Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Characteristics of Biofilm-Forming Ability and Antibiotic Resistance of Cutibacterium acnes and Staphylococcus epidermidis from Acne Vulgaris Patients
Authors Ruchiatan K , Rizqandaru T , Satjamanggala PR, Tache N , Cahyadi AI , Rezano A , Gunawan H , Sutedja EK , Dwiyana RF , Hidayah RMN , Achdiat PA , Sutedja E , Suwarsa O , Hindritiani R
Received 26 June 2023
Accepted for publication 25 August 2023
Published 11 September 2023 Volume 2023:16 Pages 2457—2465
DOI https://doi.org/10.2147/CCID.S422486
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Kartika Ruchiatan,1,2 Trustia Rizqandaru,1 Panji Respati Satjamanggala,1 Nisrina Tache,1 Adi Imam Cahyadi,3 Andri Rezano,4,5 Hendra Gunawan,1 Eva Krishna Sutedja,1 Reiva Farah Dwiyana,1 Risa Miliawati Nurul Hidayah,1 Pati Aji Achdiat,1 Endang Sutedja,1 Oki Suwarsa,1 Reti Hindritiani1
1Department of Dermatology and Venereology, Faculty of Medicine, Universitas Padjadjaran-Dr. Hasan Sadikin Hospital, Bandung, West Java, Indonesia; 2Doctorate Program, Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia; 3Division of Microbiology, Department of Biomedical Sciences, Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia; 4Division of Cell Biology, Department of Biomedical Sciences, Faculty of Medicine, Universitas Padjadjaran, Sumedang, Indonesia; 5Graduate School of Biomedical Sciences Master Program, Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia
Correspondence: Kartika Ruchiatan, Department of Dermatology and Venereology, Faculty of Medicine, Universitas Padjadjaran - Dr. Hasan Sadikin Hospital, Jl. Pasteur 38, Bandung, West Java, 40161, Indonesia, Tel +62811247932, Email [email protected]
Introduction: Acne vulgaris (AV) is a common and chronic disorder of the pilosebaceous unit and has a multifactorial pathology, including activities of Cutibacterium acnes (C. acnes) and Staphylococcus epidermidis (S. epidermidis). Antibiotic resistance has become a major concern in dermatology daily practice, and the ability of biofilm formation by both bacteria is suggested to increase antibiotic resistance in acne.
Purpose: Our aim was to analyze the comparison of antibiotic resistance between biofilm-forming (BF) and non-biofilm-forming (NBF) strains of C. acnes and S. epidermidis towards seven antibiotics commonly used for acne.
Methods: This is a cross-sectional analytical study involving 60 patients with AV. Samples were obtained from closed comedones on the forehead using the standardized skin surface biopsy (SSSB) method at the Cosmetic Dermatology Clinic Dr. Hasan Sadikin in Bandung, Indonesia. Isolates were cultured and identified before undergoing the biofilm-forming test using the tissue culture plate method. Antibiotic susceptibility testing for each antibiotic was then performed using the disc diffusion method.
Results: The incidence of antibiotic resistance to clindamycin in BF and NBF C. acnes isolates was 54.5% (p=1.00), while in BF and NBF S. epidermidis isolates, it was 54.5% and 45.5% respectively (p=0.67). The incidence of antibiotic resistance to erythromycin and azithromycin in BF and NBF C. acnes isolates was 54.5% and 63.6% respectively (p=1.00), whereas for S. epidermidis BF and NBF isolates, it was 54.5% (p=1.00). There was no resistance observed to tetracycline, doxycycline, levofloxacin, and cotrimoxazole in all groups.
Conclusion: There were no significant differences in resistance against seven antibiotics between the C. acnes and S. epidermidis in BF and NBF groups. Furthermore, although statistically not significant, some resistances were observed against clindamycin, erythromycin, and azithromycin. Consequently, the use of these three antibiotics should be judiciously regulated.
Keywords: acne vulgaris, antibiotic resistance, biofilm, Cutibacterium acnes, Staphylococcus epidermidis
Introduction
Acne vulgaris (AV) is a common multifactorial disorder of the pilosebaceous unit.1,2 Follicular epidermal hyperplasia, sebum production, the presence and activity of bacteria, most frequently Cutibacterium acnes (C. acnes), inflammation, and immune response are the four essential components of the pathophysiology of acne vulgaris.3 A study by Bek-Thomsen et al4 showed that C. acnes and Staphylococcus epidermidis (S. epidermidis) were the predominant bacteria in AV, and they have the ability to form biofilms.4,5 Biofilm refers to a surface-attached, structured microbial community embedded in a self-produced extracellular matrix that adheres to a biotic or abiotic surfaces.4–6 In vitro research suggests that bacteria within a biofilm’s protected microenvironment are 50–500 times more resistant to antimicrobial treatments compared to free-floating bacteria.5 Factors contributing to this resistance include restricted penetration of antimicrobials, decreased growth rate, expression of resistance genes, and the presence of resistant “persister” cells.1 The rise of antibiotic resistance in acne has become a major concern in dermatology practice, and the ability of C. acnes and S. epidermidis to form biofilms is believed to contribute to increased antibiotic resistance in acne.7 Reports of antibiotic resistance in C. acnes and S. epidermidis in AV have been documented in various countries and have shown an increasing trend over the years.7
In this study, we examined the in vitro capacity of C. acnes and S. epidermidis to form biofilms. We then assessed the resistance of biofilm-grown bacteria to commonly used antimicrobial drugs for acne treatment. To date, no studies have compared the antibiotic resistance between biofilm-forming C. acnes and S. epidermidis species. Therefore, the aim of this study is to analyze and compare the antibiotic resistance in biofilm-forming C. acnes and S. epidermidis in AV patients at Dr. Hasan Sadikin Bandung, Indonesia.
Materials and Methods
Patients
This study involved 60 patients who visited the Cosmetic Dermatology Clinic, Department of Dermatovenerology, Hasan Sadikin Bandung General Hospital, Indonesia. The inclusion criteria for our study were as follows: female patients clinically diagnosed with AV, aged between 18 to 24 years, and presenting with closed comedones on the forehead, along with positive bacterial culture results for C. acnes or S. epidermidis. Patients were excluded if they were pregnant, had received topical antibiotics in the past week and/or systemic antibiotics in the past two weeks, were using hormonal therapy or contraceptives and anti-androgenic treatment for the past three months, or had allergies to cyanoacrylate. The study protocol was approved by the Board and Ethics Committee, and written informed consent was obtained from all patients.
Bacterial Culture and Identification
The standardized skin surface biopsy (SSSB) method was used to collect microbiological samples from each patient who had closed comedones on the forehead. This method involves the use of high-bond glue (cyanoacrylate) to collect the follicular contents and the superficial portion of the horny layer, including pilosebaceous units and comedones. The collected tissue was then cultivated in fluid thioglycolate medium (FTM) and blood agar to detect C. acnes growth, and in tryptic soy broth (TSB), blood agar, and MacConkey agar to detect S. epidermidis growth. Identification of the isolates was performed using the Vitek®2 compact machine (bioMerieux, France).
Biofilm Formation Assay
In this study, the protocols for the biofilm formation assay were based on the method described by Kuehnast et al with modifications. C. acnes isolates were inoculated into 10 mL Brain-Heart Infusion + 1% glucose (BHIglu) and incubated at 37°C for 72 hours under anaerobic conditions. The incubation product was then diluted with BHIglu in a 1:100 ratio or until the opacity reached 0.5 McFarland. The diluted sample was divided into non-treated 96-well U-bottom tissue culture plates, with each well containing 0.15 mL of the sample. Each clinical isolate was cultivated in triplicates across 3 culture plates and incubated at 37°C for 72 hours under anaerobic conditions. BHIglu was used as the negative control and underwent a similar process as the isolates.
Staphylococcus epidermidis isolates were inoculated into 10 mL Tryptic Soy Broth + 1% glucose (TSBglu) and incubated at 37°C for 24 hours. The incubation product was then diluted with TSBglu in a 1:100 ratio. The diluted sample was divided into non-treated 96-well flat-bottom tissue culture plates, with each well containing 0.2 mL of the sample. Each clinical isolate was cultivated in triplicates across 3 culture plates and incubated at 37°C for 24 hours. TSBglu was used as the negative control and underwent a similar process as the isolates.
At the end of the incubation process, the remaining medium was discarded by gently tapping the base of the plate. The samples were washed with 0.2 mL of phosphate buffer saline (PBS) three times. The remaining biofilms at the base of the wells were dried for 50 minutes at 60°C and then fixed with 100 µL of methanol (for C. acnes) or with 0.2 mL of 2% CH3COONa (for S. epidermidis) for 10 minutes. The samples were stained with 0.15 mL of 0.1% safranin for 2 minutes (Figure 1). Afterward, the cells were washed again with 0.2 mL of PBS three times, and 0.2 mL of isopropanol was added to the plates. The biofilm-forming abilities were presented as optical density (OD), measured by a spectrophotometer (MultiskanTM FC Microplate Photometer) at a wavelength (λ) of 492 nm, and further classified into biofilm-forming (BF) and non-biofilm-forming (NBF) categories (Table 1).
 |
Table 1 Optical Density Measurement and Classification of Biofilm |
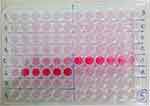 |
Figure 1 Biofilm production assay. Tissue culture plates show different color intensities for BF and NBF cells and measured with spectrophotometer as optical density (OD). |
Antibiotic Resistance Test
The disc diffusion method was used to examine the susceptibility of each antibiotic to the bacterial isolates. A bacterial colony suspension was spread onto Mueller Hinton Agar plates. Antibiotic discs containing tetracycline, doxycycline, clindamycin, erythromycin, azithromycin, levofloxacin, and cotrimoxazole were placed on top of the agar surface using sterile techniques in triplicates (Figure 2A and B). The antibiotic resistance results were determined by measuring the zone of inhibition around the antibiotic discs after an incubation period of 24 hours for C. acnes and 72 hours for S. epidermidis. The data obtained from this procedure were analyzed according to the Clinical and Laboratory Standards Institute (CLSI) standards.
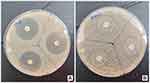 |
Figure 2 Antibiotic susceptibility testing with disc diffusion method. A sensitive antibiotic produce zones of inhibition (A), while a resistant antibiotic not produce zone of inhibition (B). |
Data Analysis
All our data were analyzed using the Pearson Chi-square test, with Fisher’s exact test used as an alternative when the expected value was less than 5. A p-value ≤ 0.05 was considered statistically significant. Data processing was performed using the IBM® SPSS® (Statistical Package for the Social Sciences) application, version 24.0, by the International Business Machines Corporation.
Results
Subject Characteristics
Out of the 60 patients, 68 bacterial isolates were obtained, including 36 isolates of C. acnes and 32 isolates of S. epidermidis. Among the C. acnes isolates, 20 (55.6%) were BF and 16 (44.4%) were NBF. For S. epidermidis isolates, 11 (34.4%) were BF and 21 (65.6%) were NBF. Antibiotic susceptibility testing was performed on 11 isolates from each group.
The distribution of antimicrobial susceptibilities is presented in Table 2. Clindamycin, erythromycin, and azithromycin exhibited higher resistance rates among both C. acnes and S. epidermidis strains compared to other tested antibiotics. None of the isolates were resistant to tetracycline, doxycycline, levofloxacin, or cotrimoxazole. In the C. acnes BF group, 54.5% of the isolates were resistant to the three aforementioned antibiotics. In the NBF group, 54.5% were resistant to clindamycin, 63.6% to erythromycin, and 63.6% to azithromycin. The resistance rates in the S. epidermidis BF group to clindamycin, erythromycin, and azithromycin were 54.5%, while in the NBF group, the resistance rates to clindamycin, erythromycin, and azithromycin were 45.5%, 54.5%, and 54.5%, respectively.
 |
Table 2 Comparison of Antibiotic Resistance Between Biofilm-Forming and Non-Biofilm Forming of C. Acnes and S. Epidermidis |
The Chi-Square test was performed to analyze the resistance to tetracycline, doxycycline, clindamycin, levofloxacin, and cotrimoxazole, while the Fisher’s exact test was used for assessing resistance to erythromycin and azithromycin. The results of these tests showed p-values greater than 0.05, indicating no significant difference in antibiotic resistance between the C. acnes BF and NBF groups, S. epidermidis BF and NBF groups, as well as among the four groups.
Discussion
Staphylococcus epidermidis and C. acnes are two major bacterial strains that are commonly isolated and are known to contribute as pathogenic factors in AV.7,8 Studies have shown that C. acnes is the most prevalent microbe in the pilosebaceous unit, with up to 107 viable organisms found in a single sebaceous unit.5 However, S. epidermidis counts can be equal to or higher than C. acnes counts in some follicles, as observed in studies that examined pooled samples of excised follicles.8 In our previous study at this hospital, S. epidermidis was ranked as the second most common bacteria found in comedones of AV patients.9 The presence of biofilms was found to be more frequent in comedone lesions compared to other inflammatory lesions, as reported in studies by Dreno et al10 and Hindritiani et al11 which also revealed higher rates of antibiotic resistance in closed comedones compared to skin smears or pustules. Hence, for this study, samples were obtained specifically from comedone lesions.
According to our study, out of the total number of C. acnes isolates, 20 (55.6%) were biofilm-forming (BF) strains, while 16 (44.4%) were non-biofilm-forming (NBF) strains. BF strains were more frequently isolated from acne sufferers compared to NBF strains. These findings support the conclusion of Jahns et al,12 who visualized large biofilms of C. acnes in 14 out of 18 AV patients. However, this is in contrast to a study conducted by Loss et al,13 which found C. acnes biofilms in only nine out of 39 samples (23%).
A total of S. epidermidis isolates, 11 (34.4%), were biofilm-forming (BF), including 2 (18.1%) isolates characterized as strong biofilm producers, while 21 (65.6%) were non-biofilm-forming (NBF). The proportion of NBF strains was higher than BF strains isolated from acne patients. This result contrasts with a study conducted by Farran et al14 which found biofilm-forming S. epidermidis in 91.4% of samples. In their study, they used PCR to screen for the presence of the intracellular adhesion (ica) operon in S. epidermidis isolates, which is correlated with the prevalence of biofilm formation. However, this was not assessed in our study.
Our study showed that the BF group of S. epidermidis was more frequently resistant to antibiotics compared to the NBF group. On the other hand, in C. acnes strains, the NBF group showed a tendency to be more resistant to antibiotics, although this difference was not statistically significant. A comparative study on antibiotic resistance and biofilm formation ability between C. acnes and S. epidermidis isolates, as used in this study, has not been previously assessed.
The topic remains controversial, as other authors have reached radically different conclusions using various approaches and different species of therapeutically relevant bacteria. A study by Donadu et al15 in Italy found that 51.7% of S. aureus and 62.8% of non-aureus staphylococcal strains were strong biofilm producers, but they found no variations in biofilm formation based on methicillin resistance. Similar findings were reported in a study by Gajdacs et al16 which found no significant connections between the rate of biofilm formation and antibiotic resistance in 302 isolates of Pseudomonas aeruginosa.
Cutibacterium acnes and S. epidermidis are known to be biofilm producers.1,5,17 Biofilms are aggregates of mono- or multispecies bacterial communities, consisting of diverse exopolysaccharides (EPS), environmental DNA, and other biomolecules such as lipids, proteins, and carbohydrates.4 Biofilms provide protection against shear forces, maintain an inflammatory environment in vivo, and promote the transformation of C. acnes and S. epidermidis into metabolically dormant persister cells.5,14
Cutibacterium acnes and S. epidermidis possess an extensive repertoire of virulence factors. AV is caused by C. acnes virulence factors including camp5, gehA, tly, sialidases, neuraminidase, and endoglycoceramidases. The lipoglycan-based cell envelope and the extracellular secreted lipase, particularly triacylglycerol lipase encoded by the gehA gene, aid in the adherence and colonization of the bacterium to the sebaceous follicle. Additionally, the gehA gene product contributes to acne formation by damaging host tissue.18 One of the key virulence factors produced by S. epidermidis is the fatty acid modifying enzyme, which converts bactericidal fatty acids in the skin into cholesterol. S. epidermidis also secretes the exopolysaccharide intercellular adhesin (PIA), responsible for adhesion and biofilm formation on the skin surface, providing protection against components of the human innate host defense. These biofilms create favorable anaerobic conditions necessary for the growth of C. acnes.14
Apart from their propensity to form biofilms, the ability of C. acnes and S. epidermidis to establish chronic infections and persist in vivo enhances their survival in adverse environmental conditions.14,18 In chronic infections, where C. acnes and S. epidermidis establish long-term persistence within biofilms, the expression of virulence factors is downregulated to accommodate the lower metabolic activity within the EPS. This can result in therapeutic failure and a decreased quality of life for affected patients.4 Furthermore, the chemical composition of biofilms inhibits the diffusion of antimicrobials, acting as a pharmacokinetic barrier to these drugs. Consequently, the minimum inhibitory concentrations (MICs) required to target biofilm-embedded bacteria may be 101−104 times higher than those needed for planktonic bacteria.14
The environment has a significant impact on biofilm formation, and researchers are intrigued by the mechanisms through which gene expression in individual cells influences biofilm formation. Environmental factors determine whether a cell forms a biofilm or not.18 Additionally, the structure of biofilms is highly dependent on the surrounding environment, indicating that biofilms adapt to local conditions. Second messengers, such as cAMP and c-di-GMP, play a crucial role in the interplay between environmental factors and gene regulation. Cell-to-cell communication, known as quorum sensing (QS), is a vital component in biofilm formation.19 QS is one of the primary mechanisms responsible for regulating the expression of virulence factors and biofilm formation.6,19 However, several antibiotics can also affect these QS systems in C. acnes and S. epidermidis by directly influencing gene expression or degrading the signal molecules involved.19
Based on our experimental data, we did not find any significant differences or notable relationship between biofilm formation and antibiotic resistance. This could be explained by the fact that microorganisms adapt their virulence factor expression only in survival-critical situations, or it may suggest that our in vitro methodologies were not sophisticated enough to detect their association. Additionally, while most laboratory biofilm studies are conducted under static conditions, natural conditions exhibit fluctuations.16
The emergence of C. acnes and S. epidermidis isolates with varying capacities to form biofilms may provide insights into the genetic heterogeneity within these species, which is a crucial factor in the effectiveness of AV infection.19 However, it is not yet fully understood how this heterogeneous gene expression contributes to the adaptability and flexibility of biofilms in different environments.16 Additionally, discrepancies in phenotypes and susceptibility patterns may also be attributed to the geographical origins of these isolates.6,19
Cutibacterium acnes phylogenetic groups exhibit distinct genetic and phenotypic characteristics.20 To date, six recognized phylotypes of C. acnes exist. Kuehnast et al17 conducted a study to compare the dynamics of biofilm formation, biofilm morphology, and adherence ability among these six phylotypes of C. acnes. The results revealed a correlation between biofilm formation and the phylotypes of C. acnes. The IA1 phylotype was found to have thicker biofilms, and IA1, IA2, and IC phylotypes exhibited higher adhesion ability to abiotic surfaces. The study concluded that the C. acnes phylotype determines the quality of biofilm formation. Rachmawati et al21 conducted a study that demonstrated a significant correlation between the expression of the icaA and icaD genes, encoding intracellular adhesion proteins, and S. epidermidis biofilm formation in vitro. However, our study did not assess the phylotype of C. acnes or the expression of the icaA and icaD genes in S. epidermidis, thus limiting our ability to evaluate their biofilm-forming capacity.
The emergence of C. acnes and S. epidermidis isolates with varying capacities to form biofilms may provide insights into the genetic heterogeneity within these species, which is a crucial factor in the effectiveness of AV infection.19 However, it is not yet fully understood how this heterogeneous gene expression contributes to the adaptability and flexibility of biofilms in different environments.16 Additionally, discrepancies in phenotypes and susceptibility patterns may also be attributed to the geographical origins of these isolates.6,19
Treatment with oral antibiotics should be avoided due to the high rates of antimicrobial resistance reported in AV worldwide.7 A study conducted by Platsidaki et al20 in the UK, Spain, Italy, Greece, Sweden, and Hungary reported that out of 664 patients, the prevalence of C. acnes resistance rates ranged from 50.8% to 93.6% to various antibiotics (tetracycline, macrolide, lincosamide, and streptogramin B). A previous study conducted in Bandung in 2019 revealed that C. acnes and S. epidermidis were the most commonly found bacteria in AV, and they exhibited high resistance rates towards clindamycin (62.5%), azithromycin (60.7%), and erythromycin (57.1%), respectively.9 These results are similar to our findings. Macrolide-resistant C. acnes is frequently isolated from AV patients, with the majority of resistant isolates having the 23S rRNA mutation.7,22 Clindamycin has become the most commonly used antibiotic for acne treatment.22 However, the uncontrolled use of clindamycin can lead to a high frequency of antimicrobial resistance among AV patients.7 The rate of tetracycline resistance was lower compared to clindamycin and erythromycin.20 Additionally, some antibiotics require specific conditions to work effectively. For example, clindamycin and erythromycin need to be in a basic environment (pH > 7) to be effective. In infected tissues, this condition may not always occur, resulting in a poor therapeutic outcome.23
Conclusion
There were no significant differences in antibiotic resistance against tetracycline, doxycycline, clindamycin, erythromycin, azithromycin, levofloxacin, and cotrimoxazole between the C. acnes and S. epidermidis in BF and NBF groups (p > 0.05). The use of clindamycin, erythromycin, and azithromycin should be judiciously regulated, while tetracycline, doxycycline, levofloxacin, and cotrimoxazole remain sensitive antibiotic treatments for AV.
Ethical Statement
This study complies with the Declaration of Helsinki and was performed according to the approval from the Research Ethics Committee of Universitas Padjadjaran (No. 164/UN6.KEP/EC/2021).
Consent Statement
The authors certify that they have obtained all appropriate patient consent forms.
Acknowledgments
The authors would like to thank all staff in the Dermatology and Venereology Department, Faculty of Medicine, Universitas Padjadjaran – Dr. Hasan Sadikin General Hospital, Bandung, West Java, Indonesia, and the Division of Microbiology, Department of Biomedical Sciences, Faculty of Medicine, Universitas Padjadjaran.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Coenye T, Peeters E, Nelis HJ. Biofilm formation by Propionibacterium acnes is associated with increased resistance to antimicrobial agents and increased production of putative virulence factors. Res Microbiol. 2007;158(4):386–392. doi:10.1016/j.resmic.2007.02.001
2. Zaenglein AL, Thiboutot DM. Acne Vulgaris. In: Bolognia JL, Schaffer JV, Cerroni L, editors. Dermatology. Elsevier; 2017:588–603.
3. Goh C, Cheng C, Agak G, et al. Acne Vulgaris. In: Kang S, Amagai M, Bruckner AL, et al. editors. Fitzpatrick’s Dermatology.
4. Brandwein M, Steinberg D, Meshner S. Microbial biofilms and the human skin microbiome. NPJ Biofilms and Microbiomes. 2016;2(1):1–6. doi:10.1038/s41522-016-0004-z
5. Burkhart CG, Burkhart CN. Expanding the microcomedone theory and acne therapeutics: Propionibacterium acnes biofilm produces biological glue that holds corneocytes together to form plug. Elsevier. 2007;722–724.
6. Vasudevan R. Biofilms: microbial cities of scientific significance. J Microbiol Exp. 2014;1(3):1–16.
7. Moon SH, Roh HS, Kim YH, Kim JE, Ko JY, Ro YS. Antibiotic resistance of microbial strains isolated from Korean acne patients. The Journal of Dermatology. 2012;39(10):833–837. doi:10.1111/j.1346-8138.2012.01626.x
8. O’Neill AM, Gallo RL. Host-microbiome interactions and recent progress into understanding the biology of acne vulgaris. Microbiome. 2018;6(1):1–16. doi:10.1186/s40168-018-0558-5
9. Ruchiatan K, Rahardja JI, Rezano A, Hindritiani R, Sutedja E, Gunawan H. A five-year clinical acne patients profiles and its management based on Indonesian acne expert guideline in Bandung, Indonesia. Journal of Pakistan Association of Dermatology. 2020;30(2):229–234.
10. Dréno B, Bettoli V, Ochsendorf F, Layton A, Mobacken H, Degreef H. European recommendations on the use of oral antibiotics for acne. Eur J Dermatol. 2004;14(6):391–399.
11. Hindritiani R, Soedarwoto A, Ruchiatan K, et al. Resistensi antibiotik Propionibacterium acnes dari berbagai lesi kulit akne vulgaris di rumah sakit dr. Hasan Sadikin Bandung. Media Dermato-Venereologica Indonesiana. 2017;38:15–19.
12. Jahns AC, Lundskog B, Ganceviciene R, et al. An increased incidence of Propionibacterium acnes biofilms in acne vulgaris: a case–control study. British Journal of Dermatology. 2012;167(1):50–58. doi:10.1111/j.1365-2133.2012.10897.x
13. Loss M, Thompson KG, Agostinho‐Hunt A, et al. Noninflammatory comedones have greater diversity in microbiome and are more prone to biofilm formation than inflammatory lesions of acne vulgaris. Int J Dermatol. 2021;60(5):589–596. doi:10.1111/ijd.15308
14. Farran CE, Sekar A, Balakrishnan A, Shanmugam S, Arumugam P, Gopalswamy LJ. Prevalence of biofilm-producing Staphylococcus epidermidis in the healthy skin of individuals in Tamil Nadu, India. Indian J Med Microbiol. 2013;31(1):19–23. doi:10.4103/0255-0857.108712
15. Donadu MG, Ferrari M, Mazzarello V, et al. No correlation between biofilm-forming capacity and antibiotic resistance in environmental Staphylococcus spp.: in vitro results. Pathogens. 2022;11(4):471. doi:10.3390/pathogens11040471
16. Gajdács M, Baráth Z, Kárpáti K, et al. No correlation between biofilm formation, virulence factors, and antibiotic resistance in Pseudomonas aeruginosa: results from a laboratory-based in vitro study. Antibiotics. 2021;10(9):1134. doi:10.3390/antibiotics10091134
17. Kuehnast T, Cakar F, Weinhäupl T, et al. Comparative analyses of biofilm formation among different Cutibacterium acnes isolates. Int J Med Microbiol. 2018;308(8):1027–1035. doi:10.1016/j.ijmm.2018.09.005
18. Kumar B, Pathak R, Mary PB, Jha D, Sardana K, Gautam HK. New insights into acne pathogenesis: exploring the role of acne-associated microbial populations. Dermatologica sinica. 2016;34(2):67–73. doi:10.1016/j.dsi.2015.12.004
19. Toyofuku M, Inaba T, Kiyokawa T, Obana N, Yawata Y, Nomura N. Environmental factors that shape biofilm formation. Bioscience, Biotechnology, and Biochemistry. 2016;80(1):7–12. doi:10.1080/09168451.2015.1058701
20. Platsidaki E, Dessinioti C. Recent advances in understanding Propionibacterium acnes (Cutibacterium acnes) in acne. F1000Research. 2018;7:1953. doi:10.12688/f1000research.15659.1
21. Rachmawati D, Alimsardjono L. The correlation between icaA and icaD genes with biofilm formation Staphylococcus epidermidis in vitro. Folia Medica Indonesiana. 2019;55(4):251–259. doi:10.20473/fmi.v55i4.24388
22. Nakase K, Nakaminami H, Takenaka Y, Hayashi N, Kawashima M, Noguchi N. Relationship between the severity of acne vulgaris and antimicrobial resistance of bacteria isolated from acne lesions in a hospital in Japan. J Med Microbiol. 2014;63(5):721–728. doi:10.1099/jmm.0.067611-0
23. Yadav AK, Bhooshan S, Johnson A, Asati DP, Nema S, Biswas D. Association of Antimicrobial Susceptibility and Treatment Outcome in Acne Vulgaris Patients: a Pilot Study. Journal of Laboratory Physicians. 2020;12(04):233–238. doi:10.1055/s-0040-1720943
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
