Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Characteristics of Xerosis, Pruritus, and Pallor in Stage 5 Chronic Kidney Disease Patients Undergoing Hemodialysis at Dr. Hasan Sadikin General Hospital, Bandung
Authors Dwiyana RF , Tsaqilah L , Sukesi L, Setiawan, Avriyanti E , Suhada KU, Zahira NI
Received 11 June 2023
Accepted for publication 5 September 2023
Published 21 September 2023 Volume 2023:16 Pages 2613—2621
DOI https://doi.org/10.2147/CCID.S418776
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Reiva Farah Dwiyana,1,* Laila Tsaqilah,1,* Lilik Sukesi,2,* Setiawan,3 Erda Avriyanti,1 Kamelia Utami Suhada,1 Nazya Irene Zahira4
1Department of Dermatology and Venereology, Faculty of Medicine, Universitas Padjadjaran-Dr. Hasan Sadikin Hospital, Bandung, West Java, Indonesia; 2Department of Internal Medicine, Faculty of Medicine, Universitas Padjadjaran-Dr. Hasan Sadikin Hospital, Bandung, West Java, Indonesia; 3Department of Biomedical Science, Faculty of Medicine, Universitas Padjadjaran, Sumedang Regency, West Java, Indonesia; 4Undergraduate Program, Faculty of Medicine, Universitas Padjadjaran, Sumedang Regency, West Java, Indonesia
*These authors contributed equally to this work
Correspondence: Reiva Farah Dwiyana, Department of Dermatology and Venereology, Faculty of Medicine, Universitas Padjadjaran – Dr. Hasan Sadikin Hospital, Jl. Pasteur 38, Bandung, West Java, 40161, Indonesia, Email [email protected]
Purpose: This study aims to delineate the demographic and clinical characteristics of xerosis, pruritus, and pallor among patients with stage 5 chronic kidney disease (CKD) undergoing hemodialysis at Dr. Hasan Sadikin General Hospital, Bandung.
Patients and Methods: This cross-sectional, descriptive study involved the analysis of 139 selected medical records of patients with stage 5 CKD who underwent hemodialysis between July and August 2022. A comprehensive examination was conducted by a dermatovenereologist, and the findings were duly recorded in the patients’ medical records. The documentation encompassed gender, age, employment status, as well as the clinical characteristics of xerosis, pruritus, and pallor. The collected data were analyzed using descriptive statistical methods.
Results: Out of the 139 patients, 70 (50.4%) were male, while 69 (49.6%) were female. The mean (SD) age was 47.6 (11.8) years. The majority of the patients were unemployed (n=96, 69.1%). The median (IQR) duration of hemodialysis was 48 (96.0– 24.0) months. The predominant findings were xerosis (n=84, 60.4%) and pallor (n=83, 59.7%), followed by pruritus (n=56, 40.3%). Instances of xerosis were more frequently observed in males, whereas pallor was more prevalent in females. Xerosis and pruritus exhibited higher prevalence in the ≥ 65 years age group, whereas pallor was more common in the 18– 44 years age group. In contrast to xerosis, pruritus and pallor were more frequently noted in the unemployed group. Xerosis was predominantly mild with overall dry skin (ODS) score of one, and it was mainly observed on the patients’ legs. Among those experiencing pruritus, over half displayed a moderate severity with visual analogue scale (VAS) scores ranging from ≥ 3 to < 7. Patients with pallor mostly exhibited hemoglobin levels below 10 g/dL.
Conclusion: Xerosis, pruritus, and pallor were prevalent among patients with stage 5 CKD undergoing hemodialysis. These disorders presented with distinct demographic and clinical characteristics. Timely diagnosis and intervention have the potential to enhance the quality of life for these patients.
Keywords: CKD, dialysis, dry skin, itch, pale skin
Introduction
Chronic kidney disease (CKD) can be defined as a persistent decline in kidney function resulting from prolonged injury to the kidney parenchyma. It represents a significant global health concern and ranks among the top 20 causes of death worldwide.1 The burden of CKD is projected to steadily increase on a global scale, primarily due to the escalating prevalence of its leading contributor, diabetes. This surge in burden is expected to be most pronounced in Asia. Approximately 434.3 million adults across Eastern, Southern, and South-Eastern Asia are affected by CKD.2 Within Indonesia, according to data from the Basic Health Research (Riskesdas) in 2018, there was a 1.8% rise in CKD prevalence per thousand individuals aged 15 years and older, compared to data recorded in 2013. Among the 34 provinces under study, West Java displayed the 8th highest CKD prevalence.3
CKD is classified into five stages based on the glomerular filtration rate (GFR). The fifth stage, characterized by a GFR <15 mL/min/1.73m2, is also referred to as end-stage renal disease (ESRD).4 At this stage, permanent kidney failure occurs, and without kidney replacement therapy (KRT), it can be fatal.5 Dialysis stands as the primary KRT modality in many nations, with hemodialysis being the most widely employed method.6,7 In 2018, 98% of stage 5 CKD patients in Indonesia underwent hemodialysis as their routine dialysis treatment. It is noteworthy that the number of of new hemodialysis patients doubled compared to the previous year, with 14,796 (22.27%) of the 66,433 new hemodialysis patients originating from West Java.8
Symptoms in the early stages of CKD are typically absent, becoming noticeable as patients reach stages 4 or 5.9 As the disease progresses and kidney function deteriorates, there is a buildup of uremic retention solutes, with those eliciting biological effects termed uremic toxins. This gives rise to uremic syndrome, which represents the clinical manifestation of the impacts exerted by uremic solutes on nearly all organ systems.10–12 Various organ systems affected include the cardiovascular, endocrine, gastrointestinal, neurological, and dermatological systems.12
A range of cutaneous disorders can be found in CKD patients, particularly those undergoing hemodialysis.13 The prevalence of these skin disorders often increases with the duration and severity of kidney disease.14 These manifestations can occur before or after the initiation of hemodialysis.15,16 However, the likelihood of encountering new skin conditions is greater in hemodialysis patients,16 attributable to their extended life expectancy due to the benefits of hemodialysis, affording more time for the development of novel skin disorders. Studies highlight a heightened prevalence of skin disorders in hemodialysis patients.17 Several investigations have identified the presence of at least one skin disorder in patients undergoing hemodialysis.18–20
Skin disorders among CKD patients undergoing dialysis can be categorized into specific and nonspecific conditions. Acquired perforating dermatosis, calcific uremic arteriolopathy, bullous diseases, and nephrogenic fibrosing dermopathy fall under the category of specific skin disorders. Nonspecific disorders include pruritus, xerosis, pallor, pigmentary changes, purpura, nail disorders, hair disorders, oral disorders, and uremic frost.19 Xerosis, pruritus, and pallor have exhibited high prevalence in certain studies.5,15,21 Aggarwal et al22 also discovered a significant increase in the occurrence of these skin disorders from stage 2 CKD to stage 5 CKD with hemodialysis. Pruritus in CKD is linked to sleep disturbances and impaired quality of life.23 Xerosis leads to discomfort, significantly impacting psychosocial well-being, and moderately to severely affecting quality of life.24 However, both conditions are often inadequately treated in hemodialysis patients.25 Conversely, pronounced pallor can serve as an indicator of severe anemia.26 In CKD patients, anemia is associated with lower quality of life and increased morbidity and mortality.27
The escalating number of CKD cases in Indonesia could potentially lead to an increase in its manifestations, including skin disorders that are notably common in stage 5 CKD patients undergoing hemodialysis. Xerosis, pruritus, and pallor are frequently reported and are also correlated with impaired quality of life. In 2018, West Java significantly contributed to the rise in CKD prevalence and the number of new hemodialysis patients. Despite being the city with the highest population density in the West Java province,28 research on the characteristics of these three skin disorders in Bandung remains limited. Thus, the objective of this study is to delineate the characteristics of xerosis, pruritus, and pallor among stage 5 CKD patients undergoing hemodialysis at Dr. Hasan Sadikin General Hospital, Bandung.
Materials and Methods
Study Design
This descriptive study employed a cross-sectional design and was conducted at the Medical Record Unit of Dr. Hasan Sadikin General Hospital, Bandung, from October to December 2022. The study aimed to provide a descriptive account of the demographic and clinical attributes of xerosis, pruritus, and pallor in stage 5 CKD patients undergoing hemodialysis. Secondary data sourced from patients’ medical records constituted the basis for this descriptive depiction.
Study Population
The data were extracted from medical records of stage 5 CKD patients undergoing hemodialysis, encompassing information derived from history-taking and physical examinations conducted by a dermatovenereologist. These examinations were carried out at the Hemodialysis Unit of Dr. Hasan Sadikin General Hospital, Bandung, from July to August 2022. Inclusion criteria encompassed medical records of patients aged ≥18 years who underwent hemodialysis at least twice weekly for a minimum of three months between July and August 2022. Excluded were medical records that were either inaccessible, incomplete, or pertaining to patients with a history of kidney transplant, positive human immunodeficiency virus-acquired immunodeficiency syndrome (HIV-AIDS), or other skin disorders. The sample size for this study was determined through total sampling.
Study Variables
This study described the demographic and clinical characteristics of pruritus, and pallor. Demographic variables included gender, age, employment status, and hemodialysis duration. Clinical characteristics differed for each skin disorder. Xerosis was graded based on overall dry skin (ODS) score. Lesion locations were categorized as chest, back, forearm, and legs, determining localized or generalized xerosis. Pruritus severity was assessed using the visual analogue scale (VAS). Pallor was objectively assessed through hemoglobin levels categorized as <10 g/dL and ≥10 g/dL.
The overall dry skin (ODS) score, developed by the European Group on Efficacy Measurement of Cosmetics and other Topical Products (EEMCO), is a scoring method evaluating all major and minor signs of xerosis or ichthyosis. Scores range from 0 (indicating absence of xerosis) to 4 (indicating extensive scales, notable roughness, presence of redness, eczematous changes, and cracks).29 In this study, each score was utilized to represent the extent of xerosis, ranging from mild to very severe.
The Visual analogue scale (VAS) is one of the unidimensional scales employed to subjectively measure the intensity of pruritus. Other available unidimensional scales include the numerical rating scale (NRS) and verbal rating scale (VRS). A recommendation exists to convert the cut off values of VAS and NRS into VRS values. These values are grouped into three categories: >0-<3 points indicate mild pruritus, ≥3-<7 points indicate moderate pruritus, ≥7-<9 points signify severe pruritus, and ≥9 points represent very severe pruritus.30
Pallor, characterized by the absence of color in the skin and mucous membranes due to low circulating hemoglobin levels, signifies the primary physical indication of anemia.31 Anemia is indicated if a patient’s GFR is below 20 mL/min and the hemoglobin levels are less than 10 g/dL.32 According to the United States Renal Data System (USRDS) Annual Data Report of 2022, the average hemoglobin level among incident ESRD patients in 2020 was 9.3 g/dL.33
Statistical Analysis
All collected data were entered into a Microsoft Excel spreadsheet. Statistical analysis was conducted using both Microsoft Excel and the IBM statistical package SPSS for Windows version 23. Categorical variables were presented as frequencies and percentages, while continuous variables were assessed using the Kolmogorov–Smirnov test for normality. Data that followed normal and non-normal distributions were subsequently reported as mean with standard deviation (SD) and median with interquartile range (IQR), respectively.
Ethics
The study protocol and ethical approval were obtained from the Health Research Ethics Committee of Dr. Hasan Sadikin General Hospital, Bandung, Indonesia, in accordance with the Declaration of Helsinki (ethical clearance number: LB.02.02/X.2.2.1/24859/2022). All patients were informed about the study’s purpose, and their informed consent was secured before their participation. Data confidentiality and patients’ privacy were rigorously upheld.
Results
A total of 139 patients’ medical records were included in this study. The male-to-female patient ratio was nearly balanced, with 70 (50.4%) male patients and 69 (49.6%) female patients. Their ages ranged from 19 to 76 years, with a mean age (SD) of 47.6 (11.8) years. A majority of the patients were unemployed, and over half of them were housewives. The duration of hemodialysis spanned from 3 months to 19 years, with a median duration (IQR) of 48 (96.0–24.0) months. Further details regarding the demographic characteristics of the patients can be found in Table 1.
 |
Table 1 Demographic Characteristics of Study Population |
In terms of frequency, xerosis and pallor were more prevalent compared to pruritus. Xerosis was identified in 84 (60.4%) patients. The demographic and clinical characteristics of xerosis are outlined in Table 2. Predominantly, xerosis presented as mild in severity, with the most frequently observed ODS score being 1, accompanied by a with median (IQR) ODS score of 1 (1.0–1.0). All patients exhibiting xerotic lesions displayed them on their legs.
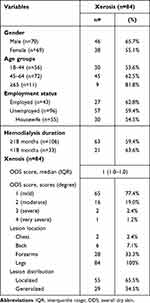 |
Table 2 Demographic and Clinical Characteristics of Xerosis |
The demographic and clinical attributes of pruritus are detailed in Table 3. Pruritus was reported in 56 (40.3%) patients. The median (IQR) VAS score was 5 (7.5–3.0). The majority of patients experiencing pruritus fell within the ≥3-<7 VAS score range.
 |
Table 3 Demographic and Clinical Characteristics of Pruritus |
Table 4 presents the demographic and clinical characteristics of pallor. Among all the patients included in this study, 83 (59.7%) displayed pallor. Most patients with pallor exhibited a hemoglobin level below 10 g/dL, with a median (IQR) of 8.4 (8.9–7.9) g/dL.
 |
Table 4 Demographic and Clinical Characteristics of Pallor |
Discussion
A high prevalence of mucous and skin disorders was observed among patients undergoing hemodialysis.19 Various skin disorders are prevalent in stage 5 CKD patients undergoing hemodialysis. This study specifically collected data on xerosis, pruritus, and pallor.
The prevalence of xerosis in hemodialysis patients varies between 54% and 91%.19 In congruence with these estimates, our study identified xerosis in 60.4% of patients. Similar findings were reported by Nasir et al,17 who observed xerosis in 60% of their patients. Conversely, Bouhamidi et al18 noted a lower prevalence of 40.9% in their study. Discrepancies in prevalence could stem from geographic and environmental differences.16 Our study took place in Indonesia, a tropical country with increased sun exposure that might contribute to skin dehydration, hence intensifying skin dryness.34
Xerosis was present in 46 (65.7%) males and 38 (55.1%) females. This dry skin condition was more common among the employed group (62.8%), the ≥65 age group (81.8%), and those undergoing hemodialysis for <18 months (63.6%). The development of xerosis is attributed to three primary mechanisms: cutaneous dehydration, altered barrier function, and heightened irritability to external agents.24 In CKD, dysfunctional sebaceous and apocrine sweat glands35 lead to reduced skin lipid levels, thereby compromising skin hydration. Additionally, skin barrier dysfunction impairs water content in the stratum corneum.24 These dermal changes are closely tied to uremia,36 possibly explaining the higher prevalence of xerosis in patients undergoing hemodialysis for <18 months; those with longer hemodialysis durations may have better hemodialysis adequacy, as suggested by Rezaiee et al.37 Factors such as excessive diuretic dosages, vitamin A metabolism disruptions, glycerol deficiency-induced dehydration, and chemical irritants might also contribute to xerosis development in hemodialysis patients.19 Socioeconomic status, dust and detergent exposure, and inadequate emollient use may also be contributory.34 This could elucidate why xerosis was more prevalent in the employed group, as they engage in outdoor activities more frequently, exposing them to dusts, detergents, and other external substances. While housewives are categorized as unemployed, they are still susceptible to detergent exposure and indoor air pollution from cooking. This may account for the relatively high occurrence of xerosis in housewives (54.4%) and the unemployed group (59.4%). Males might exhibit a higher tendency for dry skin due to suboptimal emollient use, compared to females who generally exhibit greater cosmetic awareness. Additionally, advanced age can exacerbate xerosis.24 This could contribute to the increased prevalence of dry skin among individuals aged ≥65 years in this study.
In accordance with the ODS score, most patients exhibited mild xerosis. In contrast, Kurniawan et al25 found severe xerosis (n=18, 46.2%) most commonly among their 39 patients. This condition typically affects the extensor surfaces of the forearms, legs, and, thighs, similar to observations by Udayakumar et al20 in their study.16 Existing literature indicates that xerosis in maintenance dialysis patients frequently manifests across a range of locations, especially on the legs, back, chest, and hands.24 In this study, all patients displayed xerosis on their legs, with the forearms being the second most affected body part. Only a small subset of patients exhibited xerosis on their chest and back. Ana-Sonia et al38 reported a similar pattern, where xerosis predominantly affected the chest, back, and extensor surfaces of the extremities. In terms of lesion distribution, most patients presented with localized xerosis (65.5%), confined to their legs.
Patients undergoing hemodialysis often experience pruritus, an uncomfortable symptom.34 However, pruritus was the least frequent finding in the present study, accounting for 40.3% of the total study population. This prevalence was lower than what has been reported in previous studies.5,15–19,21,34 According to the VAS score, moderate pruritus was the most frequently observed severity in this study. Similarly, Ariyani and Robby39 noted that moderate pruritus (n=13, 34%) was the highest, with an equal frequency to severe pruritus (n=13, 34%). The higher frequency of severe pruritus in their study compared to ours might be due to the exclusion of patients who were on anti-pruritic therapy.
The exact cause of pruritus in CKD is not well understood, but it’s likely related to the gradual accumulation of pruritogens, the nature of which remains unknown.20 Several mechanisms, including uremic toxins, immune dysregulation, neuropathy, and opioid imbalance, are considered possible contributors.23 Hemodialysis can trigger immune responses, such as interleukin-1 production by leukocytes, which is believed to act as a pruritogen.40 This mechanism could explain the higher occurrence of pruritus in the hemodialysis duration ≥18 months group (42.4%) in our study. Advanced age is generally associated with an increased tendency for pruritus due to physiological changes in elderly skin, resulting in reduced hydration and altered nerve function.39 This could account for the higher prevalence of pruritus was more frequent in the ≥65 years old group (72.7%). Employment status might also influence pruritus. Satti et al41 found an association between employment and pruritus, indicating that employed males had significantly lower 5-D itch scores than unemployed males. This might be due to the psychological distraction provided by a regular job, suppressing the urge to itch. Housewives, on the other hand, face factors that can exacerbate pruritus, such as heat23 from cooking and skin dryness23 caused by detergent and indoor pollution exposure, thus explaining the common occurrence of pruritus among housewives (45.4%). Taken together, these factors could contribute to the higher frequency of pruritus in the unemployed group (44.8%).
Pallor in CKD patients is primarily a consequence of anemia.17 Skin pallor is considered a hallmark of CKD and an early observable sign.20,21 In our study, pallor (59.7%) was the second most prevalent finding. Other studies reported pallor prevalence ranging from 60% to 100%.5,15,17,21 Pallor was more frequent in females (68.1%) and the 18–44 age group (60.7%). Although there was only a slightly higher prevalence of pallor in the unemployed group (60%) compared to the employed group (59.1%), a higher prevalence was noted among housewives (65.1%) within the unemployed group. Among the 83 patients with pallor, 77 (92.77%) had hemoglobin levels below 10 g/dL. In a study by Udayakumar et al,20 64% of patients with pallor exhibited hemoglobin levels below 8 g/dL. Anees et al5 reported a statistically significant association between pallor and hemoglobin levels, with 112 (70.8%) patients with pallor having hemoglobin levels below 11 g/dL. Anemia in CKD arises from various factors, including nutritional deficiencies,5 such as iron, folic acid, and vitamin D,38 reduced erythrocyte survival, erythropoietin deficiency due to uremic toxin-induced suppression of erythropoietin synthesis,42 and blood loss due to coagulation disorders.5 The higher frequency of pallor in females, the 18–44 age group, and particularly housewives in our study might be attributed to a greater risk of iron deficiency due to menstrual blood loss in premenopausal women. Lower dietary iron intake could also be contributory.43 Pallor was also more prevalent in patients with hemodialysis durations below 18 months (66.7%). This could be due to the possibility that patients with longer hemodialysis durations achieve better hemodialysis adequacy, leading to improved clearance of uremic toxins and enhanced erythropoietin synthesis.
Conclusion
This study revealed a high prevalence of xerosis and pallor. Although pruritus exhibited a lower frequency compared to the previous two disorders, it remained a common symptom, often occurring in patients undergoing hemodialysis. The insights provided in this research can significantly assist medical professionals in promptly identifying these skin conditions. Given their frequent occurrence among stage 5 CKD patients undergoing hemodialysis, this information should be factored into the diagnostic process for stage 5 CKD. Additionally, the demographic characteristics data for each disorder could facilitate further investigations into potential correlations with the presence of these disorders. The clinical insights presented here are valuable for physicians, enabling them to tailor appropriate management strategies for each condition. Ultimately, such interventions have the potential to enhance patients’ quality of life and reduce morbidity and mortality rates.
Acknowledgments
The authors would like to express their gratitude to the staff of Department of Dermatology and Venereology and Department of Internal Medicine, Faculty of Medicine, Universitas Padjadjaran as well as as all the staff of Medical Record Unit in Dr. Hasan Sadikin General Hospital, Bandung, West Java, Indonesia.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ke C, Liang J, Liu M, Liu S, Wang C. Burden of chronic kidney disease and its risk-attributable burden in 137 low-and middle-income countries, 1990–2019: results from the global burden of disease study 2019. BMC Nephrol. 2022;23(1):1–12. doi:10.1186/S12882-021-02597-3/FIGURES/4
2. Liyanage T, Toyama T, Hockham C, et al. Prevalence of chronic kidney disease in Asia: a systematic review and analysis. BMJ Glob Health. 2022;7(1):e007525. doi:10.1136/BMJGH-2021-007525
3. National Institute of Health Research and Development. Main Results of Riskesdas 2018; 2018. Available from: https://kesmas.kemkes.go.id/assets/upload/dir_519d41d8cd98f00/files/Hasil-riskesdas-2018_1274.pdf.
4. Vadakedath S, Kandi V. Dialysis: a review of the mechanisms underlying complications in the management of chronic renal failure. Cureus. 2017;9(8):e1603. doi:10.7759/CUREUS.1603
5. Anees M, Butt G, Gull S, Nazeer A, Hussain I, Ibrahim M. Factors affecting dermatological manifestations in patients with end stage renal disease. J Coll Physicians Surg Pak. 2018;28(2):98–102. doi:10.29271/JCPSP.2018.02.98
6. Queeley GL, Campbell ES. Comparing treatment modalities for end-stage renal disease: a meta-analysis. Am Health Drug Benefits. 2018;11(3):127. doi:10.1016/j.jval.2014.03.1690
7. Yilmaz U, Inci A, Akarsu A, Maden U. Factors affecting the choice of renal replacement therapy in patients with end stage renal failure. Osmangazi J Med. 2022;44(4):499–507. doi:10.20515/OTD.1059206
8. Indonesian Renal Registry (IRR). 11th Report Of Indonesian Renal Registry 2018; 2018. Available from: https://www.indonesianrenalregistry.org/data/IRR%202018.pdf.
9. Vaidya SR, Aeddula NR, Doerr C. Chronic Renal Failure (Nursing). StatPearls Publishing; 2022. Available form: https://www.ncbi.nlm.nih.gov/books/NBK568778/.
10. Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet. 2017;389(10075):1238–1252. doi:10.1016/S0140-6736(16)32064-5
11. Nigam SK, Bush KT. Uraemic syndrome of chronic kidney disease: altered remote sensing and signalling. Nat Rev Nephrol. 2019;15(5):301–316. doi:10.1038/s41581-019-0111-1
12. Vanholder R, Fouque D, Glorieux G, et al. Clinical management of the uraemic syndrome in chronic kidney disease. Lancet Diabetes Nephrol. 2016;4(4):360–373. doi:10.1016/S2213-8587(16)00033-4
13. Falodun O, Ogunbiyi A, Salako B, George AK. Skin changes in patients with chronic renal failure. Saudi J Kidney Dis Transpl. 2011;22(2):272.
14. Khanna D, Singal A, Kalra OP. Comparison of cutaneous manifestations in chronic kidney disease with or without dialysis. Postgrad Med J. 2010;86(1021):641–647. doi:10.1136/PGMJ.2009.095745
15. Farhood IG, Naief ZR. Re-evaluation the frequency of cutaneous manifestations in patients on hemodialysis. Iraqi J Med Sci. 2013;11(4):388–392.
16. Kolla PK, Desai M, Pathapati RM, et al. Cutaneous manifestations in patients with chronic kidney disease on maintenance hemodialysis. ISRN Dermatol. 2012;2012:1–4. doi:10.5402/2012/679619
17. Nasir A, Shehzad A. Dermatological manifestations in patients with chronic kidney disease on regular hemodialysis. J Pak Assoc Dermatol. 2017;27(3):263–269.
18. Bouhamidi A, Amraoui M, Rafik H, Boui M, Hjira N. Dermatologic manifestations in patients on chronic hemodialysis. J Dermatol Res Ther. 2019;5(1):069. doi:10.23937/2469-5750/1510069
19. Mourad B, Hegab D, Okasha K, Rizk S. Prospective study on prevalence of dermatological changes in patients under hemodialysis in hemodialysis units in Tanta University hospitals, Egypt. Clin Cosmet Investig Dermatol. 2014;7:313–319. doi:10.2147/CCID.S70842
20. Udayakumar P, Balasubramanian S, Ramalingam KS, Lakshmi C, Srinivas CR, Mathew AC. Cutaneous manifestations in patients with chronic renal failure on hemodialysis. Indian J Dermatol Venereol Leprol. 2006;72(2):119–125. doi:10.4103/0378-6323.25636
21. Iftikhar U, Anees M, Nadeem M, Aman S, Kazmi AH. Frequency of cutaneous manifestations in patients of end stage renal disease on hemodialysis. Ann King Edw Med Univ. 2015;21(2):61. doi:10.21649/AKEMU.V21I2.699
22. Aggarwal H, Jain D, Yadav L, Aggarwal A, Singh J. Spectrum of skin manifestations in CKD: a tertiary care center experience from North India. Indian J Clin Pract. 2022;32(11):56.
23. Cheng AY, Wong LS. Uremic pruritus: from diagnosis to treatment. Diagnostics. 2022;12(5):1108. doi:10.3390/DIAGNOSTICS12051108
24. Szepietowski JC, Reich A, Schwartz RA. Uraemic xerosis. Nephrol Dial Transplant. 2004;19(11):2709–2712. doi:10.1093/NDT/GFH480
25. Kurniawan M, Regina R, Iryaningrum MR. The correlation between pruritus and xerosis with the quality of life of patients undergoing hemodialysis in Atma Jaya Hospital. J Pak Assoc Dermatol. 2022;32(2):288–292.
26. Kalicki RM, Farese S, Uehlinger DE. The validation of a new visual anaemia evaluation tool HemoHue HH1 in patients with end-stage renal disease. Anemia. 2013;2013:1–6. doi:10.1155/2013/424076
27. Anees M, Ibrahim M. Anemia and hypoalbuminemia at initiation of hemodialysis as risk factor for survival of dialysis patients. J Coll Physicians Surg Pak. 2009;19(12):776–780.
28. Population and Civil Registry Office. Population density based on district/city in West Java. Open Data Jabar. Available from: https://opendata.jabarprov.go.id/id/dataset/kepadatan-penduduk-berdasarkan-kabupatenkota-di-jawa-barat.
29. Serup J. EEMCO guidance for the assessment of dry skin (xerosis) and ichthyosis: clinical scoring systems. Skin Res Technol. 1995;1(3):109–114. doi:10.1111/J.1600-0846.1995.TB00029.X
30. Manenti L, Leuci E. Do you feel itchy? A guide towards diagnosis and measurement of chronic kidney disease-associated pruritus in dialysis patients. Clin Kid J. 2021;14(suppl. 3):i8–i15. doi:10.1093/CKJ/SFAB143
31. da Silva RM, Machado CA. Clinical evaluation of the paleness: agreement between observers and comparison with hemoglobin levels. Rev Bras Hematol Hemoter. 2010;32(6):444–448. doi:10.1590/S1516-84842010000600007
32. Bhatta S, Aryal G, Kafle R. Anemia in chronic kidney disease patients in predialysis and postdialysis stages. J Pathol Nepal. 2011;1(1):26–29. doi:10.3126/JPN.V1I1.4446
33. United States Renal Data System. 2022 USRDS Annual Data Report: epidemiology of Kidney Disease in the United States; 2022. Available from: https://usrds-adr.niddk.nih.gov/2022.
34. Maskey A, Kumar A, Shrestha R. Study of cutaneous manifestations in end stage kidney disease undergoing hemodialysis. Nepal J Dermatol Venereol Leprol. 2020;18(1):37–43. doi:10.3126/NJDVL.V18I1.29568
35. Agarwal P, Garg V, Karagaiah P, Szepietowski JC, Grabbe S, Goldust M. Chronic kidney disease-associated pruritus. Toxins. 2021;13(8):527. doi:10.3390/toxins13080527
36. Shafiee MA, Akbarian F, Memon KK, Aarabi M, Boroumand B. Dermatologic manifestations in end-stage renal disease. Iran J Kidney Dis. 2015;9(5):339–353.
37. Rezaiee Rezaiee O, Shahgholian N, Shahidi S. Assessment of hemodialysis adequacy and its relationship with individual and personal factors. Iran J Nurs Midwifery Res. 2016;21(6):577–582. doi:10.4103/1735-9066.197673
38. Ana-Sonia I, Mirela G, Iudita-Maria B, Mihail-Alexandru B, Elena L, Sliviu-Horia M. Cutaneous findings in chronic kidney disease and hemodialysis. Rom J Clin Exp Dermatol. 2015;2(3):182–189.
39. Ariyani H, Robby A. Pruritus overview of chronic kidney failure patients using visual analogue scale application in Hemodialysis Unit General Hospital dr. Soekardjo, Tasikmalaya City, West Java. J Phys Conf Ser. 2020;1477(6):62014. doi:10.1088/1742-6596/1477/6/062014
40. Daraghmeh M, Badran M, Janajreh A, et al. Prevalence of pruritus associated with hemodialysis and its association with sleep quality among hemodialysis patients: a multicenter study. BMC Nephrol. 2022;23(213):23. doi:10.1186/s12882-022-02838-z
41. Satti MZ, Arshad D, Javad H, et al. Uremic pruritus: prevalence and impact on quality of life and depressive symptoms in hemodialysis patients. Cureus. 2019;11(7):e5178. doi:10.7759/cureus.5178
42. Hamza E, Metzinger L, Metzinger-le Meuth V. Uremic toxins affect erythropoiesis during the course of chronic kidney disease: a review. Cells. 2020;9(9):2039. doi:10.3390/cells9092039
43. Coad J, Pedley K. Iron deficiency and iron deficiency anemia in women. Scand J Clin Lab Invest. 2014;74(suppl. 244):82–89. doi:10.3109/00365513.2014.936694
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
