Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 14
Characteristics and Treatment Response of Patients with HIV Associated Kaposi’s Sarcoma in Central Kenya
Authors McLigeyo A, Owuor K, Ng'ang'a E, Mwangi J, Wekesa P
Received 20 January 2022
Accepted for publication 1 April 2022
Published 25 April 2022 Volume 2022:14 Pages 207—215
DOI https://doi.org/10.2147/HIV.S359278
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Olubunmi Akindele Ogunrin
Angela McLigeyo,1 Kevin Owuor,1 Evelyne Ng’ang’a,1 Jonathan Mwangi,2 Paul Wekesa1
1Center for Health Solutions - Kenya, Nairobi, Kenya; 2Division of Global HIV & TB, Centers for Disease Control and Prevention (CDC), Nairobi, Kenya
Correspondence: Paul Wekesa, Centre for Health Solutions – Kenya, CVS Plaza, 4th Floor, Kasuku Road, off Lenana Road, P.O. Box 23248-00100 GPO, Nairobi, Kenya, Tel +254 20 271 0077, Email [email protected]
Introduction: Kaposi’s sarcoma (KS) is the most common HIV-associated malignancy in Sub Saharan Africa. In 2018, it was the 7th most common cancer and the 10th most common cause of cancer death in Kenya. This study aimed to describe the baseline and clinical characteristics and treatment response observed following combined antiretroviral treatment (ART) and chemotherapy in KS patients.
Methods: This was a descriptive analysis of patients aged ≥ 15 years treated for KS and HIV at 11 treatment hubs in Central Kenya between 2011 and 2014. Data on baseline and clinical characteristics, ART and chemotherapy regimens as well as treatment responses were collected from patient files and KS registers.
Results: A total of 95 patients presenting with clinically suspected KS with no history of prior treatment with chemotherapy were reviewed. All had histological diagnostic samples taken with 67 (71%) having confirmed KS. All were on ART, either newly initiated or continuing on ART, and 63 of the 67 (94.0%) confirmed to have KS received chemotherapy. Among the 67 patients with confirmed KS, mean age was 37.2 years (± 13.2) and 40 (59.7%) were male. More than 80% had normal baseline and follow-up BMI, and 34 (50.7%) were on a TDF-based regimen, 52 (77.6%) were treated with the Adriamycin, bleomycin and vinblastine protocol, and 55 (82.1%) had KS diagnosis before HIV diagnosis. All 67 patients had mucocutaneous lesions. Complete, partial response and stable disease occurred in 27 (40.3%), 10 (14.9%) and 7 (10.4%), respectively, 11 (16.4%) defaulted care during treatment, six patients died during treatment, four patients died before treatment while two patients had progressive disease during chemotherapy.
Conclusion: The diagnosis of KS preceded HIV in the majority of cases reviewed, with histology helpful to reduce misdiagnosis. Patients generally complied with their chemotherapy, with overall good response rate for this intervention implemented at primary health-care facilities.
Keywords: HIV, Kaposi’s sarcoma, characteristics, treatment, response, adults
Introduction
Kaposi’s sarcoma (KS) is a multi-centric angio-proliferative malignancy. On the basis of clinical and epidemiological features, four types of KS have been recognized: classic, endemic (African), iatrogenic and epidemic (AIDS related). Clinical presentation may vary within the sub-types, but histology is similar. KS has a predilection for the skin and mucous membranes where it may present with papules, macules, nodules, ulcers, plagues, and fungating lesions. Epidemic KS is the most common opportunistic malignancy associated with HIV infection.1 All types of KS are associated with Human Herpes Virus-8 (HHV-8) infection.2,3 According to Globocan 2018, globally there were 41,799 incident KS cases and 19,902 deaths due to KS in 2018.4 In Kenya, KS ranked as the 7th most common cancer, with an estimated 1,782 new cases, and was the 10th most common cause of death in 2018.4
Epidemic KS tends to affect young patients with a median age of 34 years.5,6 Epidemic KS has also been reported in African children with prevalence rates as high as 36.0% in Uganda and 9.0% in South Africa.7,8 Clinical evaluation is key to making the initial diagnosis of KS. A study in Nigeria reported that KS lesions commonly developed on the lower limbs (46.0%), conjunctiva (12.0%) and trunk (10.0%).9 A South African study reported that almost all patients had skin disease, either localized or disseminated.10
The malignancy has traditionally been associated with severe immunosuppression in patients who are antiretroviral treatment (ART) naive. Patients who develop KS during ART typically have low CD4 cell counts and the KS develops within 6 months of ART initiation.11 A study in South Africa reported a median CD4 count of 82 cells/uL at KS diagnosis.5 Combination ART alone is quite effective for mild KS and has been associated with KS lesion regression in size of KS lesions and significant improvement in patient survival.12 The initial type of ART does not affect treatment response and no specific class of ART is preferred.13 There is no mortality or treatment response benefit to using ART plus chemotherapy versus using ART alone in mild KS.14
Prior to 2019, patients with KS were treated with single agent vincristine, single agent gemcitabine, or combination therapy using doxorubicin, bleomycin and vincristine, either as doublet or triplet therapy in Kenya.15 A study conducted among 70 patients in Western Kenya, in 2014, looking at 3 year survival outcomes after gemcitabine or bleomycin and vincristine therapy reported survival of 85.7%.16 In 2019, the Kenya National Cancer Treatment Protocols directed that patients with HIV and KS be initiated on anti-retroviral therapy as well as chemotherapy using pegylated liposomal doxorubicin as first line and paclitaxel as second line.17 A clinical trial by Krown et al in 2020, which compared progression-free survival (PFS) at 48 weeks among patients on bleomycin and vincristine, or oral etoposide vs paclitaxel found PFS to be much higher with the paclitaxel than with the other agents.18
The World Health Organization (WHO) has described the clinical response of HIV-associated KS following chemotherapy, as either complete response (CR), partial response (PR), progressive disease, or stable disease.19 A study conducted in Zambia among 38 patients reported CR rates of 47.0% and PR rates of 53.0% after 6 cycles of adriamycin, bleomycin and vinblastine (ABV).20
Researchers from Conakry - Guinea reported that among 225 patients with KS followed up for a three-year period and receiving a median of 8 cycles of treatment with chemotherapy, CR was achieved in 28.9%, PR in 23.6%, stable disease in 6.7%, and unknown response in 40.9% patients who dropped out of care.21 In another study conducted in Chile among 17 patients with KS treated with ART and chemotherapy, seven achieved CR, five achieved a PR, two remained stable and three died.22
Complications that may be fatal in patients co-infected with HIV and KS include KS-Immune Reconstitution Inflammatory Syndrome (K-IRIS), which develops following immune recovery resulting in rapid clinical deterioration, and KS Inflammatory Cytokine Syndrome (KICS) which presents with lymphadenopathy, pancytopenia, KSHV viremia and signs of systemic inflammatory syndrome.23
We aimed to determine the baseline and clinical characteristics of HIV-associated KS and clinical response to chemotherapy among patients with both KS and HIV infection in Kenya.
Methods
Study Setting
Primary health-care facilities in Kenya lack the capacity to diagnose and treat KS, a stage IV opportunistic infection among people living with HIV (PLHIV). Prior to 2011, it took approximately two to six weeks for a histological diagnosis to be availed to patients. Clinicians relied predominantly on a clinical diagnosis of KS because of the prolonged turn-around-time and high cost associated with histological diagnosis. The disease was managed using ART consisting of Tenofovir/Lamivudine/Efavirenz24 and vincristine monotherapy as per the Kenya guideline for the management of HIV related opportunistic infections and conditions at that time.15 Centre for Health Solutions – Kenya (CHS), a local indigenous non-governmental organization, through the Tegemeza Project, initiated a program to streamline the process of KS diagnosis and treatment in 2011. This project was funded by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for disease control and prevention (CDC) in Kenya. Details of the project setting including outcomes have been published elsewhere.25–27
The study was conducted at 11 health facilities, considered treatment hubs, in Central Kenya, and included Engineer, Jomo Kenyatta University of Science and Technology, Juja Farm, Karatina, Kiandutu, Muranga, Nyahururu, Nyeri, Olkalou, Ruiru and Thika hospitals. The primary health facilities acted as spokes. The physician assistants and nurses at the spokes were trained to recognize mucocutaneous lesions that were suggestive of KS and refer to the hubs. Once at the hub, trained physician assistants conducted punch biopsies which were preserved in formalin. Samples were transported to a reference laboratory in Nairobi for review and reporting by a pathologist. Results were availed to the hubs via email, after which patients were alerted to return and initiate chemotherapy, which was also administered by trained physician assistants. Clinical monitoring was conducted during each chemotherapy session until completion of all cycles. Response was documented four weeks after completion of six cycles of chemotherapy.
Study Design
This was a retrospective cohort analysis of patients managed for HIV-associated KS in 11 health facilities in Central Kenya between 2011 and 2014.
Study Population
This study enrolled all patients aged 15 years and above that were diagnosed with HIV-associated KS during study period. Patients with a prior history of treatment with chemotherapy for KS, pregnant women and patients with no histological confirmation of KS were excluded.
Variables
Baseline participant characteristics included age, sex, marital status. Clinical characteristics included baseline body mass index (BMI), calculated from height and weight. Patients were categorized using BMI as underweight (<18.5), normal weight (18.5–25.0), and overweight or obese (>25.0). Baseline CD4 count was categorized as <200, 201–500, >500 cells/uL, and median CD4 count, date of KS diagnosis and date of HIV diagnosis were used to calculate the time interval between KS and HIV diagnosis. ART regimen was categorized based on the NRTI backbone. All patients were on ART with some having initiated prior to developing KS and others initiating ART after KS diagnosis. Viral loads (VL) were also collected with viral suppression classified as VL <1000 copies/mL and VL values ≥1000 as not suppressed based on the laboratory diagnostic capacity at the time.
The type of chemotherapy was categorized as either ABV or bleomycin and vinblastine (BV) protocols. Follow-up CD4 counts were collected until the year 2014 when the country ceased to monitor CD4 counts. Follow up BMI until 2018 or at time of event was monitored.
Data Management and Statistical Analysis
Data on sociodemographic, clinical and laboratory characteristics, as well as data on treatment response were extracted from patient files. Anonymity and confidentiality of patient data were ensured by use of a de-identified dataset. Data entry was done in a Microsoft Excel worksheet, and imported into Stata version 15.1 (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC.) for data management and descriptive analysis. Continuous data such as age and CD4 were presented using means [standard deviations (SD)] or medians [interquartile range (IQR)] as appropriate. Counts and corresponding percentages were used for categorical variables such as sex and marital status of participants.
Ethical Considerations
Ethical approval was obtained before the start of the study. Local ethical approval was obtained from the Kenyatta National Hospital and University of Nairobi institutional review board (P339/06/2013). The protocol was also reviewed by the Associate Director of Science, Center for Global Health at the US Centers for Disease Control and Prevention (CDC) in line with human research protection procedures and was determined to be research, but CDC investigators did not interact with human subjects or have access to identifiable data for research purposes. Anonymity and confidentiality of data was strictly maintained. All the study procedures were done in conformity with Kenya Government, Ministry of Health, CDC, local IRB regulations and the Declaration of Helsinki.
Results
A total of 95 patients with clinically suspected KS were included. All had histologic diagnosis, and 67 had confirmed KS using histology. All received ART, and 63 patients received chemotherapy with either the dual BV protocol or the ABV protocol. Four patients died before initiating chemotherapy. Twenty eight (30%) patients who would have been treated clinically for KS were confirmed to have an alternative diagnosis after confirmatory histology. Subsequent analysis included only the 67 patients with confirmed KS on histology.
Descriptive Analysis
Clinical Characteristics of Participants
The mean age at KS diagnosis was 35 years. Over half of the patients were male 40 (59.7%). The majority of the patients were married, 42 (63.6%). Median baseline CD4 count was 108 cells/uL (IQR 35–256). The majority of patients, 46 (68.7%) had severe immunosuppression at diagnosis, with a baseline CD4 cell counts of less than 200 cell/uL. None of the patients had a CD4 cell count above 500 cells/uL. Follow up CD4 cell counts showed improvement, with 41 (67.2%) of the 61 patients having a CD4 cell count above 200 cells/uL, with a median of 280 cells/uL (IQR 175–421) (Table 1). Most of the study participants 56 (83.5%), had a baseline normal BMI, with only 3 (4.4%) of the participants diagnosed with wasting. The BMI during follow-up in 2018 or at the time of event improved, with 60 (93.8%) of the 64 patients with a BMI result reported as normal (18–25). Of the patients with documented VL results in 2018, more than half of the study participants, 37 (55.2%) showed VL suppression with and 11.9% VL not virally suppressed. Nine of the participants did not have a viral load result and therefore their viral suppression status could not be ascertained. More than half of the patients 34 (50.7%) were on TDF-based regimens, followed by 19 (28.4%) on D4T-based regimes, and 12 (17.9%) on AZT-based regimens. The majority of study participants 55 (82.1%) presented with KS as the initial AIDS defining illness. Overall, the median number of months on KS treatment was 44.5 months (IQR 6.5–60.5). Seventy seven percent of the patients with KS received systemic chemotherapy with the ABV protocol and 3 (6.0%) died prior to the administration of chemotherapy (Table 1).
 |
Table 1 Characteristics of Patients with HIV Associated Kaposi’s Sarcoma in Central Kenya |
Treatment Response and Outcomes
Complete response (CR) was observed in 27 (40.3%) of the study participants during the follow-up period, 10 (14.9%) had a partial response (PR), 7 (10.0%) had stable disease. Eleven patients (16.4%) defaulted care and did not complete the full 6 cycles of chemotherapy. Six patients died during treatment (9.0%), four patients died before treatment while two patients progressed despite treatment with systemic chemotherapy. Overall, the number of patients with complete or partial response was 37 (55.2%) among the 67 study participants (Table 2).
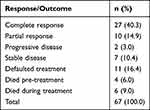 |
Table 2 Study Participants Treatment Response and Outcomes |
Discussion
The project offered comprehensive KS and HIV treatment services to 67 patients with HIV-associated KS at county health facilities. The implementation model was dependent on the existing health systems established for the HIV service delivery. The project further supported the facilities to improve histological diagnosis of KS, with quick turnaround time and improved diagnostic accuracy. Combination chemotherapy that was previously unavailable was also provided to the KS treatment sites.
Males constituted 59% of the patients. The male to female ratio was 3:2. This is similar to data from other studies. Studies from Malawi and Botswana reported that males constituted 58% of the 545 participants that were diagnosed with HIV infection and KS.28,29 Another study conducted in Botswana among 207 patients with HIV-associated KS reported that 63% of the participants were male.30 In contrast, HIV-associated KS in a South African study had an equal female-to-male ratio with females being younger and having more severe disease.31 Males may be predominantly affected by Kaposi’s sarcoma because of a higher HHV-8 sero-prevalence.32–34
The predominance of KS observed in younger age groups may be attributed to the co–transmission of HHV-8 with HIV virus among sexually active young people.35
The mean age of our study participants was 37 years. A study conducted at two hospitals in Paris among 138 patients with HIV-associated KS reported a median age of 43 years.12 In a Kenyan study, the age-group with the largest number of KS patients was between 31 and 40 years36 while in Malawi, the median age was 33 years.28
The marital status of our study population is reflective of that reported by the Kenya demographic health survey on marital status.37 Studies conducted in the country have reported high incidence rates of HIV among married heterosexual couples.44 Eighty three percent (83%) had normal baseline BMI defined as 18.5 to 25. Eight patients were classified as overweight. This is in contrast to case reports from early years in the HIV pandemic that documented low BMI in patients with KS in the setting of HIV infection.38 A normal BMI reflects the revolution in HIV care that has in turn resulted in improvements in nutritional care. A study conducted in Nigeria reported similar BMI in HIV-infected patients with KS and without KS.39
The median baseline CD4 count has been reported to be low in KS patients. In our study, the median baseline CD4 count was 108 cells/uL and 68% of the patients had a baseline CD4 cell count below 200 cells/uL. Studies conducted in Malawi, Botswana, and South Africa reported median CD4 cell counts of 180, 190, and 74 cells/uL, respectively.28,30,40 In contrast, certain case reports have documented severe and disseminated HIV-associated KS in patients with high CD4 cell counts.41 A normal immune system can keep the HHV-8 infection controlled hence suppressing the development of KS lesions42 whereas a weakened immune system predisposes to KS.
In this study, 82% of the patients presented with KS as their initial presenting illness. Similar to our study, Botswana reported that 68% of their patients were not on ART at KS diagnosis.30 In contrast, a study conducted in the USA reported that KS developed within 6 months of initiating ART.43 KS may be the initial presenting illness due to late diagnosis of HIV (which may be caused by many factors such as care seeking, stigma, etc). Low baseline CD4 counts is an observation of late HIV diagnosis/presentation.
Combination ART without chemotherapy has been reported to result in the resolution of early stage KS lesions regardless of the class of ART used.44,45 ART is also associated with a prolonged time to KS treatment failure as seen in a study that followed KS patients for 12 months after ART initiation.46 All patients in this study were on ART.
The systemic chemotherapy administered to our patients consisted of ABV or BV protocols in 77% and 16%, respectively. CR was 40%, PR 14.9%, SD 10%, and PD 3%. Similarly, a prospective study of 50 patients with HIV-associated KS on vincristine monotherapy and ART reported an overall response rate of 64% at 6 weeks of follow-up.47 A study from Malawi reported that patients who received vincristine monotherapy had a treatment response of 29% compared to 53% for patients on bleomycin/vincristine.48 Likewise, a study conducted in Zambia among 38 patients reported a complete response rate of 47% and partial response rates of 53% after 6 cycles of adriamycin, bleomycin and vinblastine (ABV).20 In contrast, researchers from Conakry - Guinea reported inferior treatment responses compared to our study. Among 225 patients with KS followed over a three-year period who received a median of 8 cycles of treatment with chemotherapy, CR was achieved in 28.9%, PR in 23.6%, SD in 6.7% and unknown response in 40.9% patients who dropped out of care.21
The overall response rate in our study was 55%. The overall response rate reported in our study could be due to the combination of chemotherapy and ART used in our patients. ART results in the improvement of CD4 counts and a reduction in viral load which in turn slows KS progression. Similar data have been reported in a Cochrane systematic review.49 Bihl et al likewise, in a prospectively randomized trial comparing ART alone to ART plus combination chemotherapy with “ABV” regimen every 2 weeks reported a trend toward improved KS treatment response for patients treated with ART plus chemotherapy compared to ART alone.50
Limitations
We were unable to present data on stage of KS at diagnosis due to missing variables. Further, we did not collect nor analyze data on date of ART initiation, ART status and its impact on KS outcomes. This may have affected our results as all these can confound the response to chemotherapy. The sample size is not robust and may affect the generalizability of the results. Another limitation was the use of retrospective data with a few missing data points, which was determined to not significantly influence the results.
Conclusion
The study aimed to determine the characteristics, diagnosis, treatment and response of HIV-associated KS among patients with both KS and HIV infection at hospitals in Central Kenya.
Overall treatment response rate was 55%. Majority of the patients had KS diagnosed prior to HIV diagnosis. Access to histological diagnosis was beneficial for ensuring accurate and timely diagnosis of KS. Patients generally complied with their chemotherapy protocol. Health systems can be supported to diagnose and treat common HIV comorbidities such as HIV – associated KS. Further, safer and more efficacious regimens can improve outcomes of KS patients in the country.
Disclaimer
The findings and conclusions in this study are those of the authors and do not necessarily represent the official position of the funding agencies.
Data Sharing Statement
The dataset used for this analysis is a dataset of individual level routine HIV care and treatment data and is not currently publically available as it is property of Ministry of Health and Government of Kenya. However, the deidentified dataset can be obtained from the corresponding author based on a reasonable request.
Acknowledgments
We acknowledge the help accorded to us in the routine data collection and management by all health-care workers and their leadership working for CDC funded CHS Tegemeza project and Kenya ministry of Health in Central Kenya. We also thank HIV positive clients in Central Kenya we served without whose data this manuscript would not have been written.
Funding
This study was supported by the US President’s Emergency Plan for AIDS Relief (PEPFAR) through the US Centers for Disease Control and Prevention (CDC) under the terms of cooperative agreement NU2GGH002024-01-00.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. La Ferla L, Pinzone MR, Pellicanò GF. Kaposi’s sarcoma in HIV-infected patients: a review of the literature. Infect Dis Trop Med. 2016;2(1):e239.
2. Chang Y, Cesarman E, Pessin MS. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865–1869. doi:10.1126/science.7997879
3. Whitby D, Howard MR, Tenant Flowers M, et al. Detection of Kaposi’s sarcoma associated herpesvirus in peripheral blood of HIV infected individuals and progression to Kaposi’s sarcoma. Lancet. 1995;346:799–802. doi:10.1016/S0140-6736(95)91619-9
4. Freddie B, Jacques F, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi:10.3322/caac.21492
5. Chu K, Mahlangeni G, Swannet S. AIDS-associated Kaposi’s sarcoma is linked to advanced disease and high mortality in a primary care HIV programme in South Africa. J Int AIDS Soc. 2010;13:13–23. doi:10.1186/1758-2652-13-13
6. Bohlius J, Valeri F, Maskew M, Prozesky H. Kaposi’s sarcoma in HIV-infected patients in South Africa: a multi-cohort study in the antiretroviral therapy era. Int J Cancer. 2014;135(11):2644–2652. doi:10.1002/ijc.28894
7. Mayama S, Cuevas LE, Sheldon J, et al. Prevalence and transmission of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) in Ugandan children and adolescents. Int J Cancer. 1998;77:817–820. doi:10.1002/(SICI)1097-0215(19980911)77:6<817::AID-IJC2>3.0.CO;2-X
8. Tukeia VJ, Kekitiinwa A, Beasley RP. Prevalence and outcome of HIV-associated malignancies among children. AIDS. 2011;25:1789–1793. doi:10.1097/QAD.0b013e3283498115
9. Kagu MB, Nggada HA, Garandawa HI, Askira BH, Durosinmi MA. AIDS-associated Kaposi’s sarcoma in Northeastern Nigeria. Singapore Med J. 2006;47(12):1069–1074.
10. Stein ME, Spencer D, Kantor A, Ruff P, Haim N, Bezwoda WR. Epidemic AIDS-related Kaposi’s sarcoma in Southern Africa: experience at the Johannesburg General Hospital. Trans R Soc Trop Med Hyg. 1994;88(4):434–436. doi:10.1016/0035-9203(94)90419-7
11. Mocroft A, Kirk O, Clumeck N. The changing pattern of Kaposi sarcoma in patients with HIV, 1994–2003: the EuroSIDA study. Cancer. 2004;100:2644–2654. doi:10.1002/cncr.20309
12. Martinez V, Caumes E, Gambotti L, Ittah H. Remission from Kaposi’s sarcoma on HAART is associated with suppression of HIV replication and is independent of protease inhibitor therapy. Br J Cancer. 2006;94(7):1000–1006. doi:10.1038/sj.bjc.6603056
13. Martin J, Laker-Oketta M, Walusana V, Orem J, Wabinga H. Antiretrovirals for Kaposi’s sarcoma (ARKS): a randomized trial of protease inhibitor-based antiretroviral therapy for AIDS-associated Kaposi’s sarcoma in sub-Saharan Africa.
14. Mosam A, Shaik F, Uldrick TS, et al. A randomized controlled trial of highly active antiretroviral therapy versus highly active antiretroviral therapy and chemotherapy in therapy-naive patients with HIV-associated Kaposi sarcoma in South Africa. J Acquir Immune Defic Syndr. 2012;60(2):150–157. doi:10.1097/QAI.0b013e318251aedd
15. Ministry of Health. National guidelines for cancer management Kenya. 2013.
16. Busakhala N, Kigen G, Waako P, et al. Three year survival among patients with aids-related Kaposi sarcoma treated with chemotherapy and combination antiretroviral therapy at Moi teaching and referral hospital, Kenya. Infect Agents Cancer. 2019;14:24. doi:10.1186/s13027-019-0242-9
17. Kenya national cancer treatment protocols. Available from: https://www.health.go.ke/wp-content/uploads/2019/09/National-treatment-Protocols-2019.pdf.
18. Krown SE, Moser CB, MacPhail P, et al.; A5263/AMC066 protocol team. Treatment of advanced AIDS-associated Kaposi sarcoma in resource-limited settings: a three-arm, open-label, randomised, non-inferiority trial. Lancet. 2020;395(10231):1195–1207. doi:10.1016/S0140-6736(19)33222-2
19. Ollivier L, Padhani AR, Leclère J. International criteria for measurement of tumour response. Cancer Imaging. 2001;2(1):31–32.
20. Mtonga W, Mujajati A, Munkombwe D. A therapeutic outcomes in AIDS-associated Kaposi’s sarcoma patients on antiretroviral therapy treated with chemotherapy at two tertiary hospitals in Lusaka, Zambia. Curr HIV Res. 2018;16(3):231–236. doi:10.2174/1570162X16666180711103610
21. Bekolo CE, Soumah MM, Tiemtore OW, Diallo A. Assessing the outcomes of HIV-infected persons receiving treatment for Kaposi sarcoma in Conakry-Guinea. BMC Cancer. 2017;17(1):806. doi:10.1186/s12885-017-3771-x
22. Lasso M, Pérez J, Noriega L, Malebrán A, Espinoza S. Kaposi sarcoma in HIV patients. Response to antiretroviral treatment and chemotherapy. Rev Med Chil. 2003;131(5):483–490.
23. Dumic I, Radovanovic M, Igandan O, et al. Case of Kaposi Sarcoma Immune Reconstitution Syndrome (KS-IRIS) complicated by Kaposi Sarcoma Inflammatory Cytokine Syndrome (KICS) or Multicentric Castleman Disease (MCD): a case report and review. Am J Case Rep. 2020;21:e926433. doi:10.12659/AJCR.926433
24. National AIDS/STI Control Program (NASCOP). Guidelines for Antiretroviral Therapy in Kenya.
25. Wekesa P, McLigeyo A, Owuor K, Mwangi J, Isavwa L, Katana A. Temporal trends in pre-ART patient characteristics and outcomes before the test and treat era in Central Kenya. BMC Infect Dis. 2021;21(1):1. doi:10.1186/s12879-021-06706-3
26. Wekesa P, McLigeyo A, Owuor K, Mwangi J, Nganga E, Masamaro K. Factors associated with 36-month loss to follow-up and mortality outcomes among HIV-infected adults on antiretroviral therapy in Central Kenya. BMC Public Health. 2020;20(1):1. doi:10.1186/s12889-020-8426-1
27. McLigeyo A, Wekesa P, Owuor K, Mwangi J, Isavwa L, Mutysia I. Factors associated with treatment outcomes among children and adolescents living with HIIV receiving antiretroviral therapy in central Kenya. AIDS Res Hum Retroviruses. 2022. doi:10.1089/aid.2021.0112
28. Mwinjiwa. E, Isaakidis P, Van den Bergh R, et al. Burden, characteristics, management and outcomes of HIV-infected patients with Kaposi’s sarcoma in Zomba, Malawi. Public Health Action. 2013;3(2):180–185. doi:10.5588/pha.13.0003
29. Christa Williams S, Grover V, Bigger S, et al. A retrospective review of patients with Kaposi’s sarcoma in Botswana. Int J Dermatol. 2019;doi:10.1111/ijd.14305
30. Elmore N, Bigger S, Kayembe E, et al. Demographic characteristics and preliminary outcomes in a cohort of HIV-positive patients with Kaposi’s sarcoma in a high ART coverage setting: a report from Botswana. J Glob Oncol. 2016. doi:10.1200/JGO.2016.004218
31. Mosam A, Hurkchand HP, Cassol E, et al. Characteristics of HIV-1-associated Kaposi’s sarcoma among women and men in South Africa. Int J STD AIDS. 2008;19:400–405. doi:10.1258/ijsa.2008.007301
32. Koski L, Ngoma T, Mwaiselage J, Le L, Soliman AS. Changes in the pattern of Kaposi’s sarcoma at Ocean Road Cancer Institute in Tanzania (2006–2011). Int J STD AIDS. 2015;26(7):470–478. doi:10.1177/0956462414544724
33. Begré L, Rohner E, Mbulaiteye ME, Bohlius J. Is human herpesvirus 8 infection more common in men than in women? Systematic review and meta-analysis. Int J Cancer. 2016;139(4):776–783. doi:10.1002/ijc.30129
34. Klein SL. The effects of hormones on sex differences in infection: from genes to behavior. Neurosci Biobehav Rev. 2000;24:627–638. doi:10.1016/S0149-7634(00)00027-0
35. Mwanda OW, Fu P, Collea R, Whalen C, Remick SC. Kaposi’s sarcoma in patients with and without human immunodeficiency virus infection, in a tertiary referral centre in Kenya. Ann Trop Med Parasitol. 2005;99(1):81–91. doi:10.1179/136485905X19928
36. Melbye M, Cook PM, Hjalgrim H, et al. Risk factors for Kaposi’s sarcoma-associated herpesvirus seropositivity in a cohort of homosexual men, 1981–1996. Int J Cancer. 1998;77:543–548. doi:10.1002/(SICI)1097-0215(19980812)77:4<543::AID-IJC12>3.0.CO;2-7
37. Kenya National Bureau of Statistics, Ministry of Health. Kenya demographic and health survey. 2014.
38. Moloi MW, Zhou F, Baliki K, Kayembe MK, Cainelli F, Vento S. Atypical presentation of Kaposi’s sarcoma in an HIV-infected patient. Isr Med Assoc J. 2013;15:459–460.
39. Agaba PA, Sule HM, Ojoh RO, et al. Presentation and survival of patients with AIDS-related Kaposi’s sarcoma in Jos, Nigeria. Int J STD AIDS. 2009;20(6):410–413. doi:10.1258/ijsa.2008.008353
40. Maskew M, Fox MP, van Cutsem G, et al. Treatment response and mortality among patients starting antiretroviral therapy with and without Kaposi Sarcoma: a Cohort study. PLoS One. 2013;8(6):e64392. doi:10.1371/journal.pone.0064392
41. Tang ASO, Teh YC, Chea CY, Yeo ST, Chua HH. Disseminated AIDS-related Kaposi’s sarcoma. Oxf Med Case Rep. 2018;2018(12). doi:10.1093/omcr/omy107
42. Mocroft A, Furrer HJ, Miro JM, Reiss P, Mussini O. The incidence of AIDS-defining illnesses at a current CD4 count ≥200 cells/µL in the post–combination antiretroviral therapy era. Clin Infect Dis. 2013;57(7):1038–1047. doi:10.1093/cid/cit423
43. Yanik EL, Napravnik S, Cole SR, Achenbach CJ, Gopal S. Incidence and timing of cancer in HIV-infected individuals following initiation of combination antiretroviral therapy. Clin Infect Dis. 2013;57(5):756–764. doi:10.1093/cid/cit369
44. Gill J, Bourboulia D, Wilkinson J, et al. Prospective study of the effects of antiretroviral therapy on Kaposi sarcoma–associated herpesvirus infection in patients with and without Kaposi sarcoma. J Acquir Immune Defic Syndr. 2002;31(4):384–390. doi:10.1097/00126334-200212010-00003
45. Asiimwe F, Moore D, Were W, et al. Clinical outcomes of HIV-infected patients with Kaposi’s sarcoma receiving nonnucleoside reverse transcriptase inhibitor-based antiretroviral therapy in Uganda. HIV Med. 2012;13(3):166–171. doi:10.1111/j.1468-1293.2011.00955.x
46. Bower M, Fox P, Fife K, Gill J, Nelson M, Gazzard B. Highly active anti-retroviral therapy (HAART) prolongs time to treatment failure in Kaposi’s sarcoma. AIDS. 1999;13(15):2105–2111. doi:10.1097/00002030-199910220-00014
47. Francis H, Bates MJ, Kalilani L. Prospective study assessing tumor response, survival, and palliative care outcomes in patients with HIV-related Kaposi’s sarcoma at Queen Elizabeth Central Hospital, Blantyre, Malawi. AIDS Res Treat. 2012;2012:312–564.
48. Mwafongo AA, Rosenberg NE, Ng’ambi W, et al. Treatment outcomes of AIDS-associated Kaposi’s sarcoma under a routine antiretroviral therapy program in Lilongwe, Malawi: bleomycin/vincristine compared to vincristine monotherapy. PLoS One. 2014;9(3):e91020. doi:10.1371/journal.pone.0091020
49. Gbabe OF, Okwundu CI, Dedicoat M, Freeman EE, Harris DH, Hessol NA. Treatment of severe or progressive Kaposi’s sarcoma in HIV-infected adults. AIDS. 2011;25(4):463–471. doi:10.1097/QAD.0b013e32834344e6
50. Bihl F, Mosam A, Henry LN, et al. Kaposi’s sarcoma-associated herpesvirus-specific immune reconstitution and antiviral effect of combined HAART/chemotherapy in HIV clade C-infected individuals with Kaposi’s sarcoma. AIDS. 2007;21:1245–1252. doi:10.1097/QAD.0b013e328182df03
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
