Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 16
Characterizing Gas Exchange Physiology in Healthy Young Electronic-Cigarette Users with Hyperpolarized 129Xe MRI: A Pilot Study
Authors He M, Qing K, Tustison NJ , Beaulac Z, King TW, Huff TB, Paige M, Earasi K, Nunoo-Asare R, Struchen S, Burdick M, Zhang Z, Ropp A, Miller GW, Patrie JT, Mata JF, Mugler JP 3rd, Shim YM
Received 15 June 2021
Accepted for publication 25 October 2021
Published 23 November 2021 Volume 2021:16 Pages 3183—3187
DOI https://doi.org/10.2147/COPD.S324388
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Richard Russell
Mu He,1 Kun Qing,2,3 Nicholas J Tustison,2 Zach Beaulac,4 Tabitha W King,5 Thomas B Huff,5 Mikell Paige,4 Kranthikiran Earasi,1 Roselove Nunoo-Asare,1 Sarah Struchen,2 Marie Burdick,1 Zhimin Zhang,1 Alan Ropp,2 Grady W Miller,2 James T Patrie,6 Jaime F Mata,2 John P Mugler 3rd,2 Yun Michael Shim1,2
1Department of Medicine, Division of Pulmonary and Critical Care, University of Virginia, Charlottesville, VA, USA; 2Department of Radiology & Medical Imaging, University of Virginia, Charlottesville, VA, USA; 3Radiation Oncology, Cancer Institute of New Jersey, Rutgers University, New Brunswick, NJ, USA; 4Department of Chemistry, George Mason University, Fairfax, VA, USA; 5Shared Research Instrumentation Facility, George Mason University, Manassas, VA, USA; 6School of Public Health, University of Virginia, Charlottesville, VA, USA
Correspondence: Yun Michael Shim
Department of Medicine, Division of Pulmonary and Critical Care, University of Virginia, PO Box 800546, Charlottesville, VA, 22908-0546, USA
Email [email protected]
In recent years, electronic-cigarette (e-cigarette) use has spread to the point of a public health emergency. E-cigarettes deliver well-known toxic materials such as nicotine, carbonyls, tobacco-specific n-nitrosamines, and heavy metals.1 While these toxic materials likely cause pulmonary epithelial and endothelial dysfunction, their effects on the human lungs are poorly understood, especially for healthy young adults during the early exposure period. Recent epidemiologic studies have raised serious concern for at-risk groups of adolescents and young adults, especially considering the exponentially increasing consumption and immense popularity of e-cigarettes.2,3 Numerous cases of severe respiratory failure linked to e-cigarettes have been reported in young adults and adolescents who use e-cigarette containing tetrahydrocannabinol (THC) oil.4 Now, emerging cases link e-cigarette use in adolescents to confusing pictures of risk for acute lung injury during the days of COVID-19.5 These events have raised an urgent need to understand potential pathology caused by e-cigarettes so that appropriate warnings can be provided to the users, scientific communities, and regulatory authorities. Although some regulatory restrictions on e-cigarettes have started, the medical community needs to educate the public with scientific information to adjust the public perception regarding the risk of e-cigarettes.
Early-stage pulmonary abnormalities in these young e-cigarette users are too subtle to be detected by traditional diagnostic tools. Hyperpolarized xenon-129 magnetic resonance imaging (HP-129Xe MRI) is a robust tool capable of acquiring 3-dimensional images of pulmonary gas distribution and gas exchange capacity with high resolution. Previous studies employing HP-129Xe MRI have demonstrated its capability to visualize regions of airflow obstruction and parenchymal disease in patients with pulmonary pathologies, including chronic obstructive pulmonary disease (COPD).6 We anticipate that HP-129Xe MRI can help characterize early-stage pulmonary pathophysiology in three distinctive microcompartments of the e-cigarette users’ lungs: airspaces, alveolar-interstitial tissues, and capillaries.7 This knowledge can inform us about subclinical but potentially significant health effects related to e-cigarette use.
The University of Virginia Institutional Review Board approved this research study protocol, which was conducted in accordance with the Declaration of Helsinki. All subjects signed informed consent. In this exploratory study, five young, healthy e-cigarette subjects (age = 21.0±6.0 years [median ± interquartile range (IQR)]) were enrolled for HP-129Xe MRI evaluations and compared to ten age-matched healthy controls with no smoking history (age = 21.0 ± 2.8 years). All e-cigarette users were asymptomatic. One user smoked traditional cigarettes for four years (1 pack per day) before transitioning to e-cigarette. All e-cigarette users smoked e-cigarette from one to five years. None of the users were dual users. One subject used the Joyetech pen, and four used the Pod system. Three subjects used no flavoring, and two used mint flavor. None of the subjects used THC products. The dose of e-cigarette use was 1.0±1.0 mL/day for 1.5±3.5 years, with a 5% nicotine content. All e-cigarette users were asked to refrain from smoking overnight before the study. Pulmonary Function Test (PFT) results were performed on the day of the MRI.
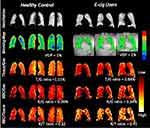
All e-cigarette users underwent HP-129Xe ventilation and gas exchange scans, acquired during two separate breath-hold imaging sessions, using a 1.5-Tesla MR scanner (Dot Avanto, Siemens Healthineers, Malvern, PA, USA).6 For the control subjects, ventilation maps were derived from the data acquired during HP-129Xe gas-exchange scans. HP-129Xe ventilation scans visualized and quantified the ventilation defect percentage (VDP) (Figure 1, second row), which reflects the percentage of lung volume with no ventilation (red).8 HP-129Xe gas-exchange scans quantified three gas-exchange capacities: Tissue-to-Gas ratio (Tissue/Gas), which reflects xenon gas movement from airspaces to alveolar-interstitial tissues; RBC-to-Gas ratio (RBC/Gas), which reflects xenon gas movement from airspaces to capillary RBCs; and RBC-to-Tissue ratio (RBC/Tissue), which reflects the efficiency of the xenon gas movement from the alveolar-interstitial tissues to capillary RBCs. As shown in Figure 1 (rows 3–5), the color gradient from red to yellow indicates low to high gas exchange capacity detectable by HP-129Xe.
Heparinized venous blood was collected at the time of MRI for toxicology analysis. Plasma from e-cigarette users was processed to remove proteins and phospholipids interfering with LC-MS/MS analysis using 1 mL Resprep PLR cartridges containing 25 mg of sorbent (Restek Corporation, State College, PA, USA). Quality control was maintained by adding 25 μL of a 4 μg/mL solution containing (±)-Nicotine-D4, DL-Cotinine-D3 trans-3ʹ-Hydroxycotinine (3HC)-D3 internal standards. Samples were analyzed on a Shimadzu Nexera ultra-high-performance liquid chromatography (UHPLC) with an 8050 triple quadrupole mass spectrometer (Shimadzu Scientific Instruments Corporation, Columbia, MD, USA). Nicotine, cotinine, and nicotine metabolite 3HC levels were measured.
PFT were normal in healthy controls vs e-cigarette users: Forced Expiratory Volume in 1 second (FEV1) 3.2±0.2 L vs 3.4±0.7 L (p=0.32), Forced Vital Capacity (FVC) 3.8±0.6 L vs 4.2±1.6 L (p=0.28), percent predicted value of FEV1 (%FEV1) 99.5±12.0% vs 103.0±9.0% (p=0.74), percent predicted value of FVC (%FVC) 107.0±11.8% vs 101.0±7.0% (p=0.97), and FEV1/FVC ratio 82.8±5.5% vs 85.0±4.0% (p=0.69). Diffusion capacity for carbon monoxide (DLCO) was measured only for the e-cigarette users. DLCO was 27.2±3.9 (Range [24.1, 35.3]) (mL/min/mm Hg), and the percent predicted DLCO (%DLCO) was 94.0±15.0% (Range [80%, 118%]). Four active e-cigarette users had detectable levels of nicotine (7.9±12.2 ng/mL), cotinine (171.6±61.4 ng/mL), and nicotine metabolite 3HC (83.4±70.1 ng/mL).
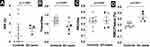
Gas distribution (VDP) was similar between e-cigarette users (1.5±0.2%) and healthy controls (0.6±1.5%) (p=0.24, Figure 2A). We observed a significantly smaller amount of xenon gas diffusing from airspaces to alveolar-interstitial tissues in e-cigarette users than healthy controls: Tissue/Gas healthy controls 1.18±0.26% vs e-cigarette users 0.88±0.27%, p=0.04, (Figure 2B). However, a similar amount of xenon gas diffused from airspaces to capillary RBCs in e-cigarette users compared to healthy controls: RBC/Gas healthy controls 0.35±0.07% vs e-cigarette users 0.41±0.16%, p>0.999, (Figure 2C). RBC/Tissue showed a significantly increased proportion of xenon gas diffusing from alveolar-interstitial tissues to capillary RBCs in e-cigarette users compared to healthy controls: RBC/Tissue, healthy controls 0.28±0.06% vs e-cigarette users 0.45±0.10%, p=0.0007, (Figure 2D).
Our exploratory study has characterized five healthy asymptomatic young adults with normal PFTs and less than five years of e-cigarette use. In these e-cigarette users, HP-129Xe MRI ventilation scans did not detect abnormal gas distribution. However, despite normal PFT %DLCO values, HP-129Xe MRI scans detected a significantly reduced gas exchange from airspaces to alveolar-interstitial tissues, illustrating the abnormal alveolar-interstitial tissues. We previously published a strong correlation between HP-129Xe MRI and %DLCO, and the reduced Tissue/Gas was found with loss of gas-exchange capacity in patients with emphysema or interstitial lung diseases.7 In our pilot cohort, the reduced Tissue/Gas in e-cigarette users suggests possible early interstitial lung abnormalities otherwise undetectable by the diffusion capacity measurement of the PFT.6,7 However, we could not discern the type of tissue pathology (emphysema vs interstitial lung disease).6,7,9 Surprisingly, the overall pulmonary gas exchange capacity, measured by the RBC/Gas, showed comparable levels in e-cigarette users and healthy controls. The preserved overall gas exchange capacity (RBC/Gas) despite the abnormally low Tissue/Gas indicates that a disproportionally higher amount of xenon in the alveolar-interstitial tissues is picked up by the capillary RBCs, likely due to increased pulmonary capillary perfusion (“hyperemic capillaries”). Even though clinically determined to have normal %DLCO (ranges between 80% and 118%), HP-129Xe MRI suggests potentially significant sub-clinical changes in the e-cigarette users’ respiratory physiology. Recent work points out that V/Q mismatch is significant in e-cigarette users, but cannot be detected by PFT, highlighting the need for a better diagnostic tool to assess these at-risk patients.10 We have recently reported a strong correlation among HP-129Xe MRI gas exchange metrics, %DLCO, Chest CT, and MRI with gadolinium.6,7 As an emerging tool, our HP-129Xe MR imaging detected abnormal alveolar-interstitial compartment in e-cigarette users.
Conclusion
We speculate that the pulmonary “hyperemia” suggested by HP-129Xe MRI has a potential to predispose e-cigarette users to acute lung injury under the right clinical circumstances of “second” hits, such as infection or tetrahydrocannabinol. We speculate that exhaustion of reserve ventilation-perfusion match may lead to loss of activity tolerance if e-cigarette users continue to smoke. We need to note that our work lacks chest CT to verify structural and perfusion findings. A small number of subjects limits the extent of our conclusion, warranting careful interpretation. However, our results are significant, in our opinion, because healthy asymptomatic young adults have abnormal respiratory physiology in their 20s with completely normal PFTs after a relatively short duration of e-cigarette use. Considering the urgency of the e-cigarette epidemic among adolescents and young adults, we feel compelled to present this exploratory data to stimulate discussion on the potentially harmful effects of e-cigarettes even during the early period of use. Our study also compels us to plan a larger clinical trial to determine the pathologic effects of e-cigarette use by applying HP-129Xe MRI.
Abbreviations
EC, Electronic Cigarette; VDP, ventilation defect percentage; LVP, low ventilation percentage; RBC, red blood cell; HP-129Xe MRI, hyperpolarized xenon-129 magnetic resonance imaging; COPD, Chronic Obstruction Pulmonary Disease; IQR, interquartile range; PFT, Pulmonary Function Test; FEV1, Forced Expiratory Volume in 1 second; FVC, Forced Vital Capacity; %FEV1, percent predicted value of FEV1; %FVC, percent predicted value of FVC; FEV1/FVC ratio, Forced Expiratory Volume in 1 second/Forced Vital Capacity ratio; DLCO, diffusing capacity for Carbon Monoxide; %DLCO, percent predicted diffusing capacity for Carbon Monoxide; LC-MS/MS analysis, liquid chromatography-mass spectrometry; (3HC)-D3, DL-Cotinine-D3 trans-3ʹ-hydroxycotinine; UHPLC, ultra-high-performance liquid chromatography; PLR, phospholipid removal; T/G, Tissue to Gas ratio; R/G, Red blood cell to Gas ratio; R/T, Red blood cell to Tissue ratio.
Data Sharing Statement
All data is available upon request.
Ethics Approval and Consent to Participate
Ethical approval was granted from the University of Virginia Institutional Review Board (IRB) #13740. All subjects signed informed consent prior to the study and was conducted in accordance with the Declaration of Helsinki.
Consent for Publication
All subjects signed informed consent prior to the study.
Acknowledgments
We acknowledge Lukasz Myc, Zaid Obaida, Jamie MacLeod, and Chunzi Song for their work on the imaging and patient data acquisition and analysis and assistance during the manuscript preparation.
Author Contributions
MH, KQ, NJT, MB, GWM, JFM, JPM, & YMS contributed in all areas of the work. AR contributed execution of data and analysis and interpretation. JTP contributed statistical analysis and interpretation. ZB, TWK, TBH, MP, ZZ contributed study design, execution, acquisition of data and analysis. KE, RN-A and SS contributed execution and acquisition of data. All authors have drafted or written, or substantially revised or critically reviewed the article. All authors have agreed on the journal to which the article will be submitted, reviewed and agreed on all versions of the article before submission, during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage. All authors agree to take responsibility and be accountable for the contents of the article.
Funding
NIH/NHLBI HL132177 (KQ, YMS). NIH/NHLBI HL132287 (YMS, MP). GMU Presidential Scholarship. R01-CA172595 (JFM). S10-OD018079 (JFM). 3UL1TR003015-02S4 (YMS, JPM).
Disclosure
Professor John P Mugler 3rd reports grants from Siemens Healthcare, outside the submitted work. The authors declare that they have no other competing interests.
References
1. Jensen RP, Luo W, Pankow JF, Strongin RM, Peyton DH. Hidden formaldehyde in e-cigarette aerosols. N Engl J Med. 2015;372(4):392–394. doi:10.1056/NEJMc1413069
2. Cullen KA, Gentzke AS, Sawdey MD, et al. E-cigarette use among youth in the United States, 2019. JAMA. 2019;322(21):2095–2103. doi:10.1001/jama.2019.18387
3. Wilson FA, Wang Y. Recent findings on the prevalence of E-cigarette use among adults in the U.S. Am J Prev Med. 2017;52(3):385–390. doi:10.1016/j.amepre.2016.10.029
4. Kalininskiy A, Bach CT, Nacca NE, et al. E-cigarette, or vaping, product use associated lung injury (EVALI): case series and diagnostic approach. Lancet Respir Med. 2019;7(12):1017–1026. doi:10.1016/S2213-2600(19)30415-1
5. Darmawan DO, Gwal K, Goudy BD, Jhawar S, Nandalike K. Vaping in today’s pandemic: e-cigarette, or vaping, product use-associated lung injury mimicking COVID-19 in teenagers presenting with respiratory distress. SAGE Open Med Case Rep. 2020;8:2050313X20969590.
6. Qing K, Tustison Nj, Mugler JP
7. Myc L, Qing K, He M, et al. Characterisation of gas exchange in COPD with dissolved-phase hyperpolarised xenon-129 MRI. Thorax. 2021;76(2):178–181.
8. He M, Driehuys B, Que LG, Huang Y-CT. Using hyperpolarized 129Xe MRI to quantify the pulmonary ventilation distribution. Acad Radiol. 2016;23(12):1521–1531. doi:10.1016/j.acra.2016.07.014
9. Wang JM, Robertson SH, Wang Z, et al. Using hyperpolarized (129)Xe MRI to quantify regional gas transfer in idiopathic pulmonary fibrosis. Thorax. 2018;73(1):21–28. doi:10.1136/thoraxjnl-2017-210070
10. Kizhakke Puliyakote AS, Elliott AR, Sá RC, Anderson KM, Crotty Alexander LE, Hopkins SR. Vaping disrupts ventilation-perfusion matching in asymptomatic users. J Appl Physiol. 2021;130(2):308–317. doi:10.1152/japplphysiol.00709.2020
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.