Back to Journals » Clinical Optometry » Volume 13
Characterization of Retinal Thickness in Individuals with Albinism: Baseline Data for a Black South African Population
Authors Pillay E, Naidoo T, Asmal K, Maliwa L , Mchunua S, van Staden DB , Rampersad N
Received 4 September 2020
Accepted for publication 3 November 2020
Published 20 January 2021 Volume 2021:13 Pages 15—22
DOI https://doi.org/10.2147/OPTO.S273141
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Mr Simon Berry
Ethan Pillay, Thiroshnee Naidoo, Khadija Asmal, Lilitha Maliwa, Sinenhlanhla Mchunua, Diane Beverly van Staden, Nishanee Rampersad
Discipline of Optometry, School of Health Sciences, University of KwaZulu-Natal, Durban X54001, South Africa
Correspondence: Nishanee Rampersad
Discipline of Optometry, School of Health Sciences, University of KwaZulu-Natal, Westville Campus, Private Bag, Durban X54001, South Africa
Tel +27 31 260 7562
Fax +27 31 260 7666
Email [email protected]
Introduction: The central retina is responsible for several visual functions and continues to develop postnatally. In albinism, which is a genetic disorder characterized by impaired melanin biosynthesis, the development of the central retina is prematurely arrested and results in foveal hypoplasia. Retinal thickness measurements can be determined non-invasively using optical coherence tomography systems. This article reports on the retinal thickness measurements of individuals with albinism in South Africa to aid in the assessment and management of affected individuals.
Methods: The study used a comparative research design and included 60 individuals (30 albinism and 30 controls) aged from 10 to 30 years who accessed the eye clinic at a tertiary institution in KwaZulu-Natal, South Africa. The Optovue iVue100 optical coherence tomographer was used to measure retinal thickness in the nine Early Treatment Diabetic Retinopathy Study (ETDRS) sectors including the central foveal, parafoveal and perifoveal regions. Study data were analysed using descriptive and inferential statistics.
Results: The mean central foveal thickness was significantly higher in individuals with albinism compared with controls (289 μm versus 239 μm, p < 0.001). In contrast, control participants showed thicker retinal thickness measurements in the other ETDRS sectors (p < 0.001). The nasal and temporal quadrants were thickest and thinnest, respectively, in the parafoveal and perifoveal regions for the albinism and control groups.
Conclusion: Individuals with albinism, aged from 10 to 30 years, have higher central foveal thickness but thinner retinal thickness measurements in the parafoveal and perifoveal regions. Optometric personnel should consider these measurements when assessing individuals with albinism with foveal retinal diseases.
Keywords: albinism, fovea, foveal hypoplasia, optical coherence tomography, retinal thickness
Introduction
The term albinism, which is derived from the Latin word albus meaning white, refers to an inherited genetic disorder characterised by impaired melanin biosynthesis.1,2 Oculocutaneous albinism, an autosomal recessive condition, involves the skin, hair and eyes whereas in ocular albinism, an x-linked condition, only the eyes are involved.3 Ocular characteristics of albinism include fundus hypopigmentation, foveal hypoplasia, nystagmus, iris translucency, high refractive errors, non-progressive reduced vision and chiasmal misrouting.4–7 Even though recent data related to the incidence and prevalence of albinism in Africa is lacking, it is recognised as a common condition that affects thousands of people with an estimated prevalence ranging from 1 in 5000–15,000 across Africa.8,9 Specifically in South Africa, Kromberg et al9 estimated that approximately 14,000 people of the total population in the country have albinism.
The retina, which is derived from the Latin word rete, consists of cellular and synaptic layers that collectively form the neurosensory retina and a single layer of pigment epithelium cells.10,11 The macular is one of the landmark structures on the retina and at its centre, referred to as the fovea, contains only cone photoreceptors that are responsible for high spatial resolution acuity.10,12 With the introduction of optical coherence tomography (OCT), it is possible to non-invasively measure retinal thickness and document the retinal layers particularly at the fovea.10,13–15 In healthy individuals scanned using OCT technology, the fovea shows a distinctive morphology characterised by a depression that is formed by the peripheral displacement of the inner retinal layers (ganglion cell layer, inner plexiform layer and inner nuclear layer) and densely packed cone photoreceptors.12,15,16 In albinism, the development and maturation of the visual system and structures therein are altered because of melanin abnormalities.9,15,17 Particularly the normal foveal developmental process, which begins early in gestation and continues postnatally, is prematurely arrested in individuals with albinism.15,18 Consequently, foveal hypoplasia, which refers to the fovea being absent or partially developed, is a common clinical characteristic in individuals with albinism.12,19
The aim of this study is to characterise retinal thickness in individuals with albinism. It is hoped that the study results will provide insight into the retinal profile in albinism as this information can aid in the assessment and management of affected patients.
Materials and Methods
The study was approved (BE139/19) by the Biomedical Research and Ethics Committee at the University of KwaZulu-Natal, South Africa, and used a cross-sectional comparative research design. The study conformed to the tenets of the Declaration of Helsinki and all ethical guidelines were adhered to. The study sample consisted of individuals with albinism who attended the optometry low vision clinic and were recruited using convenience sampling. Control participants consisted of individuals who attended the optometry general clinic and were recruited using purposive sampling as they were matched for demographic characteristics (age, gender and race) to participants with albinism. The nature of the study and procedures involved were explained to participants and guardians of minors prior to participation. Thereafter, written informed consent and assent for minors were obtained from all participants and guardians.
All participants underwent screening that consisted of subjective observation (hair, iris and skin colour), case history, visual acuity (VA) using the Low Vision Resource Centre (LVRC) chart and Bailey-Lovie word reading chart for distance and near, respectively, cover test, refraction and ocular health assessment with ophthalmoscopy, slit lamp biomicroscopy and tonometry. Participants with strabismus and/or nystagmus were allowed to use their preferred head posture and in the latter instance, a fogging technique using a high powered convex lens was used for monocular testing. The screening tests were performed by the same examiner to ensure standardization of test procedures and recording of results. Participants were excluded if they presented with any condition other than albinism, had any ocular surgery or trauma, were taking any medication or aged younger than 10 years and older than 30 years. The age range of 10 to 30 years was selected to maximise the co-operation of participants, particularly for the tests that required subjective responses, and to minimise the influence of age on the retinal thickness measurements.
The diagnosis of albinism was based on the recommendation by Kruijt et al1 as individuals with albinism show variability in clinical signs and may not always present with all of the characteristics associated with the disorder.1,2,17 Consequently, Kruijt et al1 recommend that in the absence of any molecular assessment, the diagnosis of albinism can be made using major and minor clinical diagnostic criteria. The major criteria include ocular (iris and fundus) hypopigmentation, foveal hypoplasia and chiasmal misrouting while the minor criteria include nystagmus, skin and hair hypopigmentation, fundus hypopigmentation and foveal hypoplasia. As molecular and genetic testing were not performed in this study, the diagnosis of albinism was made based on the presence of two major and two minor criteria as has been done previously.17
The Optovue iVue100 spectral-domain OCT device was used to scan and measure retinal thickness. This device has a scan acquisition rate of 26,000 axial scans (A-scans) per second and resolution of 5 µm.20 The fast scanning speed in spectral-domain OCT devices is advantageous to minimise the effect of motion artefacts in instances of poor fixation and nystagmus.4,14 The retina map scan protocol was used and is made up of a raster pattern of 13 horizontal line scans that are six millimetres in length and comprise 512 A-scans each. This scanning protocol also consists of an additional seven horizontal line scans within the centre 1.5 mm zone that comprise 1024 A-scans each. Retinal thickness is measured as the distance from the inner limiting membrane to the retinal pigment epithelium layer and is determined automatically by the algorithm in the device.20 The iVue100 OCT device produces a retinal map (Figure 1) that displays the numerical values of the average retinal thickness measurements in the nine Early Treatment Diabetic Retinopathy Study (ETDRS) sectors (bottom left) and the en face images of the seven raster scans (bottom right). In the retinal map, the central foveal thickness is defined as the centre circle with a 1 mm diameter. The two outer circles, with diameters of 3 mm and 5 mm, represent the parafoveal and perifoveal regions, respectively, and are made up of four quadrants (superior, inferior nasal and temporal) each. In this study, the average parafoveal and average perifoveal retinal thickness measurement was computed as the average thickness of the four quadrants within each region.
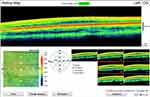 |
Figure 1 Retinal map for a participant with albinism showing the mean retinal thickness (µm) in the nine ETDRS sectors and the seven en face images. |
Both eyes of each participant were scanned three times with the OCT device and the average of the readings was computed. The same examiner, who was trained and had experience in using the iVue100 OCT device, performed all the OCT scans for standardization. During scanning, the participant’s head was stabilised using the chin and forehead rests while fixating on the internal fixation target. For participants with nystagmus, the scans were taken while they focused on the fixation target with their habitual head posture as this allowed for the null point of the nystagmus and their preferred retinal locus of fixation. The examiner performing the OCT scanning verified that each scan was acceptable prior to proceeding to the next scan by reviewing the scan for any motion artefacts, confirming that the segmentation lines were correct and reviewing the scan quality index. Scans that had motion artefacts, incorrectly placed segmentation lines, a scan quality index that was below the manufacturer’s recommendation of 40 or were labelled as “poor” were not accepted and scanning was repeated.
Data were captured and analysed using the Statistical Package for Social Sciences (SPSS) version 25. Normality of the retinal thickness measurements was checked using histograms and the Kolmogorov–Smirnov test. The data are presented using summary statistics including means, standard deviations, range and percentage. The independent sample t-test was used to assess differences in retinal thickness measurements between the albinism and control groups. A probability (p) value of less than 0.05 was considered statistically significant.
Results
The study sample consisted of 60 participants with 30 each in the albinism and control groups. The mean age of all participants was 20.20 ± 4.14 years and ranged from 13 to 30 years. All participants were Black South African and there was an equal number of males (n = 9) and females (n = 21) in each group. All control participants (n = 30) had dark brown skin, hair and iris colour. In contrast, participants with albinism had either pale or very pale creamy white skin colour (n = 30) and light brown (n = 3) or wheat blond (n = 27) hair colour. One participant each in the albinism group had dark brown, blue or hazel iris colour while the majority (n = 20) presented with a grey iris colour followed by seven participants that had a “combination” iris colour as there were at least two different colours (either brown, blue, hazel or grey) present simultaneously. Fifty percent of participants with albinism (n = 15) had a manifest strabismus that was most often esotropia (n = 12). All participants with albinism presented with horizontal nystagmus, iris transillumination and fundus hypopigmentation.
Table 1 shows the age and ocular characteristics of participants in the two groups. There was no difference in mean age (p = 0.793) but participants with albinism had significantly poorer distance and near VA (p ≤ 0.02). The difference in distance monocular VA was almost eight lines on the LogMAR chart in the two eyes. The near binocular VA, which was recorded in M-notation, was assessed at the participants’ preferred working distance and ranged from 8 cm to 40 cm in the two groups. More than two-thirds of participants with albinism (n = 21) and all control participants had near binocular VA of 1 M or better. The control participants had slightly higher mean IOP measurements in the two eyes. Even though the difference in mean IOP measurements reached statistical significance in the left eye (p = 0.006), this difference was less than 2 mmHg and may not be clinically significant.
 |
Table 1 Means and Standard Deviations for Age and Ocular Characteristics in the Two Study Groups |
Table 2 summarises the mean retinal thickness measurements in the albinism and control groups for the nine ETDRS sectors. The mean central foveal thickness was 50 µm higher in the albinism group compared with the control group (289 µm versus 239 µm, p < 0.001). With the exception of the central foveal region, control participants had significantly higher retinal thickness measurements in the other ETDRS sectors within the parafoveal and perifoveal regions (Table 2). The average parafoveal and perifoveal thickness for the albinism group was 283 µm and 268 µm respectively. In the control group, the average parafoveal and perifoveal thickness was 305 µm and 285 µm, respectively. This implies that control participants had higher average parafoveal and perifoveal retinal thickness measurements and the thickness difference was 22 µm and 17 µm, respectively. The nasal and temporal quadrants were thickest and thinnest, respectively, in the parafoveal and perifoveal regions for the albinism and control groups (Table 2).
 |
Table 2 Means and Standard Deviations for Retinal Thickness (µm) in the Nine Early Treatment Diabetic Retinopathy Study Sectors in the Two Study Groups |
Discussion
The findings of this study provide baseline data on retinal thickness measurements in individuals with albinism in KwaZulu-Natal, South Africa. As previous studies involving individuals with albinism in South Africa have focused on their vitamin D levels,21 use of services (related to eye care, skin care and social development),22 effects of coloured overlays when reading23 and refractive error as well as ocular characteristics,24 the results of the present study add to the growing body of international literature documenting retinal thickness in individuals with albinism.4,14,25–27 Investigating retinal thickness measurements and morphology in individuals with albinism and matched controls allows for an enhanced understanding of the central retinal changes that occur in albinism. This knowledge could be useful for better understanding the mechanisms of the impaired visual functions such as loss of high-resolution acuity and stereopsis that are usually associated with this condition.
Albinism is a life-long condition that has been shown to have a negative influence on education, employment and overall quality of life from discrimination and stigmatization particularly in a context where the characteristic phenotype is easily distinguished as being different.9,19,28 At present, there is no treatment for this condition and supportive strategies are used in the management of affected individuals which comprise spectacles to correct refractive error, filters to alleviate photosensitivity, assistive devices to improve functionality, education on effective sun protection and surgery for strabismus and/or nystagmus.2 Studies focused on understanding the retina, specifically the macular and fovea, in terms of its morphology and thickness in individuals with albinism are becoming more evident in the literature.7,15,27,29 This may be as retinal thickness data may aid in our understanding of the pathophysiology and natural history of albinism. Moreover, there is growing interest in investigating the potential effect of drug therapies in restoring visual function in albinism as recent preliminary studies have reported positive results in animal and human samples.3,30 For example, Adams et al30 reported an increase in pigmentation (skin and hair) and the number of letters read on a LogMAR chart in a small sample (n = 5) of adults with albinism when treated with oral nitisinone for one year. Despite these promising results, the relationship between gains in visual function and retinal structure in individuals with albinism remains uncertain and needs to be investigated in future studies. McAllister et al15 used OCT technology to image the fovea and showed that foveal development in albinism is arrested rather than absent with the resulting anomalous morphology representing a continuum of the stages of normal foveal development. Consequently, an enhanced understanding of foveal thickness and anomalous morphology in albinism may aid to better inform pharmacological drug therapy interventions, aimed specifically at melanin pigment production and/or supplementation, to recover foveal maturity and improve visual function in affected individuals.
In the present study, the mean central foveal thickness was significantly higher in participants with albinism than matched controls and this finding is consistent with results of other studies.14,25,27 Studies using OCT devices have shown that individuals with albinism have anomalous foveal morphology owing to foveal hypoplasia whereby the foveal depression is either missing or shallow and there is continuity of the inner retinal layers across the fovea.4–6,12,14,18,31 To this extent, Meyer et al5 and Holmström et al14 reported that OCT images in albinism show a widespread thickening of the macular region with no differentiation of the central fovea from the rest of the retina because the ganglion cell and nerve fibre layers extend across the central fovea without thinning.4,17 However, other studies have noted that there is significant variability in the fovea of individuals with albinism whereby some individuals have low-grade foveal hypoplasia and some of the features associated with normal foveal development.4,7,15 For this reason, it has been suggested that foveal hypoplasia and the resulting morphology in albinism be considered as representing a continuum of foveal development rather than absence of a foveal depression.15 Normal foveal morphology consists of a sloping foveal depression and absence of the inner retinal layers (nerve fibre layer, ganglion cell layer, inner plexiform layer, inner nuclear layer and outer plexiform layer) at the centre of the fovea. Consequently, the abnormal foveal morphology with the inner retinal layers being present in individuals with albinism accounts for the higher central foveal thickness measurements when compared with controls.4,26,27 It is also likely that the reduced or absent melanin pigment in affected individuals contributes to the higher central foveal thickness measurements as it has been suggested that eyes with greater choroidal pigmentation have thinner central foveal thickness measurements.32 The central foveal thickness difference between the two groups was 50 µm which is comparable to the value reported by Holmström et al14 (45 µm). In contrast, Hirota et al25 reported a 17 µm difference in central foveal thickness measurements between participants with albinism and controls which is smaller than the value noted in the present study. This discrepancy could be the result of differences in the extent of foveal hypoplasia noted in the participants with albinism whereby the cohort in the present study probably had more severe foveal hypoplasia than the cohort in the study by Hirota et al.25
The mean central foveal thickness measurements noted for the albinism and control groups in this study are interesting when compared with values reported in other studies worldwide. In this study, the mean central foveal thickness was 289 µm for the albinism group. This value is comparable to the measurements reported by Hirota et al25 (274 µm) and Neri et al31 (295 µm) for their participants with albinism. It is interesting to speculate that the similarity in foveal thickness measurements could be as these two studies25,31 also used spectral-domain OCT devices like in the present study to measure retinal thickness. Izquierdo et al26 used a time-domain OCT device and reported a mean thickness of 240 µm in 20 individuals with albinism in Peurto Rico. Similarly, Jethani et al33 reported mean values ranging between 195 µm and 244 µm in 10 individuals with albinism while Holmström et al14 noted a mean foveal thickness of 249 µm when using a Stratus time-domain OCT device. Consequently, differences in the technical specifications of the OCT devices used to measure retinal thickness, particularly those related to scanning speed and resolution, could account for the discrepancy in values noted between the different studies. In this study, the mean central foveal thickness was 239 µm for the control group and this is almost identical to the value reported by Murugan et al34 (238 µm). The study by Murugan et al34 included a similar profile of young healthy Black participants in the same setting and used the same OCT device to measure retinal thickness which may account for the similarity in mean central foveal thickness measurements.
The pattern of retinal thickness measurements in the parafoveal and perifoveal regions was different to that of the central foveal region as control participants had significantly thicker retinal thickness measurements than participants with albinism. In this way, the trend of retinal thickness measurements beyond the central fovea supports the findings of another study that imaged the parafovea and perifovea.14 In their study, Holmström et al14 reported an average thickness difference of 33 µm and 17 µm in the parafovea and perifovea, respectively, which is comparable with the thickness differences (22 µm and 17 µm) noted in the present study. It has been speculated that the thinner retinal thickness measurements in the parafoveal and perifoveal regions may be related to the aetiology and development of foveal hypoplasia in individuals in albinism.14 It is interesting to note that the thickness differences between the two groups were smaller for the parafoveal and perifoveal regions. Moreover, the nasal and temporal quadrants of both the parafoveal and perifoveal regions were thickest and thinnest, respectively, in the two groups as has been noted in other studies.13,34 The implication of these two findings, related to the parafoveal and perifoveal regions, is that although there are differences in the central foveal region, the morphology of the retina beyond the central fovea in individuals with albinism may be similar to individuals without albinism. However, this speculation would need to be confirmed in future OCT studies that use wide-field imaging of the retina beyond the central macular region in albinism.
The clinical findings in participants with albinism were not unexpected and included reduced visual acuity, hypopigmentation of the skin, hair, iris and fundus, iris transillumination, nystagmus and strabismus. Reduced visual acuity is a common finding in individuals with albinism and may be accounted for by retinal changes such as foveal hypoplasia and the influence of excessive light scattering owing to reduced ocular pigmentation in the choroid, iris and pigment epithelium layer in addition to nystagmus, uncorrected refractive error and strabismus.4,5,26,33 In the visual pathway, fibres from the temporal retina are normally projected to the ipsilateral hemisphere at the optic chiasm. However, in albinism, there is abnormal decussation of fibres at the optic chiasm whereby the temporal retinal fibres project to the contralateral hemisphere.2 The latter, which is referred to as chiasmal misrouting and detected using visually evoked potential (VEP) testing, is thought to account for poor levels of binocularity and the likelihood of strabismus in individuals with albinism.7,19,30 Nystagmus, which develops within the first two months of life, is a common finding in albinism9,14,19,29 and may be explained by several factors including foveal hypoplasia, amblyopia and chiasmal misrouting.8 Participants also presented with hypopigmentation of the skin, hair, iris and fundus as has been reported in other studies involving individuals with albinism.4,18,24
Limitations of this study include that there was no molecular or genetic testing for the albinism diagnosis and that iris transillumination, fundus hypopigmentation and foveal hypoplasia were assessed as a dichotomous variable (present or absent) and not classified according to any grading scales. In addition, axial length was not measured and used in the correction of the lateral scale of the retinal OCT images. Consequently, the retinal thickness measurements in the nine ETDRS sectors, particularly for the participants with albinism who are likely to have varying axial lengths, should be interpreted with caution. As the study only included participants aged from 10 to 30 years, the retinal thickness measurements found may not be generalized to younger and older South African individuals. The study reports on the total retinal thickness only and therefore future studies should investigate the thickness of individual retinal layers as this is likely to be important particularly for foveal hypoplasia in albinism. Furthermore, in instances of young children and nystagmus, it is not always easy to ensure that the fovea is imaged when scanning with an OCT device. To account for this, participants with nystagmus were asked to look at the fixation target with their head positioned to coincide with the null point and all scans were examined for motion artefacts and scan quality indices before being accepted. Moreover, studies have used OCT devices to measure retinal thickness in individuals with albinism where nystagmus was present.5,15,18 Strengths of the study include the use of a relative large sample of participants with albinism than has been previously reported on, a control group that was matched for demographic characteristics to the albinism group and the use of a spectral-domain OCT device with good repeatability for repeated retinal thickness measurements.35
Conclusion
This is the first study to report on retinal thickness measurements in a cohort of individuals with albinism in KwaZulu-Natal, South Africa. Consistent with previous studies, the central foveal thickness is thicker in individuals with albinism. The retinal thickness measurements reported in this study may be useful to optometric personnel when assessing for retinal diseases and structural abnormalities in individuals with albinism. The results of this study add to the literature on retinal thickness and morphology in albinism and may aid in our understanding of the pathophysiology and natural history of albinism. Moreover, data on retinal thickness measurements and morphology in individuals with albinism is important because if pharmacological drug therapy can result in even slight improvements in visual function, it would have a considerable effect on the quality of life of affected individuals.
Acknowledgments
The authors acknowledge Mr Nyika Mtemeri for assistance with the statistical analysis.
Disclosure
The authors declare that they have no financial or personal relationship(s) that may have inappropriately influenced them in writing this article. The author reports no conflicts of interest in this work.
References
1. Kruijt CC, de Wit GC, Bergen AA, Florijn RJ, Schalij-Delfos NE, van Genderen MM. The phenotypic spectrum of albinism. Ophthalmology. 2018;125(12):1953–1960. doi:10.1016/j.ophtha.2018.08.003
2. Struck MC. Albinism: update on ocular features. Curr Ophthalmol Rep. 2015;3(4):232–237. doi:10.1007/s40135-015-0083-7
3. Lee H, Scott J, Griffiths H, Self JE, Lotery A. Oral levodopa rescues retinal morphology and visual function in a murine model of human albinism. Pigment Cell Melanoma Res. 2019;32(5):657–671.
4. Chong GT, Farsiu S, Freedman SF, et al. Abnormal foveal morphology in ocular albinism imaged with spectral-domain optical coherence tomography. Arch Ophthalmol. 2009;127(1):37–44. doi:10.1001/archophthalmol.2008.550
5. Meyer CH, Lapolice DJ, Freedman SF. Foveal hypoplasia in oculocutaneous albinism demonstrated by optical coherence tomography. Am J Ophthalmol. 2002;133(3):409–410. doi:10.1016/S0002-9394(01)01326-5
6. Paul R, Kulshrestha M, Shanmugalingam S, Barr D. Foveal hypoplasia in oculocutaneous albinism and the role of OCT. Adv Ophthalmol Vis Syst. 2015;2(3):89–90.
7. Wilk MA, MaAllister JT, Cooper RF, et al. Relationship between foveal cone specialization and pit morphology in albinism. Invest Ophthalmol Vis Sci. 2014;55(7):4186–4198. doi:10.1167/iovs.13-13217
8. Hong ES, Zeeb H, Repacholi MH. Albinism in Africa as a public health issue. BMC Public Health. 2006;6(1):1–7. doi:10.1186/1471-2458-6-212
9. Kromberg JGR, Manga P, Kerr R. Children with oculocutaneous albinism in Africa: characteristics, challenges and medical care. SAJCH. 2020;14(1):50–54.
10. Freddo TF, Chaum E. Anatomy of the Eye and Orbit: The Clinical Essentials. Philadelphia: Wolters Kluwer; 2018.
11. Pavan-Langston D. Manual of Ocular Diagnosis and Therapy.
12. Woertz EN, Omoba BS, Dunn TM, et al. Assessing ganglion cell layer topography in human albinism using optical coherence tomography. Invest Ophthalmol Vis Sci. 2020;61(3):1–13. doi:10.1167/iovs.61.3.36
13. Adhi M, Aziz S, Muhammad K, Adhi MI. Macular thickness by age and gender in healthy eyes using spectral domain optical coherence tomography. PLoS One. 2012;7(5):e37638.
14. Holmström G, Eriksson U, Hellgren K, Larsson E. Optical coherence tomography is helpful in the diagnosis of hypoplasia. Acta Ophthalmol. 2010;88(4):439–442.
15. McAllister JT, Dubis AM, Tait A, et al. Arrested development: high-resolution imaging of foveal morphology in albinism. Vision Res. 2010;50(8):810–817. doi:10.1016/j.visres.2010.02.003
16. Provis JM, Hendrickson AE. The foveal avascular region of developing human retina. Arch Ophthalmol. 2008;126(4):507–511. doi:10.1001/archopht.126.4.507
17. Brücher VC, Heiduschka P, Grezebach U, Eter N, Biermann J. Distribution of macular ganglion cell layer thickness in foveal hypoplasia: a new diagnostic criterion for ocular albinism. PLoS One. 2019;14(11):1–13. doi:10.1371/journal.pone.0224410
18. Thomas MG, Kumar A, Mohammad S, et al. Structural grading of foveal hypoplasia using spectral domain optical coherence tomography; a predictor of visual acuity? Ophthalmology. 2011;118(8):1653–1660. doi:10.1016/j.ophtha.2011.01.028
19. Summers CG. Albinism: classification, clinical characteristics, and recent findings. Optom Vis Sci. 2009;86(6):659–662.
20. Optovue. iVue100 User’s Manual Version 1.9. USA: Optovue Inc; 2011.
21. van der Walt JEC, Sinclair W. Vitamin D levels in patients with albinism compared with those in normally pigmented Black patients attending dermatology clinics in the Free State province, South Africa. Int J Dermatol. 2016;55(9):1014–1019. doi:10.1111/ijd.13119
22. Zungu Z, Mashige KP. Utilisation of eye and skin care, and social services among persons with albinism in Ulundi, KwaZulu-Natal, South Africa. Afr Vis Eye Health. 2019;78(1):1–5. doi:10.4102/aveh.v78i1.484
23. Makgaba NT, Oduntan OA. Perceived effects of coloured overlays on reading material in persons with albinism. S Afr Optom. 2008;67(3):118–124.
24. Jhetam S, Mashige KP. Ocular findings and vision status of learners with oculocutaneous albinism. Afr Vis Eye Health. 2019;78(1):1–6. doi:10.4102/aveh.v78i1.466
25. Hirota L, Almeida MS, de Almeida Manzano RP, Sano RY. Analysis of foveal thickness in patients with oculocutaneous albinism using OCT with eyetracker. Invest Ophthalmol Vis Sci. 2018;59(9):1110.
26. Izquierdo NJ, Emanuelli A, Izquierdo JC, García M, Candilla C, Berrocal MH. Foveal thickness and macular volume in patients with oculocutaneous albinism. Retina. 2007;27:1227–1230. doi:10.1097/IAE.0b013e3180592b48
27. Lee H, Purohit R, Sheth V, et al. Retinal development in albinism: a prospective study using optical coherence tomography in infants and young children. Lancet. 2015;385(suppl 1):14. doi:10.1016/S0140-6736(15)60329-4
28. Ang M, Chong W, Tay WT, et al. Anterior segment optical coherence tomography study of the cornea and anterior segment in adult ethnic South Asian Indian eyes. Invest Ophthalmol Vis Sci. 2012;53(1):120–125. doi:10.1167/iovs.11-8386
29. McCafferty BK, Wilk MA, McAllister JT, et al. Clinical insights into foveal morphology in albinism. J Pediatr Ophthalmol Strabismus. 2015;52(3):167–172. doi:10.3928/01913913-20150427-06
30. Adams DR, Menezes S, Jauregui R, et al. One-year pilot study on the effects of nitisinone on melanin in patients with OCA-1B. JCI Insight. 2019;4(2):1–15. doi:10.1172/jci.insight.124387
31. Neri A, Camparini M, Tedesco SA, Lamedica A, Delfini E, Macaluso C. A high-resolution OCT evaluation of the foveal region in human albinism. Invest Ophthalmol Vis Sci. 2009;50(13):2141.
32. Wagner-Schuman M, Dubis AM, Nordgren RN, et al. Race- and sex-related difference in retinal thickness and foveal pit morphology. Invest Ophthalmol Vis Sci. 2011;52(1):625–634. doi:10.1167/iovs.10-5886
33. Jethani J, Jethani M, Parmar S, Purohit J. Correlation of macular thickness, multifocal ERG with visual acuity in oculocutaneous albinism. Health. 2014;6:2109–2114. doi:10.4236/health.2014.616244
34. Murugan C, Golodza BZ, Pillay K, et al. Retinal thickness in black and Indian myopic students at the University of KwaZulu-Natal. Afr Vis Eye Health. 2015;74(1):1–7.
35. Rampersad N, Hansraj R. Repeatability of macular thickness measurements with the iVue-100 optical coherence tomographer. Afr Vis Eye Health. 2018;77(1):1–6. doi:10.4102/aveh.v77i1.413
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
