Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Characterization of Epidermal Function in Individuals with Primary Cutaneous Amyloidosis
Authors Huang F, Zhang Y, Guo J, Pan H, Liao Z, Yang B , Lu P
Received 22 June 2023
Accepted for publication 12 October 2023
Published 6 November 2023 Volume 2023:16 Pages 3193—3200
DOI https://doi.org/10.2147/CCID.S426209
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Fujuan Huang,1,* Yuling Zhang,2,* Junyi Guo,3 Hongju Pan,4 Zhigang Liao,4 Bin Yang,1 Ping Lu1
1Department of Dermatology, Dermatology Hospital of Southern Medical University, Guangzhou, People’s Republic of China; 2Department of Dermatology, The First Hospital of Jilin University, Changchun, People’s Republic of China; 3Department of Dermatology, Guangdong Provincial Dermatology Hospital, Guangzhou, People’s Republic of China; 4Guangdong Provincial Engineering Technology Research and Development Center for External Drugs, Foshan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ping Lu; Bin Yang, Department of Dermatology, Dermatology Hospital of Southern Medical University, Guangzhou, People’s Republic of China, Email [email protected]; [email protected]
Purpose: To compare epidermal biophysical properties, indicators of epidermal function, in individuals with and without primary cutaneous amyloidosis (PCA).
Patients and Methods: This study incorporated 189 patients with PCA and 166 healthy individuals. The GPSkin Barrier was employed to measure transepidermal water loss (TEWL) rates and hydration levels of the stratum corneum. The Sebumeter and the Skin pH Meter were utilized to determine the skin surface’s sebum content and pH, respectively. The severity of pruritus in participants was evaluated using the visual analog scale (VAS).
Results: Compared to the control group without PCA, individuals with PCA displayed a notable increase in skin surface pH and TEWL and a decrease in the hydration levels of the stratum corneum (p< 0.0001 for all parameters). Additionally, the sebum content was markedly lower in those with PCA than in the controls (p< 0.0001). Of particular note, both TEWL and skin surface pH at the lesion sites on the back and the shin were more elevated in lichenoid amyloidosis (LA) and in macular amyloidosis (MA), whereas hydration levels of the stratum corneum and sebum levels were diminished in LA compared to MA (p< 0.05). In conclusion, both hydration levels of the stratum corneum and sebum content exhibited an inverse relationship with pruritus severity, whereas TEWL and skin surface pH demonstrated a positive correlation with pruritus intensity.
Conclusion: The function of the epidermis is compromised in individuals diagnosed with PCA. However, the mechanisms underlying these changes await further investigation.
Keywords: cutaneous amyloidosis, epidermis, biophysical properties, pruritus
Introduction
Primary cutaneous amyloidosis (PCA) is a persistent pruritic dermatosis defined by the localized amyloid deposition in the papillary dermis. Lichenoid amyloidosis (LA) and macular amyloidosis (MA) emerge as its two predominant types. The differentiation between LA and MA rests largely on patients’ clinical and pathological manifestations. LA is mainly identified by brown, rice-sized papules on the legs, densely arranged yet separate. Its pathological characteristics include hyperkeratosis, hyperacanthosis, and substantial deposits of pink amorphous substances in the dermal papilla upon Congo red staining. This is further complemented by a fine network encompassing peripheral melanophages. In contrast, MA typically appears as “wavy” pigmented spots either in the back’s scapular area or on the lower extremities, with some spots potentially merging. The Congo red stain reveals less dense pink deposits in the dermal papillae and a delicate network of melanophages encircling amyloid.1 Both LA and MA can manifest concurrently or appear at different stages of the disease. It is crucial to differentiate PCA from conditions like systemic amyloidosis, localized pigment abnormalities, chronic simple lichen, and nodular prurigo, etc.
PCA is particularly prevalent in regions like Southeast Asia and South America, boasting a morbidity rate of approximately 7.87% in China.2,3 The exact cause of PCA remains elusive. However, around 10% of individuals with PCA have a family history, suggesting a potential genetic link.4 Factors such as race, gender, atopy, sun exposure, and the debated frictional epidermal damage have also been proposed as contributors to its etiopathogenesis.5 Treating PCA remains a formidable challenge for clinicians due to the absence of evidence-based treatment guidelines. Many treatment avenues have been explored, spanning retinoids, corticosteroids, cyclophosphamide, laser treatment, and phototherapy, etc.6
Both atopy and sun exposure have the potential to modify the function of the epidermis, mirroring alterations in TEWL and hydration of the stratum corneum.7,8 Notably, PCA’s clinical presentations, such as pruritus, dry skin, hyperpigmentation, and lichenification, all hint at potential shifts in epidermal functions.9 For example, reduced hydration levels in the stratum corneum indicate dry skin.10 Hyperpigmented skin often presents with diminished TEWL and skin surface pH.11 Furthermore, an overwhelming 40% of individuals with PCA exhibit epidermal hyperkeratosis, and about 30% display signs of inflammatory infiltration.4 In contrast, inflammatory skin conditions like eczematous dermatitis and psoriasis are marked by elevated TEWL, decreased hydration in the stratum corneum, and a rise in skin surface pH.12–15
Moreover, the frequent occurrence of pruritus in individuals with PCA can lead to scratching behaviors, potentially compromising the epidermal permeability barrier. This collective evidence points towards potential alterations in the epidermal functions of individuals with PCA.
Against this backdrop, our study aimed to contrast TEWL, hydration levels of the stratum corneum, skin surface pH, and sebum content between individuals with and without PCA. Additionally, we delved into the relationship between epidermal biophysical properties and pruritus intensity.
Materials and Methods
Participants
A cohort of 189 PCA outpatients, comprising 135 LA and 54 MA individuals, were recruited from the Dermatology Hospital at Southern Medical University between December 2017 and December 2019. Patients with PCA exhibiting systemic amyloidosis or extracutaneous organ involvement were excluded from the study. Other uncommon types, namely nodular cutaneous amyloidosis and poikiloderma-like cutaneous amyloidosis were also not considered. A group of 166 age- and sex-matched healthy volunteers were chosen as control subjects. Both PCA patients and controls were screened based on exclusion criteria, which encompassed those with a present or past history of conditions like eczematous dermatitis, psoriasis, chronic actinic dermatitis, skin infectious diseases, or skin cancer, as well as individuals who had experienced significant sun exposure during the study’s duration.
This research received approval from the institutional review board of the Dermatology Hospital at Southern Medical University (Approval No: GDDHLS-20171029). In accordance with the Declaration of Helsinki, verbal informed consent was secured from all participants before initiating the study. All participants belonged to the Chinese ethnic group.
Measurements of Epidermal Functions
The study assessed epidermal function by gauging specific biophysical properties of the epidermis. TEWL and stratum corneum hydration (SCH) measurements were acquired using the GPSkin Barrier device (G-POWER, Korea). Concurrently, the skin surface’s pH was determined using the skin pH Meter (Hanna Instruments HI 99181N, Asch, Japan), while the Sebumeter (HANNA, Italy) was employed to evaluate sebum content. Measurements were taken from lesion sites, including the shin, back (specifically the scapular area), and nape, maintaining standard ambient conditions: room temperature between 22°C to 25°C and relative humidity ranging from 40% to 60%. Before these measurements, a 20-minute adaptation period was observed. The flexor forearm, situated 10 cm above the wrist, was also evaluated as a non-involved control site in PCA patients. Control subjects underwent measurements on analogous sites. Participants were instructed to abstain from bathing and using skincare products for at least 12 h leading up to the measurements. Each measurement was replicated thrice on every site, with the mean value chosen for further analysis.
Evaluation of Pruritic Severity
The pruritus severity was gauged using a 0–10 visual analog scale (VAS), where a score of 0 represented the absence of pruritus and a score of 10 indicated extreme pruritus.16,17
Statistical Analysis
Statistical computations were conducted using the GraphPad PRISM v8.0 software. The presented data either follows the format of mean ± SEM or mean ± SD. The study employed the independent-samples t-test, Mann–Whitney U-test (2-test), and Spearman test for analytical purposes. A p-value less than 0.05 was treated as indicative of statistical significance.
Results
Demographic Characteristics of Participants
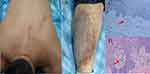
The study included 189 PCA patients and 166 healthy control subjects. Of the 166 healthy participants, 79 were male, and 87 were female, with an average age of 41 years (ranging from 25 to 65). In contrast, the PCA patient group consisted of 98 females (51.9%) and 91 males (48.1%) with an average age of 43 years (range: 25–64 years). The average age at which the PCA symptoms first appeared was 34, ranging from 14 to 50 years (refer to Table 1 for details). Within the PCA patient group, 19 individuals (12.8%) reported lesion development post-scratching, while 67 (45.3%) indicated lesion exacerbation due to scratching. The primary affected body regions were the back and shins (see Figures 1A and B). Histological verification of PCA diagnosis was achieved using Congo red stain (as depicted in Figure 1C) and HE stain (shown in Figure 1D). These tests revealed the presence of amyloid deposits in the papillary dermis for all patients.
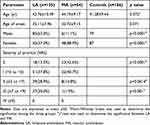 |
Table 1 Demographic Characteristics of Participant |
Alterations in Epidermal Biophysical Properties in Subjects with PCA
Within the PCA group, TEWL was determined across 283 lesion sites, including 112 on the back, 115 on the shin, 56 on the neck, and 189 on non-lesion sites of the forearm. For the control group, measurements were taken at 166 sites for each body region. All affected skin regions in PCA patients displayed increased TEWL and skin surface pH levels, diminished sebum content, and SC hydration compared to the healthy controls. However, the unaffected flexor forearm site showed no noticeable disparities in TEWL, SCH, skin surface pH, or sebum content between LA, MA, and the control group (refer to Table 2).
 |
Table 2 Comparison of Epidermal Biophysical Properties |
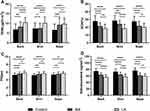
When juxtaposing LA to MA, LA presented more pronounced deviations in the epidermal biophysical properties across most body regions. The nape was an exception, where the epidermal biophysical properties were similar for both LA and MA (refer to Table 2 and Figure 2).
Association of Epidermal Biophysical Properties with the Severity of Pruritus
Given the frequent association of PCA with pruritus, the study evaluated whether changes in epidermal biophysical properties correlated with pruritus severity. Findings revealed that TEWL and skin surface pH directly correlated with pruritus severity (see Figures 3A and C). Conversely, SCH levels and skin surface sebum content exhibited an inverse relationship with the intensity of pruritus (illustrated in Figures 3B and D).
Discussion
PCA is a persistently advancing skin disorder distinguished by the accumulation of amyloid within the skin. The elements that make up amyloid encompass diverse components such as keratin, APC, APOE, glycosaminoglycan, oligomer, actin, EF, and CF, among others.18 While extensive literature does not necessarily correlate the generation of amyloid components to fluctuations in water content and lipids, specific components, particularly apolipoprotein E and E4, have ties to lipid metabolism. Significantly, hyperlipidemia has been identified as a key associated factor for PCA within the Taiwanese populace.19 Our past research utilizing whole genome sequencing on a PCA family highlighted the LRP6 gene as a prospective causative element. This gene might possess a pivotal role in the metabolism of the amyloid precursor protein and the synthesis of amyloid beta protein.20
Histologically, PCA is typified by the accumulation of eosinophilic material in the papillary dermis that reacts positively to stains like Congo red.21 This is complemented by other features such as perivascular inflammatory infiltration, epidermal hyperkeratosis, and vacuolar degeneration of basal cells.22 The global prevalence of PCA hovers around 0.2–0.3%, with MA dominating.4 Contrastingly, our findings indicated a higher prevalence of LA at 70%, echoing results from earlier Chinese research.23 This may hint at the racial influences on PCA presentation. Intriguingly, our study found an equal distribution of PCA between genders, diverging from earlier research from regions like Southeast Asia, the Middle East, and South America, where females showed a higher prevalence.24,25 This discrepancy might arise from variances in racial and geographic factors. Furthermore, as corroborated by previous studies,26–28 women were more predisposed to MA, whereas men predominantly exhibited LA. Our results concerning the age of PCA onset, averaging 34.127 ± 8.825 years, aligned with past observations.25,28
Even though the clinical and pathological features of PCA are well charted, insights into its epidermal biophysical properties are sparse. In this investigation, we established modifications in these properties, namely heightened TEWL and skin surface pH, alongside diminished SCH and sebum content in PCA patients. The exact mechanisms steering these changes in PCA remain elusive. Nonetheless, existing data hint at a couple of plausible explanations. Firstly, PCA is characterized by keratinocyte malfunction as evidenced by signs like basal cell vacuolar degeneration and hyperkeratosis. This dysfunction can impede epidermal lipid synthesis, disrupt keratinocyte differentiation, and misregulate stratum corneum acidification, reshaping TEWL, SCH, and skin surface pH values.29–32 Secondly, many PCA patients grapple with pruritus. Scratching can damage the stratum corneum, causing a surge in TEWL and skin surface pH.33 As such, keratinocyte dysfunction coupled with pruritus can collectively mold the epidermal biophysical characteristics in PCA. Moreover, factors such as dermal inflammation and hyperkeratosis can suppress sebum secretion, culminating in diminished skin surface sebum levels. Our earlier research also made an intriguing observation: the intercellular spaces of the stratum basale in PCA patients were significantly broader than in the control group. The basement membrane zone in PCA lesions also showed folds and bifurcations, and the hemidesmosomes appeared discontinuous. These changes may underpin the observed epidermal barrier dysfunction in PCA.34
Another compelling discovery from our study is the widespread occurrence of pruritus among PCA patients. This could be attributed to heightened expression levels of OSMRβ and IL-31RA.35,36 Yet, PCA lesions did not show elevated IL-31 levels in the patient’s skin and blood when juxtaposed with control subjects. Furthermore, the expression levels of nerve growth factor and its receptor, tropomyosin receptor kinase A (TrkA), remained comparable between PCA patients and controls.36 Aside from its potential role in amyloid deposition,37 pruritus-induced scratching can aggravate the epidermal permeability barrier and spur cutaneous inflammation. This observation aligns with our finding that TEWL is directly proportional to the severity of pruritus. The nefarious loop of itching leading to barrier disruption might escalate epidermal hyperproliferation and cutaneous inflammation, thereby worsening PCA. Thus, strategies focusing on curbing pruritus and fortifying the epidermal permeability barrier could be therapeutic for PCA. Nonetheless, this hypothesis necessitates validation via rigorous clinical trials. To encapsulate, PCA patients indeed exhibit deviations in epidermal functions.
Conclusion
Our research highlights that the epidermal biophysical properties in PCA patients are compromised. To help patients restore the epidermal biophysical properties may be helpful to alleviate pruritus severity. However, delving deeper into the mechanisms responsible for this epidermal damage warrants further investigation.
Acknowledgments
The research team wishes to express profound gratitude to the dedicated healthcare professionals who care for PCA patients. We also extend our thanks to the faculty of the Department of Dermatology, Guangdong Provincial Hospital, for their unwavering support throughout the study.
Funding
This study was generously sponsored by the National Natural Science Foundation of China (81972924) and the Joint Laboratory of the Dermatology Hospital, Southern Medical University, and China Resources Sanjiu Medical & Pharmaceutical Co, Ltd.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Jianzhong Z. Dermatopathology. Tianjin: Tianjin Science and Technology Translation publishing Limited Company; 2017:228.
2. Bandhlish A, Aggarwal A, Koranne RV. A clinico-epidemiological study of macular amyloidosis from north India. Indian J Dermatol. 2012;57(4):269–274. doi:10.4103/0019-5154.97662
3. Fang S, Shen X, Chen AJ, Li S, Shan K, Asnani MR. Health-related quality of life in patients with primary cutaneous amyloidosis. PLoS One. 2015;10(3):e0120623. doi:10.1371/journal.pone.0120623
4. Mehrotra K, Dewan R, Kumar JV, Dewan A. Primary cutaneous amyloidosis: a clinical, histopathological and immunofluorescence study. J Clin Diagn Res. 2017;11(8):WC01–WC051. doi:10.7860/JCDR/2017/24273.10334
5. Biswas P, Pal D, De A, et al. Clinicopathological study of primary cutaneous amyloidosis in a tertiary care center of eastern India reveals insignificant association with friction, scrubbing, and photo-exposure: how valid is the “Keratinocyte Hypothesis”? Indian J Dermatol. 2019;64(1):28–33. doi:10.4103/ijd.IJD_149_18
6. Weidner T, Illing T, Elsner P. Primary localized cutaneous amyloidosis: a systematic treatment review. Am J Clin Dermatol. 2017;18(5):629–642. doi:10.1007/s40257-017-0278-9
7. Liu Z, Fluhr JW, Song SP, et al. Sun-induced changes in stratum corneum function are gender and dose dependent in a Chinese population. Skin Pharmacol Physiol. 2010;23(6):313–319. doi:10.1159/000314138
8. Ye Y, Zhao P, Dou L, et al. Dynamic trends in skin barrier function from birth to age 6 months and infantile atopic dermatitis: a Chinese prospective cohort study. Clin Transl Allergy. 2021;11(5):e12043. doi:10.1002/clt2.12043
9. Guillet C, Steinmann S, Maul JT, Kolm I. Primary localized cutaneous amyloidosis: a retrospective study of an uncommon skin disease in the largest tertiary care center in Switzerland. Dermatology. 2022;238(3):579–586. doi:10.1159/000518948
10. Augustin M, Kirsten N, Körber A, et al. Prevalence, predictors and comorbidity of dry skin in the general population. J Eur Acad Dermatol Venereol. 2019;33(1):147–150. doi:10.1111/jdv.15157
11. Man MQ, Lin TK, Santiago JL, et al. Basis for enhanced barrier function of pigmented skin. J Invest Dermatol. 2014;134(9):2399–2407. doi:10.1038/jid.2014.187
12. Montero-Vilchez T, Cuenca-Barrales C, Rodriguez-Pozo JA, et al. Epidermal barrier function and skin homeostasis in atopic dermatitis: the impact of age. Life. 2022;12(1):132. doi:10.3390/life12010132
13. Li Q, Fang H, Dang E, Wang G. The role of ceramides in skin homeostasis and inflammatory skin diseases. J Dermatol Sci. 2020;97(1):2–8. doi:10.1016/j.jdermsci.2019.12.002
14. Montero-Vilchez T, Segura-Fernández-Nogueras MV, Pérez-Rodríguez I, et al. Skin barrier function in psoriasis and atopic dermatitis: transepidermal water loss and temperature as useful tools to assess disease severity. J Clin Med. 2021;10(2):359. doi:10.3390/jcm10020359
15. Lee Y, Je YJ, Lee SS, et al. Changes in transepidermal water loss and skin hydration according to expression of aquaporin-3 in psoriasis. Ann Dermatol. 2012;24(2):168–174. doi:10.5021/ad.2012.24.2.168
16. Reich A, Szepietowski JC. Measurement of Itch Intensity. Curr Probl Dermatol. 2016;50:29–34.
17. Murray CS, Rees JL. Are subjective accounts of itch to be relied on? The lack of relation between visual analogue itch scores and actigraphic measures of scratch. Acta Derm Venereol. 2011;91(1):18–23. doi:10.2340/00015555-1002
18. Chapman JR, Liu A, Yi SS, et al. Proteomic analysis shows that the main constituent of subepidermal localised cutaneous amyloidosis is not galectin-7. Amyloid. 2021;28(1):35–41. doi:10.1080/13506129.2020.1811962
19. Lee DD, Huang CK, Ko PC, Chang YT, Sun WZ, Oyang YJ. Association of primary cutaneous amyloidosis with atopic dermatitis: a nationwide population-based study in Taiwan. Br J Dermatol. 2011;164(1):148–153. doi:10.1111/j.1365-2133.2010.10024.x
20. Zhang YL, Lu P, Wu FF, et al. Whole genome sequencing results and preliminary analysis of a family with primary cutaneous amyloidosis. J Acta Univ Med Anhui. 2019;54(8):1308–1312.
21. Lu P, Wu FF, Rong ZL, et al. Clinical and genetic features of Chinese patients with lichen and macular primary localized cutaneous amyloidosis. Clin Exp Dermatol. 2019;44(4):e110–e117. doi:10.1111/ced.13925
22. Kibbi A-G, Rubeiz NG, Zaynoun ST, Kurban AK. Primary localized cutaneous amyloidosis. Int J Dermatol. 1992;31(2):95–98. doi:10.1111/j.1365-4362.1992.tb03245.x
23. Wang WJ, Chang YT, Huang CY, Lee DD. Clinical and histopathological characteristics of primary cutaneous amyloidosis in 794 Chinese patients. Zhonghua Yi Xue Za Zhi. 2001;64(2):101–107.
24. Tan T. Epidemiology of primary cutaneous amyloidoses in Southeast Asia. Clin Dermatol. 1990;8(2):20–24. doi:10.1016/0738-081X(90)90083-D
25. Rasi A, Khatami A, Javaheri SM. Macular amyloidosis: an assessment of prevalence, sex, and age. Int J Dermatol. 2004;43(12):898–899. doi:10.1111/j.1365-4632.2004.01935.x
26. Hashimoto K, Ito K, Kumakiri M, Headington J. Nylon brush macular amyloidosis. Arch Dermatol. 1987;123(5):633–637. doi:10.1001/archderm.1987.01660290101025
27. Eswaramoorthy V, Kaur I, Das A, Kumar B. Macular amyloidosis: etiological factors. J Dermatol. 1999;26(5):305–310. doi:10.1111/j.1346-8138.1999.tb03476.x
28. Lu P, Wu F, Man M, Rong Z, Zhang Y, Yang B. Sex differences of Chinese patients with primary localized cutaneous amyloidosis. J Dermatol. 2019;46(7):e242–e243. doi:10.1111/1346-8138.14800
29. Feingold KR, Elias PM. Role of lipids in the formation and maintenance of the cutaneous permeability barrier. Biochim Biophys Acta. 2014;1841(3):280–294. doi:10.1016/j.bbalip.2013.11.007
30. Verdier-Sévrain S, Bonté F. Skin hydration: a review on its molecular mechanisms. J Cosmet Dermatol. 2007;6(2):75–82. doi:10.1111/j.1473-2165.2007.00300.x
31. Chan A, Mauro T. Acidification in the epidermis and the role of secretory phospholipases. Dermatoendocrinol. 2011;3(2):84–90. doi:10.4161/derm.3.2.15140
32. Hachem JP, Behne M, Aronchik I, et al. Extracellular pH Controls NHE1 expression in epidermis and keratinocytes: implications for barrier repair. J Invest Dermatol. 2005;125(4):790–797. doi:10.1111/j.0022-202X.2005.23836.x
33. Fluhr JW, Dickel H, Kuss O, Weyher I, Diepgen TL, Berardesca E. Impact of anatomical location on barrier recovery, surface pH and stratum corneum hydration after acute barrier disruption. Br J Dermatol. 2002;146(5):770–776. doi:10.1046/j.1365-2133.2002.04695.x
34. Zhang Y, Le Y, Guo J, Wu F, Li Q, Lu P. Barrier function and ultrastructure characteristics of epidermis in patients with primary cutaneous amyloidosis. J Dermatol. 2023;50(8):999–1007. doi:10.1111/1346-8138.16819
35. Tanaka A, Arita K, Lai-Cheong JE, Palisson F, Hide M, McGrath JA. New insight into mechanisms of pruritus from molecular studies on familial primary localized cutaneous amyloidosis. Br J Dermatol. 2009;161(6):1217–1224. doi:10.1111/j.1365-2133.2009.09311.x
36. Tey HL, Cao T, Nattkemper LA, Tan VW, Pramono ZA, Yosipovitch G. Pathophysiology of pruritus in primary localized cutaneous amyloidosis. Br J Dermatol. 2016;174(6):1345–1350. doi:10.1111/bjd.14391
37. Hernández-Núñez A, Daudén E, Moreno de Vega MJ, Fraga J, Aragüés M, García-Díez A. Widespread biphasic amyloidosis: response to Acitretin. Clin Exp Dermatol. 2001;26(3):256–259. doi:10.1046/j.1365-2230.2001.00808.x
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.