Back to Journals » Stem Cells and Cloning: Advances and Applications » Volume 13
Characterization and Immunomodulation of Canine Amniotic Membrane Stem Cells
Authors de Oliveira Pinheiro A, Lara VM , Souza AF , Casals JB, Bressan FF, Fantinato Neto P , Oliveira VC, Martins DS, Ambrosio CE
Received 7 November 2019
Accepted for publication 24 March 2020
Published 7 May 2020 Volume 2020:13 Pages 43—55
DOI https://doi.org/10.2147/SCCAA.S237686
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Bernard Binetruy
Alessandra de Oliveira Pinheiro,1 Valéria M Lara,1 Aline F Souza,1 Juliana B Casals,2 Fabiana F Bressan,1 Paulo Fantinato Neto,1 Vanessa C Oliveira,1 Daniele S Martins,1 Carlos E Ambrosio1
1Department of Veterinary Medicine, Faculty of Animal Science and Food Engineering, University of São Paulo, Pirassununga, São Paulo, Brazil; 2Private Veterinary Practice, Pirassununga, São Paulo, Brazil
Correspondence: Carlos E Ambrosio
Department of Veterinary Medicine, Faculty of Animal Science and Food Engineering, University of São Paulo, FZEA- Av. Duque de Caxias Norte, 225, ZMV, Pirassununga 13635-900, São Paulo, Brazil
Tel +55 19 3565-4113 Email [email protected]
Purpose: Amniotic membrane stem cells have a high capacity of proliferation, cell expansion, and plasticity, as well as immunomodulatory properties that contribute to maternal-fetal tolerance. Owing to the lack of research on human amniotic membrane at different gestational stages, the canine model is considered ideal because of its genetic and physiological similarities. We aimed to characterize the canine amniotic membrane (CAM) cell lineage in different gestational stages and evaluate the expression of immunomodulatory genes.
Materials and Methods: Twenty CAMs from early (20– 30 days) (n=7), mid- (31– 45 days) (n=7), and late gestation (46– 63 days) (n=6) stages were studied. The cell features were assessed by cell viability tests, growth curve, colony-forming units, in vitro differentiation, cell labeling for different immunophenotypes, and pluripotent potential markers. The cells were subjected to RT-PCR and qPCR analysis to determine the expression of IDO, HGF, EGF, PGE2, and IL-10 genes.
Results: CAM cells exhibited a fibroblastoid morphology and adherence to plastic with an average cell viability of 78.5%. The growth curve indicated a growth peak in the second passage and we obtained an average of 138.2 colonies. Osteogenic, chondrogenic, and adipogenic lineages were confirmed by in vitro differentiation assays. Cellular immunophenotyping experiments confirmed the presence of positive mesenchymal markers (CD90 and CD105) and the low or negative expression of hematopoietic markers (CD45 and CD34). Qualitative analysis of the immunomodulatory functions indicated the expression of the IDO, HGF, EGF5, and PGE2 genes. When stimulated by interferon-gamma, CAM cells exhibited higher IDO levels throughout gestation.
Conclusion: The CAMs from different gestational stages presented features consistent with mesenchymal stem cell lineage; better results were observed during the late gestation stage. Therefore, the gestational stage is a key factor that may influence the functionality of therapies when using fetal membrane tissues from different periods of pregnancy.
Keywords: canine stem cells, immunomodulation, fetal annexes
Introduction
Mesenchymal stem cells (MSCs) are characterized by a multipotent cell lineage with a high capacity of differentiation into different cell types, which can execute trophic, paracrine, and immunomodulatory functions in other cells based on the microenvironment.1,2
In regenerative medicine, MSCs derived from fetal annexes such as amniotic fluid, amniotic membrane, and umbilical cord blood and vessels have previously been isolated and well-characterized in humans3 and other species, such as dogs,4–7 cats,8,9 bovines,10 sheep,11,12 horses,13 rats,14 rabbits,15 and ducks,16 suggesting the advantages of using it as raw material for the creation of cell banks.17–20
Immunomodulatory activities occur through direct contact between the MSCs and tissues or through paracrine interaction mediated by interferon-gamma (IFN-γ), produced by the body’s immune cells, which act on natural killer cells, monocytes, neutrophils, and macrophages. Some of the T-helper lymphocytes, cytotoxic T lymphocytes, and B lymphocytes secret soluble factors such as TGF-β, interleukin-10 (IL-10), interleukin-6 (IL-6), indoleamine-2,3-dioxygenase (IDO), prostaglandin E2 (PGE2), and soluble human leukocyte antigen-G5 (sHLA-G5), or interact by cell-cell reactions,21 in addition to preventing the expression of proinflammatory cytokines such as IFN-γ and tumor necrosis factor-α (TNF-α).22,23
Based on the significant immunomodulatory properties of MSCs and the limited number of studies on canine amniotic membrane (CAM) stem cells from different gestational stages, we aimed to characterize the CAM stem cells derived from different gestational stages and to determine the in vitro immunomodulatory potential and to establish a cell line that can be employed in the treatment of several diseases that affect domestic animals.
Materials and Methods
This study was approved by the Ethical Committee on Animal Use (Comissão de Ética no Uso de Animais) of the Faculty of Animal Science and Food Engineering of the University of São Paulo (protocol #4,598,140,116). We follow the international guide for use of dogs in experiments. https://www.ccac.ca/Documents/Standards/Guidelines/Vol2/dogs.pdf
Isolation and Culture of Stem Cells from CAM
Twenty CAMs from different gestational stages, early (20–30 days) (n=7), mid- (31–45 days) (n=7), and late gestation (46–63 days, due parturition) (n=6), were collected from the uteri of pregnant mixed-breed domestic dogs. The amniotic membrane fragments (Figure 1A) were washed and digested with 1 mL of Collagenase type I (Sigma, St. Louis, USA). The cells were cultured in plastic plates using αMEM medium (Gibco, New York, USA) supplemented with 15% fetal bovine serum (FBS, Invitrogen, Carlsbad, USA) and 1% penicillin/streptomycin (Gibco, New York, USA), and were incubated at 38.5°C, 5% CO2, and maximum humidity.
Analysis of Cell Viability, Growth Curve, Doubling Time, and Colony-Forming Assays
To assess the cell viability, the cells were separated in triplicate at a density of 4x104 cells/cm2 and frozen. After thawing, the cells were stained with trypan blue (1:1, Sigma-Aldrich, Brazil) to assess cell viability. The viable cells were counted using a hemocytometer and a Newbauer chamber. The cells were frozen for 24 h at −80°C in Mr. Frosty Freezing container (Sigma-Aldrich, Brazil) and stored in liquid nitrogen. After freezing and thawing, the cells were counted using a Newbauer camera.
The growth curve was estimated from the triplicate and the cells were plated and counted using a hemocytometer; 3x104 cells were plated on 35-mm plates and were maintained in an incubator at 38.5°C. The cells were counted every 96 h and were subcultured at the same density until passage 5. The formula Ct/Cd was used to calculate the doubling time, where Ct represents the culture time between passage n and passage n+1 and Cd represents cell doubling. Cell doubling was calculated using the formula Cd=ln (nf/ni) ln2, where nf and ni represent the number of harvested and seeded cells, respectively. Doubling time was calculated, as described by Vidane et al.9
For the colony-forming unit assays, 1x104 cells were plated in 90-mm Petri dishes using the culture medium. The colony formation assays were conducted over 13 days, and the culture medium was changed every 3 days. After 13 days, colonies were observed and adherent cells were fixed in 4% paraformaldehyde (Sigma, St. Louis, USA) and stained for 15 min using Giemsa 0.1% stain.
In vitro Differentiation Assays (Osteogenic, Adipogenic, and Chondrogenic)
All differentiation assays were performed during passage 2. To promote osteogenic and adipogenic differentiation, 1x105 were cultured in StemXVivo™ Human/Mouse Osteogenic/Adipogenic Base Media (R&D, Minneapolis, USA) supplemented with penicillin-streptomycin; after 21 days, the plates were fixed with 4% paraformaldehyde and stained by Alizarin Red and Sudan Black, respectively. For chondrogenic differentiation, 3x105 cells were placed in Falcon tubes with 5 mL of StemXVivoTM Human/Mouse Chondrogenic (R&D). After 3 days, the cells were resuspended in 2 mL of differentiation medium (R&D). After 21 days, the pellets were fixed, stained using Alcian Blue and Masson Trichrome, and analyzed.9
Flow Cytometry
Flow cytometry was used to analyze the reactivity of mesenchymal (CD90 and CD105) and hematopoietic (CD34 and CD45) specific markers (Table 1). Cells were taken from passage 2 (1x105 cells) and incubated for 20 min at 4°C with each antibody. Samples were analyzed using the flow cytometer FACSAria-BD Cell Sorter supported by DiVa software V.6.1.2 (BD Biosciences, San Jose, CA, USA).
 |
Table 1 Specifications of the Primary and Secondary Antibodies Used for the Characterization of Mesenchymal and Hematopoietic Markers |
Evaluation of the Immunomodulatory Response
CAM cells from passage 2 at different gestational stages were cultured in triplicate at a density of 5x105. The cells were treated with 200 ng/mL IFN-γ (R&D) for 72 h, as described by Saulnier et al 24 and Russel et al.25 After this period, the cells were evaluated for IDO, HGF, EGF, PGE-2, and IL-10 expression.
Real-Time Polymerase Chain Reaction (qPCR) for Evaluation of Immunomodulatory and Pluripotency Genes
Total RNA was extracted from the CAM cells from passage 2 using the TRIzol LS reagent (Life Technologies, Carlsbad, USA) following the manufacturer’s protocol. The total cellular RNA concentration was quantified by the Nanodrop 1000 spectrophotometer (Nanodrop Technologies, Wilmington Delaware, USA). For cDNA synthesis, the mRNA was reverse transcribed using the Enzyme Reverse Transcriptase superscript III kit (Invitrogen) according to the manufacturer’s specifications. Gene expression was assessed by qPCR (Step One Plus Real-Time PCR Systems, Life Technologies). The reactions were performed using a commercial assay system (PowerUpTM SYBR® Green PCR Master Mix, Applied Biosystems®, Carlsbad, USA) with OCT4 and SOX2 (pluripotency genes) and IDO, HGF, EGF, PGE-2, and IL-10 (immunomodulatory genes) as target genes of interest. The 18S gene, a housekeeping gene, served as the control. The primer sequences are presented in Table 2. The reaction conditions consisted of 40 cycles at an annealing temperature of 60°C were quantified by normalizing the signals to the 18S signals using the 2−ΔCT method.
 |
Table 2 Sequence of Primers Used in the Evaluation of the Expression of Pluripotency and Immunomodulatory Genes |
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) for Evaluation of Immunomodulatory Genes
The CAM cells from passage 2 were subjected to RT-PCR analysis using the GoTaq® Green Master Mix Kit (Promega®, Madison, USA); the Applied Biosystems Veriti 96-well Thermal Cycler (Applied Biosystems®) was used for processing the reactions. Sequences were amplified under the following conditions: 95°C for 2 min, followed by 30 cycles of 30 s at 95°C, 30 s at 56.5°C, and 1 min at 72°C, followed by a final extension at 72°C for 5 min. At the end of the reaction, the products were analyzed in 2% agarose gel (Sigma, Carlsbad, USA) stained in solution containing SYBR® safe DNA Gel Stain (Invitrogen) to detect the IDO, HGF, EGF, PGE-2, and IL-10 genes and were compared with 100 bp (1 kb Plus DNA Ladder, Invitrogen) markers. The endogenous 18S gene (Table 2) was used as a reference.
Statistical Analysis
The data obtained from the experimental procedures were analyzed using the program Graphpad Prism®, with prior verification of residue normality by the Shapiro–Wilk test. The variables that did not meet the statistical assumptions were subjected to the logarithmic transformation [Log (X + 1)]. The original or transformed data were subjected to Analysis of Variance (p<0.05) as required. The time and treatment effects were evaluated by the Tukey-Krammer’s test. Effects were considered significant for p<0.05.
Results
Isolation and Culture of Stem Cells from CAMs
CAM cells from different gestational stages were successfully cultured, as shown in Figure 1B–D. Cells in primary culture adhered to the plastic surface after a 48-h period and a heterogeneous population of polygonal and fibroblastoid cells was formed.
Cryopreservation and Cellular Susceptibility
After cryopreservation, the CAM cells were thawed and an average viability of 83.3% was observed in cells from the early gestation stage. In the mid- and late gestation stages, the average viabilities were 80.1% and 75.22%, respectively.
Growth Curve
After a 96-h period, a confluence of 80% was observed in the culture plates. After repeated passages, the growth curve indicated high development of cells at the beginning of procedures, and notably, a predominance of proliferation was noted in passage 2, while a gradual reduction of the proliferation was observed in passage 5 (Figure 1E). The Doubling time increased with each additional passage. The gradual reduction in cell proliferation rate was in accordance with successive increments in passages (Figure 1F).
Colony-Forming Assays
Colony formation was observed after 9, 10, and 13 days in CAM cells from early, mid-, and late gestation, with 145, 142.5, and 127.3 colonies observed, respectively (Figure 1G).
In vitro Differentiation Assays
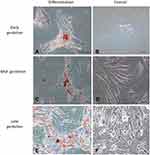
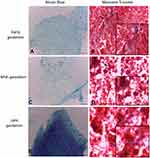
Cells cultured in the adipogenic differentiation medium exhibited morphological changes, with formation of intracytoplasmic vacuoles. Cells cultured in the osteogenic differentiation medium exhibited the deposition of extracellular amorphous mineral material. After being cultured in the chondrogenic differentiation medium, collagen fibers stained in blue were observed (Figures 2–4).
Flow Cytometry
Early gestation CAM cells exhibited expression of CD90 (31.7%) and CD105 (5.6%) and low labeling of CD34 (0.3%) and CD45 (0.4%). CAM cells from the mid-gestation stage exhibited positivity toward the CD90 (36.7%) and CD105 (6.3%) markers, with low labeling of CD34 (0.2%) and CD45 (0.4%). CAM cells from the late gestation stage exhibited positivity toward CD90 (67.1)% and CD105 (96.4%) markers, with low labeling of CD34 (4.4%) and CD45 (2.0%) (Figure 5).
 |
Figure 5 Quantification of the expression of mesenchymal (CD90 and CD105) and hematopoietic markers (CD45 and CD34) in CAM cells. |
Real-Time Polymerase Chain Reaction (qPCR) for Evaluation of Immunomodulatory and Pluripotency Genes
There was no expression of the OCT-4 transcript in the CAM cells from the three gestational stages. There was a low expression of the SOX-2 transcript at different stages, with a variability of abundance between gestational stages (p<0.05%).
In the IDO transcript analysis, there was a significant difference (p<0.05) between the stimulated and unstimulated cells from each gestational stage, with higher expression in the stimulated cells. A significant difference (p<0.05%) was observed between the stimulated cells from early gestation and other gestational stages. Unstimulated CAM cells from late gestation were significantly different from CAM cells from mid-gestation, with lower expression in cells from late gestation (Figure 6). The expression of HGF, EGF, IL-10, and PGE2 transcripts was not observed in any of the three gestational stages.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) for Evaluation of Immunomodulatory Genes
Analysis of the RT-PCR results revealed the expression patterns of IDO, HGF, EGF, PGE2, and IL-10 transcripts in CAM cells from the three different gestational stages and from canine bone marrow, as shown in Figure 7.
Discussion
MSCs are present in a majority of adult tissues and have a high proliferative capacity. In comparison to corresponding information from human and mouse lineage, there is limited information on the biology and function of canine MSCs in veterinary medicine. This lack of knowledge prevents the development of evidence-based studies using canine MSCs.25
In this study, we successfully established the CAM MSC line from early, mid-, and late gestation, based on the methodology described by Lange-Consiglio et al13 in the equine model. Initially, the CAM cells were isolated and subjected to primary culture, and they appeared to be proliferative and heterogeneous, with the presence of polygonal and fibroblastoid cells.8,13,24 After the primary culture, the cells displayed a fibroblastoid morphology with adhesion to the plastic surface, which is one of the characteristics of MSCs.1,6,11,26
Our observations from the growth curve analysis indicated a cell growth peak in the second passage, followed by a decline in proliferation in successive passages, corroborating with previous results.9,27 This decline was observed in MSCs from amniotic fluid, umbilical cord blood, and umbilical cord matrix as well, indicating a proliferation potential limited to the first passage.4,28 In contrast, Park et al reported an increasing cellular proliferation rate in amniotic membrane cells between the third and the twentieth passages, without a declining growth curve. The doubling time of CAM cells increased in terms of number of days required for cell duplication, a phenomenon observed in MSCs that undergo aging and quiescence as the number of passages progress.5,9,24,27
The three gestational stages exhibited differentiation capacity in adipogenic, chondrogenic, and osteogenic lines. The differentiation capacity is cited as one of the attributes necessary for stem cells to be classified as MSCs.1,4,5,9,11,13,24,29-32
The cellular immunophenotyping results revealed the expression of mesenchymal markers (CD90 and CD105) and non-expression of hematopoietic markers (CD45 and CD34) in CAM cells from different gestational stages. This was anticipated since the tested cells were not associated with the hematopoietic lineage. These results are consistent with findings from studies involving CAM cells that characterized MSC markers (CD29, CD44, CD90 CD105 and CD166) and negative markers for immune cells (CD3, CD11c, CD28, CD38 and CD62L), hematopoietic cells (CD34 and CD45), and platelets (CD41).5,7,13,24 Additionally, Saulnier et al24 and Cardoso et al7 reported a low expression of the CD105 marker in CAM cells.
Cells from the fetal annexes present certain advantages owing to their potential, since these can preserve embryonic characteristics by maintaining the pluripotency of original tissues.18,24,33 In our study, we reported low expression of SOX2 and no expression of OCT4 in the three gestational stages. Our results corroborated with those reported by Saulnier et al,24 wherein the noted low expression of SOX2 gene and no expression of OCT4 in CAMs, and in canine placenta and umbilical cord matrices. In contrast to our results, OCT4 gene expression was reported in CAM cells in another study.4–7 Mauro et al11 reported the expression of OCT4 in passage 1 sheep cells, with significant reduction after the passage 6. Conversely, Filioli Uranio et al4 reported a reduction in OCT4 expression between passage 1 and 2. Due to the diversity of results described in the literature, more tests should be conducted on the pluripotency of CAM.
To date, this is the first study to investigate the immunomodulatory response of CAM stem cells at different gestational stages through the expression of soluble factors such as IL-10, IDO, HGF, EGF, and PGE2. A limitation in this field is the absence of studies evaluating cells from canine fetal annexes throughout gestation.
The IDO gene, identified in some species, plays a central role in the study of in vitro immunomodulation, acting in tryptophan catabolism and inhibition of T-lymphocyte proliferation.34 Stimulation with mitogens such as INF-γ, TNF-α, interleukin 1A (IL-1A), or interleukin 1β (IL-1β) enhances the expression of immunomodulatory genes.35–37 In our results, we observed a higher expression of IDO in cells stimulated with INF-γ, irrespective of the gestational stage. Saulnier et al 24 reported that CAM, placenta, and umbilical cord matrix stimulated with IFN-γ exhibited higher IDO expression than unstimulated cells from the same tissues.
Munn et al38 and Mellor et al39 highlight the significance of IDO during gestation, since pregnant mice exposed to IDO-inhibiting drugs were unable to maintain gestation. Likewise, in unstimulated CAM cells, IDO expression was lower in late gestation when compared to that in mid-gestation. Maternal-fetal recognition and placentation take place during the early to mid-gestation stages. Therefore, a higher expression of IDO, and consequently the inhibition of the local immune system against the allogenic fetus seems to be essential to initiation and maintenance of placentation, as it is to gestation. On the contrary, in late gestation, lower expression of IDO is noted as the immune system appears to be linked to parturition, and hence, to the release of the fetal membranes as well.
The results from IFN-γ stimulation experiments confirm the findings reported by Saulnier et al,24 since CAM cells exhibit increased IDO expression levels in the presence of inflammatory stimulation. Therefore, the late gestation CAM cells should display significant immunomodulatory potential prior to inflammatory stimulation.
Quantitative evaluation of HGF, EGF, PGE2, and IL-10 expression in CAM cells yielded negative results. Conversely, immunomodulation-related soluble factors, such as IGF-1, bFGF, IL-10, TNFα, TGF-β, PGE2,40 HGF and EGF,40,41 have been identified in human amniotic membrane epithelial cells and MSCs. Kang et al42 were the first to describe the immunomodulatory potential of canine MSCs, demonstrating the expression of TGF-β, IL-6, IL-8, CCL2, CCL5, EGF, HGF and the non-expression of IL-10 genes in adipose tissue cells. In canine adipose and bone marrow cells, lymphocyte activation was suppressed by the cyclooxygenase and TGF-β pathways, as well as by the commonly known pathways mediated by NO or IDO.43 In addition, Lee et al28 reported that BM-MSCs were able to inhibit leukocyte proliferation and highlighted PGE2 as a potential antiproliferative factor.
This study involved the assessment of the immunomodulation of CAM by exclusive IFN-γ stimulation. Rossi et al44 reported that the immunological stimulation of human amniotic membrane cells is induced by soluble molecules and cell-to-cell contact. Di Nicola et al45 reported that the immunomodulatory effect on bone marrow cells is mediated exclusively by soluble factors. In contrast, Krampera et al35 reported that the immunomodulatory effect on rodent MSCs appeared to be predominantly mediated by cell-to-cell contact. Therefore, the diversity of results on the immunomodulation of MSCs may be directly related to the tissue being studied or the mechanisms inherent to each species,46 such as in dogs.47
Conclusions
This is the first study that compared the cellular characterization and gene expression patterns linked to immunomodulation in CAMs from different gestational stages. We revealed that CAM cells undergo an immunophenotypic and immunomodulatory transformation during the gestational period, with more satisfactory results observed in cells from the late gestation stage. These results suggest a distinct clinical impact on new avenues of research. Owing to the scarcity of data confirming the pathways involved in the immunomodulatory response in dogs, we conclude that a better understanding of CAM-mediated immunosuppression is necessary for future clinical applications, and this may serve as a strategy in the treatment of several immune-mediated diseases.
Abbreviations
CAM, canine amniotic membrane; IFN-γ, gamma-interferon; MSCs, mesenchymal stem cells.
Data Sharing Statement
All data generated and/or analyzed during this study are included in this published article.
Ethics and Consent Statement
This study was approved by the Ethical Committee on Animal Use (Comissão de Ética no Uso de Animais) of the Faculty of Animal Science and Food Engineering of the University of São Paulo (protocol #4,598,140,116; issued in 02/24/2016). We follow the international guide for use of dogs in experiments (https://www.ccac.ca/Documents/Standards/Guidelines/Vol2/dogs.pdf).
Acknowledgments
The authors acknowledge Clésio Gomes Mariano Junior for their technical support and manuscript editing, and Natália Juliana Gonçalves Nardelli for donating the dog’s cells lineages.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) – Finance Code 001, and the São Paulo Research Foundation (FAPESP – grant #2017/21266-0).
Disclosure
The authors declare that they have no competing interests.
References
1. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy positions statement. Cytotherapy. 2006;8(4):315–317. doi:10.1080/14653240600855905
2. Yi T, Song SU. Immunomodulatory properties of mesenchymal stem cells and their therapeutic applications. Arch Pharm Res. 2012;35(2):213–221. doi:10.1007/s12272-012-0202-z
3. In’tanker PS, Scherjon SA, Kleijburg-van Der Keur C, et al. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22(7):1338–1345. doi:10.1634/stemcells.2004-0058
4. Filioli Uranio M, Valentini L, Lange‐Consiglio A, et al. Isolation, proliferation, cytogenetic, and molecular characterization and in vitro differentiation potency of canine stem cells from fetal adnexa: a comparative study of amniotic fluid, amnion, and umbilical cord matrix. Mol Reprod Dev. 2011;78(5):361–73.
5. Park SB, Seo MS, Kim HS, Kang KS, Bauer JA. Isolation and characterization of canine amniotic membrane-derived multipotent stem cells. PLoS One. 2012;7(9):e44693. doi:10.1371/journal.pone.0044693
6. Filioli Uranio MF, Dell’aquila ME, Caira M, et al. Characterization and in vitro differentiation potency of early-passage canine amnion- and umbilical cord-derived mesenchymal stem cells as related to gestational age. Mol Reprod Dev. 2014;81(6):539–51.
7. Cardoso MT, Pinheiro AO, Vidane AS, et al. Characterization of teratogenic potential and gene expression in canine and feline amniotic membrane-derived stem cells. Reprod Domest Anim. 2017;52:58–64.
8. Rutigliano L, Corradetti B, Valentini L, et al. Molecular characterization and in vitro differentiation of feline progenitor-like amniotic epithelial cells. Stem Cell Res Ther. 2013;4(5):133. doi:10.1186/scrt344
9. Vidane AS, Souza AF, Sampaio RV, et al. Cat amniotic membrane multipotent cells are nontumorigenic and are safe for use in cell transplantation. Stem Cells Cloning. 2014;7:71–78. doi:10.2147/SCCAA.S67790
10. Campos LL, Landim-Alvarenga FC, Ikeda TL, et al. Isolation, culture, characterization and cryopreservation of stem cells derived from amniotic mesenchymal layer and umbilical cord tissue of bovine fetuses. Pesquisa Veterinária Brasileira. 2017;37(3):278–286. doi:10.1590/s0100-736x2017000300012
11. Mauro A, Turriani M, Ioannoni A, et al. Isolation, characterization, and in vitro differentiation of ovine amniotic stem cells. Vet Res Commun. 2010;34(Suppl S1):S25–S28. doi:10.1007/s11259-010-9393-2
12. Barboni B, Russo V, Curini V, et al. Gestational stage affects amniotic epithelial cells phenotype, methylation status, immunomodulatory and stemness properties. Stem Cell Rev Rep. 2014;10(5):725–741. doi:10.1007/s12015-014-9519-y
13. Lange-Consiglio UM, Corradetti B, Bizzaro D, et al. Characterization and potential applications of progenitor-like cells isolated from horse amniotic membrane. J Tissue Eng Regen Med. 2012;6(8):622–35.
14. Marcus AJ, Coyne TM, Rauch J, Woodbury D, Black IB. Isolation, characterization, and differentiation of stem cells derived from the rat amniotic membrane. Differentiation. 2008;76(2):130–44.
15. Borghesi J, Mario LC, Carreira AC, Miglino MA, Favaron PO. Phenotype and multipotency of rabbit (Oryctolagus cuniculus) amniotic stem cells. Stem Cell Res Ther. 2017;8(1):7. doi:10.1186/s13287-016-0468-z
16. Ma C, Wang K, Ji H, et al. Multilineage potential research of Beijing duck amniotic mesenchymal stem cells. Cell Tissue Bank. 2018;19(4):519–529. doi:10.1007/s10561-018-9701-6
17. Parolini O, Caruso M. Review: preclinical studies on placenta-derived cells and amniotic membrane: an update. Placenta. 2011;32(Suppl 2):S186–S195. doi:10.1016/j.placenta.2010.12.016
18. Cremonesi F, Corradetti B, Consiglio AL. Fetal adnexa derived stem cells from domestic animal: progress and perspectives. Theriogenology. 2011;75(8):1400–1415. doi:10.1016/j.theriogenology.2010.12.032
19. Iacono E, Rossi B, Merlo B. Stem cells from fetal adnexa and fluid in domestic animals: an update on their features and clinical application. Reprod Domest Anim. 2015;50(3):353–64.
20. Miki T. A rational strategy for the use of amniotic epithelial stem cell therapy for liver diseases. Stem Cells Transl Med. 2016;5(4):405–9.
21. Kariminekoo S, Movassaghpour A, Rahimzadeh A, Talebi M, Shamsasenjan K, Akbarzadeh A. Implications of mesenchymal stem cells in regenerative medicine. Artif Cells Nanomed Biotechnol. 2016;44(3):749–57.
22. Rasmusson I, Uhlin M, Le Blanc K, Levitsk V. Mesenchymal stem cells fail to trigger effector functions of cytotoxic T lymphocytes. J Leukoc Biol. 2007;82(4):887–93.
23. Marti LC, Ribeiro AAF, Hamerschlak N. Immunomodulatory effect of mesenchymal stem cells. Einstein (São Paulo). 2011;9(2):224–228. doi:10.1590/s1679-45082011rw1843
24. Saulnier N, Loriau J, Febre M, et al. Canine placenta: a promising potential source of highly proliferative and immunomodulatory mesenchymal stromal cells? Vet Immunol Immunopathol. 2016;171:47–55.
25. Russell KA, Chow NH, Dukoff D, et al. Characterization and immunomodulatory effects of canine adipose tissue- and bone marrow-derived mesenchymal stromal cells. PLoS One. 2016;11(12):e0167442. doi:10.1371/journal.pone.0167442
26. Bydlowski SP, Debes AA, Maselli LMF, Janz FL. Características biológicas das CT mesenquimais [Biological characteristics of mesenchymal stem cells]. Rev Bras Hematol Hemoter. 2009;31(Suppl1):S25–S35. doi:10.1590/S1516-84842009005000038
27. Lockhart JA. An interpretation of cell growth curves. Plant Physiol. 1971;48(3):245–248. doi:10.1104/pp.48.3.245
28. Lee WS, Suzuki Y, Graves SS, et al. Canine bone marrow-derived mesenchymal stromal cells suppress alloreactive lymphocyte proliferation in vitro but fail to enhance engraftment in canine bone marrow transplantation. Biol Blood Marrow Transplant. 2011;17(4):465–75.
29. Iida G, Asano K, Seki M, et al. Gene expression of growth factors and growth factor receptors for potential targeted therapy of canine hepatocellular carcinoma. J Vet Med Sci. 2014;76(2):301–6.
30. Wenceslau CV, Miglino MA, Martins DS, et al. Mesenchymal progenitor cells from canine fetal tissues: yolk sac, liver, and bone marrow. Tissue Eng Part A. 2011;17(17–18):2165–2176. doi:10.1089/ten.tea.2010.0678
31. Taghi GM, Mariam HGK, Taghi L, Leili H, Leyla M. Characterization of in vitro cultured bone marrow and adipose tissue-derived mesenchymal stem cells and their ability to express neurotrophic factors. Cell Biol Int. 2012;36(12):1239–1249. doi:10.1042/CBI20110618
32. Yamahara K, Harada K, Ohshima M, et al. Comparison of angiogenic, cytoprotective, and immunosuppressive properties of human amnion- and chorion-derived mesenchymal stem cells. PLoS One. 2014;9(2):e88319. doi:10.1371/journal.pone.0088319
33. Vita BD, Campos LL, Listoni AJ, et al. Anexos fetais: uma fonte alternativa de CT mesenquimais para a medicina veterinária eqüina [Fetal annexes: an alternative source of mesenchymal stem cells for equine veterinary medicine]. Veterinária e Zootecnia. 2012;19(1):8–22.
34. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–22.
35. Krampera M, Galipeau J, Shi Y, Tarte K, Sensebe L. Immunological characterization of multipotent mesenchymal stromal cells -The International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy. 2013;15(9):1054–61.
36. Krampera M, Cosmi L, Angeli R, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24(2):386–98.
37. Wheat WH, Chow L, Kurihara JN, et al. Suppression of canine dendritic cell activation/maturation and inflammatory cytokine release by mesenchymal stem cells occurs through multiple distinct biochemical pathways. Stem Cells Dev. 2017;26(4):249–62.
38. Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281(5380):1191–1193. doi:10.1126/science.281.5380.1191
39. Mellor AL, Sivakumar J, Chandler P, et al. Prevention of T cell-driven complement activation and inflammation by tryptophan catabolism during pregnancy. Nat Immunol. 2001;2(1):64–68. doi:10.1038/83183
40. Silini AR, Magatti M, Cargnoni A, Parolini O. Is immune modulation the mechanism underlying the beneficial effects of amniotic cells and their derivatives in regenerative medicine? Cell Transplant. 2017;26(4):531–9.
41. Koizumi NJ, Inatomi TJ, Sotozono CJ, Fullwood NJ, Quantoc AJ, Kinoshita S. Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res. 2000;20(3):173–177. doi:10.1076/0271-3683(200003)2031-9FT173
42. Kang JW, Kang KS, Koo HC, Park JR, Choi EW, Park YH. Soluble factors–mediated immunomodulatory effects of canine adipose tissue–derived mesenchymal stem cells. Stem Cells Dev. 2008;17(4):681–694. doi:10.1089/scd.2007.0153
43. Izumi M, Pazin BJ, Minervin CF, et al. Quantitative comparison of stem cell marker-positive cells in fetal and term human amnion. J Reprod Immunol. 2009;81(1):39–43.
44. Rossi D, Pianta S, Magatti M, Sedlmayr P, Parolini O, Rojas M. Characterization of the conditioned medium from amniotic membrane cells: prostaglandins as key effectors of its immunomodulatory activity. PLoS One. 2012;7(10):e46956. doi:10.1371/journal.pone.0046956
45. Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–3843. doi:10.1182/blood.V99.10.3838
46. Ren G, Su J, Zhang L, et al. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 2009;27(8):1954–1962. doi:10.1002/stem.118
47. Ambrosio CE, Orlandin JR, Oliveira VC, et al. Potential application of aminiotic stem cells in veterinary medicine. Anim Reprod. 2019;16(1):24–30. doi:10.21451/1984-3143-AR2018-0124
48. Gonçalves NJN, Bressan FF, Roballo KCS, et al. Generation of LIF-independent induced pluripotent stem cells from canine fetal fibroblasts. Theriogenology. 2017;92:75–82. doi:10.1016/j.theriogenology.2017.01.013
49. Kowalewski MP, Beceriklisoyb HB, Aslanb S, Agaoglub AR, Hoffmanna B. Time related changes in luteal prostaglandin synthesis and steroidogenic capacity during pregnancy, normal and antiprogestin induced luteolysis in the bitch. Anim Reprod Science. 2009;116(1-2):129–138. doi:10.1016/j.anireprosci.2008
50. Souza AF, Pieri NCG, Roballo KCS, et al. Dynamics of male canine germ cell development. PLoS One. 2018;13(2):e0193026. doi:10.1371/journal.pone.0193026
 © 2020 The Author(s). This work is published by Dove Medical Press Limited, and licensed under a Creative Commons Attribution License.
The full terms of the License are available at http://creativecommons.org/licenses/by/4.0/.
The license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
© 2020 The Author(s). This work is published by Dove Medical Press Limited, and licensed under a Creative Commons Attribution License.
The full terms of the License are available at http://creativecommons.org/licenses/by/4.0/.
The license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.