2006. | ||
World Health Organization. Skin cancers. Available from: http://www.who.int/uv/faq/skincancer/en/index1.html. Accessed March 11, 2017. | ||
Bolognia JL, Schaffer JV, Duncan KO, Ko CJ. Cutaneous melanoma. In: Dermatology Essentials. Amsterdam: Elsevier; 2014:909–928. | ||
Bolognia JL, Jorizzo JL, Rapini RP. Melanoma. In: Dermatology. Amsterdam: Elsevier; 2003:1789–1815. | ||
Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19(16):3622–3634. | ||
Barth A, Wanek LA, Morton DL. Prognostic factors in 1,521 melanoma patients with distant metastases. J Am Coll Surg. 1995;181(3):193–201. | ||
Fletcher WS, Pommier RF, Lum S, Wilmarth TJ. Surgical treatment of metastatic melanoma. Am J Surg. 1998;175(5):413–417. | ||
Lui P, Cashin R, Machado M, Hemels M, Corey-Lisle PK, Einarson TR. Treatments for metastatic melanoma: synthesis of evidence from randomized trials. Cancer Treat Rev. 2007;33(8):665–680. | ||
Maverakis E, Cornelius LA, Bowen GM, et al. Metastatic melanoma: a review of current and future treatment options. Acta Derm Venereol. 2015;95(5):516–524. | ||
Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–2516. | ||
Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367(2):107–114. | ||
Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010; 363(8):711–723. | ||
Walker LS, Sansom DM. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat Rev Immunol. 2011;11(12):852–863. | ||
Wolchok JD, Saenger Y. The mechanism of anti-CTLA-4 activity and the negative regulation of T-cell activation. Oncologist. 2008;13 (Suppl 4):2–9. | ||
Amarnath S, Mangus CW, Wang JC, et al. The PDL1-PD1 axis converts human TH1 cells into regulatory T cells. Sci Transl Med. 2011; 3(111):111ra120. | ||
Singh BP, Salama AK. Updates in therapy for advanced melanoma. Cancers (Basel). 2016;8(1):E17. | ||
Blank C, Brown I, Peterson AC, et al. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 2004;64(3):1140–1145. | ||
Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–2526. | ||
Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-abel, phase 3 trial. Lancet Oncol. 2015;16(4):375–384. | ||
National Institute for Health and Care Excellence. Ipilimumab for previously untreated advanced (unresectable or metastatic) melanoma. 2017. Available from: https://www.nice.org.uk/guidance/ta319. Accessed March 11, 2017. | ||
National Institute for Health and Care Excellence. Nivolumab in combination with ipilimumab for treating advanced melanoma. 2017. Available from: https://www.nice.org.uk/guidance/ta400. Accessed March 11, 2017. | ||
National Institute for Health and Care Excellence. Pembrolizumab for advanced melanoma not previously treated with ipilimumab. 2017. Available from: https://www.nice.org.uk/guidance/ta366. Accessed March 11, 2017. | ||
National Institute for Health and Care Excellence. Treating stage IV melanoma. 2017. Available from: http://pathways.nice.org.uk/pathways/melanoma#path=view%3A/pathways/melanoma/treating-stage-iv-melanoma.xml&content=view-node%3Anodes-immunotherapy-and-targeted-therapy. Accessed March 11, 2017. | ||
CONSORT Transparent Reporting of Trials. CONSORT 2010. 2010. Available from: http://www.consort-statement.org/consort-2010. Accessed March 11, 2017. | ||
Flaherty KT, Hennig M, Lee SJ, et al. Surrogate endpoints for overall survival in metastatic melanoma: a meta-analysis of randomised controlled trials. Lancet Oncol. 2014;15(3):297–304. | ||
Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. | ||
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. | ||
James K, Eisenhauer E, Christian M, et al. Measuring response in solid tumors: unidimensional versus bidimensional measurement. J Natl Cancer Inst. 1999;91(6):523–528. | ||
Higgins JP, Green S. Cochrane Handbook. Obtaining standard errors from confidence intervals and P values: absolute (difference) measures. Available from: http://handbook.cochrane.org/chapter_7/7_7_7_2_obtaining_standard_errors_from_confidence_intervals_and.htm. Accessed March 11, 2017. | ||
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. | ||
Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16(8):908–918. | ||
Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. | ||
Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–2017. | ||
Saenger YM, Wolchok JD. The heterogeneity of the kinetics of response to ipilimumab in metastatic melanoma: patient cases. Cancer Immun. 2008;8:1. | ||
Hales RK, Banchereau J, Ribas A, et al. Assessing oncologic benefit in clinical trials of immunotherapy agents. Ann Oncol. 2010;21(10):1944–1951. | ||
Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32(10):1020–1030. | ||
Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–7420. | ||
Luke JJ, Ott PA. PD-1 pathway inhibitors: the next generation of immunotherapy for advanced melanoma. Oncotarget. 2014;6(6):3479–3492. | ||
Dranitsaris G, Cohen RB, Acton G, et al. Statistical considerations in clinical trial design of immunotherapeutic cancer agents. J Immunother. 2015;38(7):259–266. | ||
Guo Z, Wang H, Meng F, Li J, Zhang S. Combined trabectedin and anti-PD1 antibody produces a synergistic antitumor effect in a murine model of ovarian cancer. Journal of Translational Medicine. 2015;13:247. | ||
Sznol M. Combined CTLA4 and PD-1 pathway blockade for treatment of advanced cancer. 2015. Available from: http://tatcongress.org/wp-content/uploads/2015/03/O2.3-Mario-Sznol.pdf. Accessed March 11, 2017. | ||
Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107(9):4275–4280. | ||
Dequen P, Lorigan P, Jansen JP, van Baardewijk M, Ouwens MJ, Kotapati S. Systematic review and network meta-analysis of overall survival comparing 3 mg/kg ipilimumab with alternative therapies in the management of pretreated patients with unresectable stage III or IV melanoma. Oncologist. 2012;17(11):1376–1385. | ||
Jin C, Zhang X, Zhao K, Xu J, Zhao M, Xu X. The efficacy and safety of nivolumab in the treatment of advanced melanoma: a meta-analysis of clinical trials. Onco Targets Ther. 2016;9:1571–1578. | ||
Chen R, Peng P, Wen B, et al. Anti-programmed cell death (PD)-1 immunotherapy for malignant tumor: a systematic review and meta-analysis. Transl Oncol. 2015;9(1):32–40. | ||
Yun S, Vincelette ND, Green MR, Hendrickson AE, Abraham I. Targeting immune checkpoints in unresectable metastatic cutaneous melanoma: a systematic review and meta-analysis of anti-CTLA-4 and anti-PD-1 agents trials. Cancer Med. 2016;5(7):1481–1491. | ||
Ribas A, Kefford R, Marshall MA, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;31(5):616–622. | ||
Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–2532. | ||
Bertrand A, Kostine M, Barnetche T, Truchetet ME, Schaeverbeke T. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med. 2015;13:211. | ||
Weber J, Thompson JA, Hamid O, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15(17):5591–5598. | ||
Merck Sharp & Dohme. A study of the safety and efficacy of pembrolizumab (MK-3475) in combination with trametinib and dabrafenib in participants with advanced melanoma (MK-3475-022/KEYNOTE-022). Available from: https://clinicaltrials.gov/ct2/show/NCT02130466. NLM identifier: NCT02130466. Accessed March 11, 2017. | ||
Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. | ||
Larkin J, Lao CD, Urba WJ, et al. Efficacy and safety of nivolumab in patients with BRAF V600 mutant and BRAF wild-type advanced melanoma: a pooled analysis of 4 clinical trials. JAMA Oncol. 2015;1(4):433–440. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.