Back to Journals » Clinical Ophthalmology » Volume 17
Characteristics and Treatment Patterns of Patients with Diabetic Macular Edema Non-Responsive to Anti-Vascular Endothelial Growth Factor Treatment in Ontario, Canada
Authors Somani S , Koushan K, Shah-Manek B, Mercer D, Kanagenthiran T, Zhao C, Alobaidi A
Received 6 December 2022
Accepted for publication 17 May 2023
Published 17 July 2023 Volume 2023:17 Pages 2013—2025
DOI https://doi.org/10.2147/OPTH.S399981
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Scott Fraser
Sohel Somani,1 Keyvan Koushan,2 Bijal Shah-Manek,3 Daniel Mercer,4 Thula Kanagenthiran,5 Changgeng Zhao,5 Ali Alobaidi5
1Department of Ophthalmology and Vision Sciences, University of Toronto and Uptown Eye Specialists, Brampton, Canada; 2Toronto Retina Institute, Toronto, Canada; 3Health Economics and Outcomes Research, Noesis Healthcare Technologies, Inc., Redwood City, CA, USA; 4Genesis Research, Hoboken, NJ, USA; 5Allergan, an AbbVie Company, Irvine, CA, USA
Correspondence: Sohel Somani, Email [email protected]
Purpose: To understand the demographics, clinical characteristics, treatment patterns, visual and anatomic responses of patients with diabetic macular edema (DME) initially treated with anti-vascular endothelial growth factor (anti-VEGF) agents in the real-world clinical setting.
Patients and Methods: This retrospective cohort study used electronic health records to identify consecutively presenting patients with DME who received their first documented anti-VEGF injection (index injection) on or after 1 October 2015 and before 30 September 2016 (index period) at 4 clinical sites in Ontario, Canada. Patients receiving anti-VEGF injections in the study eye were followed for ≥ 18 months. After the first 3 monthly injections, patients were classified as “responder” (≥ 20% reduction in central retinal thickness [CRT] from index date) or “nonresponder” (< 20% reduction in CRT) to anti-VEGF treatment.
Results: At 12 months, change from baseline (CFB) in best visual acuity (BVA) of responders (n = 30) was mean (SD) 12.8 (13.00) letters; CFB in nonresponders (n = 56) was 3.2 (16.3) letters. Sensitivity analyses stratified by initial BVA were supportive. Mean (SD) change in CRT (μm) was − 160.4 (111.4) in responders and − 62.2 (98.6) in nonresponders. While changes in anti-VEGF therapy were lower in responders versus nonresponders (10.0% vs 23.2%), mean number of injections was similar (8.3 in each cohort).
Conclusion: Despite receiving a substantial number of injections and requiring changes in therapy more frequently, nonresponders showed a lack of clinically meaningful change in BVA and CRT. Nonresponders could be identified after 3 anti-VEGF injections. There remains an unmet need for treatment options in patients with DME who show a nonresponse after 3 months of anti-VEGF treatment.
Keywords: real-world evidence, diabetic macular edema, drug therapy, anti-vascular endothelial growth factor, visual acuity
Introduction
Treatment of diabetic macular edema (DME) with anti-vascular endothelial growth factor (anti-VEGF) agents typically requires monthly injections for an extended period, and a substantial proportion of patients (30–65%) are partial or nonresponders.1–5 A suboptimal or nonresponse has been defined in clinical studies as patients achieving <5 letter gain in best-corrected visual acuity (BCVA) or <10% or 20% decrease in central retinal thickness (CRT).1,6–9 A post hoc analysis of the Diabetic Retinopathy Clinical Research Retina Network Protocol I study indicated that approximately 40% of eyes receiving monthly ranibizumab had suboptimal BCVA improvement at 12 weeks. Treatment over 3 years produced only modest improvement with one third of eyes having <5 letter gain.6 However, data from the Protocol T study comparing aflibercept, bevacizumab, and ranibizumab indicate that suboptimal response (BCVA <5 letters) at 12 weeks following 3 consecutive anti-VEGF injections does not necessarily preclude meaningful vision improvement from occurring in many eyes.10
Changes in anti-VEGF agent therapy because of poor response to an original treatment has not been well studied in the real-world clinical setting although data have shown marginal benefits in visual outcomes in nonresponders.11 Lim et al12 reported that patients who were refractory to 6 injections of ranibizumab/bevacizumab had improved anatomic and visual outcomes following conversion to aflibercept, while other studies have reported that the subsequent administration of aflibercept after a mean of 13.7 injections was not associated with significant visual acuity (VA) improvements but only anatomic outcomes.13 The use of another class of drugs such as intravitreal corticosteroids (dexamethasone or fluocinolone) has shown significant improvement in visual and anatomical outcomes in nonresponders to anti-VEGF therapy.4 In addition, studies have shown that early use of alternative therapy in nonresponders is beneficial.4,6 Early identification of patients with DME who are poorly or un-responsive to anti-VEGF therapy would enable more timely consideration of potential changes to their treatment regimens.1 This study reviews the use of anti-VEGF treatment for DME in a Canadian real-world population to better understand the demographics, clinical characteristics, treatment patterns, and vision and anatomic responses of patients with DME initially treated with anti-VEGF agents.
Methods
Study Design and Data Source
This retrospective, noninterventional, observational, cohort study was conducted in 4 clinical sites in Ontario, Canada. Electronic health records (EHRs) were screened to identify consecutively presenting patients with a diagnosis of DME who received their first documented anti-VEGF injection (index event) on or after 1 October 2015 and before 30 September 2016 (index date identification period; Figure 1). The study complied with International Society for Pharmacoepidemiology Good Pharmacoepidemiology Practice guidelines and the tenets of the 2013 Declaration of Helsinki. Approval of the protocol was obtained from a centralized independent institutional review board (Advarra, Columbia, MD). Since the collected data were retrospective and anonymized, written informed patient consent was waived.
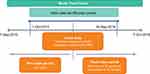 |
Figure 1 Study design. Abbreviations: Anti-VEGF, anti-vascular endothelial growth factor; DME, diabetic macular edema. |
Patient Identification
Adult patients, naïve to anti-VEGF therapy, who received ≥1 anti-VEGF injection to treat visual impairment due to DME on or after 1 October 2015 and before 30 September 2016 (index period) and who had follow-up data for ≥18 months were eligible. Patients with unconfirmed DME, and missing data on age, gender, and to assess treatment response within 60 days of the third anti-VEGF injection, and VA or optical coherence tomography (OCT) recorded at baseline (defined as no more than 30 days prior to the index date [date of index event] through 7 days after the index date) were excluded as were eyes for which laterality of the anti-VEGF injection was not identified, and eyes with concomitant retinal diagnoses commonly treated with anti-VEGF therapy (retinal vein occlusion and neovascular age-related macular degeneration as identified by International Classification of Diseases codes). If patients received anti-VEGF injection in both eyes, the eye that received the maximum number of injections was deemed the study eye.
Once eligibility was confirmed, patients were stratified into one of the two study cohorts – anti-VEGF responders or anti-VEGF nonresponders. Responders were defined as patients with a ≥20% reduction in CRT from the index date to the first CRT value within 60 days after the third anti-VEGF injection; the 60-day designated window allowed variability in visit times from patient to patient. If the index date CRT was not available, the last pre-index date measurement recorded within 60 days prior to the index date was used. Nonresponders were defined as having a <20% reduction in CRT from the index date to the first OCT value within 60 days after the third injection. If the index date CRT was not available, the last pre-index date measurement measured within 60 days prior to the index date was used.
Data Collection
Retrospective data from EHRs were anonymized and entered in an electronic data capture system by the site personnel. Patient data were collected for at least 18 months to 2 years after the index date or until 7 October 2018 (follow-up period; Figure 1). Baseline patient demographics and characteristics and data on the type, date, and frequency of anti-VEGF injections, and VA and CRT measurements performed at baseline and during the follow-up period were recorded. Snellen VA measurements were converted to approximate Early Treatment Diabetic Retinopathy Study letter scores.
Data Analysis
Outcome variables evaluated included anti-VEGF agent utilization, ie, the number of anti-VEGF injections administered/year and the use of an alternative agent; anti-VEGF agent effectiveness including change in VA and CRT from the index date to the end of follow-up (at least 18 months to 2 years after the index date); VA at baseline and at 12, 18, and 24 months and stratified by baseline VA; and eyes with ≥15 letter gain. Data were analyzed for the full population and the anti-VEGF responder and nonresponder cohorts (primary analysis).
To corroborate the results from the primary analyses, 3 sensitivity analyses were performed by changing the definitions of responder and nonresponder. In sensitivity analysis 1, in alignment with the Protocol I EARLY analysis,7,14 responders were defined as patients with a ≥10% reduction in CRT or ≥5 letter gain from baseline (index date) to the first CRT or VA value, within 60 days after the third anti-VEGF injection. For sensitivity analysis 2, patients were assigned to responder vs nonresponder cohorts after 6 anti-VEGF injections using the base case definition of responder (≥20% reduction in CRT). In sensitivity analysis 3, responders were defined as those who had either ≥20% reduction in CRT or ≥5 letter gain based on the first CRT or VA value within 60 days after the third anti-VEGF injection.
Analyses were primarily descriptive. Continuous variables were described by mean, standard deviation (SD), median, extreme values (minimum and maximum), and missing data. Categorical variables were summarized using relative frequency and percentage. Two-sided 95% confidence interval of the mean and percentage was calculated when appropriate. The study was exploratory; no formal sample size calculations were performed. Approximately 120 records were planned for inclusion based on eligible patient numbers. Statistical analyses used SAS software version 9.04.01.
Results
Patient Disposition, Baseline Demographics, and Characteristics
Of the 120 patients identified in the EHR database with a diagnosis of DME, 97 were eligible for inclusion. Eleven patients were later excluded mainly due to receiving <3 anti-VEGF injections during study period (Figure 2). The mean (SD) time to cohort allocation was 75.2 (34.1) days for the responder cohort and 73.2 (22.9) days for the nonresponder cohort. In all, 65.1% of patients (56/86) met the nonresponder to anti-VEGF treatment criteria.
Baseline demographics, medical and general ophthalmic history, and study eye characteristics are presented in Table 1 and Table 2 by cohort. In the overall population, the mean (SD) age at baseline was 64.8 (10.2) years, 55.8% of patients were ≥65 years of age, and 61.6% were male. Of the 40 patients who had reported data, 20.0% and 80.0% had type 1 and type 2 diabetes, respectively, and the overall mean baseline glycosylated hemoglobin (HbA1C) was 7.2. An equal number of right and left eyes were included. Prior to study initiation, 96.5% of study eyes were naïve to corticosteroid therapy with the remainder having no data. Of note, baseline VA was lower and CRT was higher in the responder cohort.
 |
Table 1 Patient Baseline Demographics, and Medical and Ophthalmic History |
 |
Table 2 Patient Baseline Study Eye Characteristics and Index Treatment |
Anti-VEGF Therapy Administration
In the overall population (n = 86), aflibercept was the most frequently used index treatment in the study eye (61.6%), followed by ranibizumab (26.7%), and bevacizumab (11.6%) (Table 2). Aflibercept was administered in 66.7% of the 30 eyes in the responder cohort and 58.9% of 56 eyes in the nonresponder cohort. Bevacizumab was administered as the index treatment in the nonresponder cohort only. In year 1, the mean number of injections administered to each cohort was similar; in the 18 months to 2-year period, responders received more injections than nonresponders (Table 3).
 |
Table 3 Number of Anti-VEGF Injections Administered to the Study Eye |
Some patients required alternate anti-VEGF agents during the study period. Overall, the use of an alternative anti-VEGF treatment was lower in the responder cohort (10% at both 12 and 18 months) versus the nonresponder cohort (23.2% at 12 months; 30.4% at 18 months; Figure 3). The need for an alternative agent was greater in the nonresponder cohort and mean time to switch from the first anti-VEGF agent to the second agent was longer in the responder cohort (Table 4). The majority of first switches were from bevacizumab to aflibercept in 14.3% of nonresponders, aflibercept to ranibizumab in 6.7% of responders, and ranibizumab to either bevacizumab or aflibercept in 5.4% of nonresponders (each).
 |
Table 4 Patients Requiring Alternate Anti-VEGF Agents in the Study Eye |
 |
Figure 3 Percentage of patients requiring alternative therapy at 12, 18 and 24 months.Abbreviation: anti-VEGF, anti-vascular endothelial growth factor. |
Visual Acuity Outcomes
At year 1, in the responder cohort, the mean (SD) change in BVA from the index date in the study eye was 12.8 (13.0) letters, whereas in the nonresponder group the change from baseline was 3.2 (16.3) letters. Similar benefit was maintained through 24 months in both groups (Figure 4). More patients in the responder cohort had a ≥15 letter gain than the nonresponder cohort at 12 months (36.7% vs 15.7%) and 18 months (34.5% vs 14.0%), respectively. The proportion of study eyes achieving BVA of 0.3 LogMAR or better from baseline after 12 and 18 months was 43.3%, and 31.0% in the responder cohort, and 27.5% and 30.0%, respectively, in the nonresponder cohort. The proportion of study eyes achieving a BVA of 20/40-20/200 inclusive after 12 and 18 months in the responder cohort was 46.7% and 58.6%, and in the nonresponder cohort, 58.8% and 54.0%, respectively. When patients with baseline VA were stratified by a baseline letter score of <69 or 78 to 69, mean change from baseline in letter score at 12 months was greater in the responder versus nonresponder groups. The difference was more pronounced in patients with worse VA (<69 letters) (Table 5).
 |
Table 5 Visual Acuity (ETDRS Letters) at Baseline and at 12 Months (Stratified by Baseline Visual Acuity) |
Anatomic Outcomes
Similar to VA, numerically greater improvements in CRT were observed in responders and were maintained over time. At year 1, the mean (SD) change in CRT from baseline in the study eye in responders was −160.4 (111.4) µm, whereas in the nonresponder group the change was only −62.2 (98.6) µm. Benefit was maintained through 24 months (Figure 5).
 |
Figure 5 Mean change in central retinal thickness (CRT) from baseline in overall cohort, and in responders and nonresponders. Abbreviation: SE, standard error. |
Sensitivity Analyses
Patients meeting the definitions for responder and nonresponder for each sensitivity analysis and their VA and CRT findings are summarized in Table 6. Overall, in sensitivity analysis 1, both responders and the nonresponders showed ≥10% reduction in CRT from baseline at almost all time points. Despite this reduction in CRT, the nonresponder group showed only a maximum of 3-letter gain in BVA. In comparison, there was a minimum of 6-letter to a maximum of 17-letter gain in BVA in the responder group.
 |
Table 6 Sensitivity Analyses: Change in Best Visual Acuity (BCA) and Central Retinal Thickness (CRT) |
In sensitivity analyses 2 and 3, CRT thickness decreased (27% to 39%) and BVA letters gained increased (6 to 11) in the responder group. However, in the nonresponder group, at most time points, the reduction in CRT was not ≥20%. In concert with this finding, the gain in BVA was not significant (−1.8 to 3.7). Therefore, sensitivity analyses by definition further supported the results of the primary analysis; anti-VEGF treatment did not result in a clinically meaningful improvement in VA in the nonresponder group.
Discussion
In this retrospective analysis, we identified 86 patients in the clinical practice setting who received their first documented anti-VEGF injection to treat visual impairment for DME and followed their treatment journey for a minimum of 18 months. At Index, of the 86 patients in the total cohort, 11.6% were receiving bevacizumab, 26.7% ranibizumab, and 61.6% aflibercept. When categorized as responders or nonresponders to anti-VEGF therapy based on a ≥20% or <20% reduction in CRT from baseline, respectively, after 3 injections, almost two-thirds of patients were identified as nonresponders (<20% decrease in CRT), a rate higher than typically reported in other studies.1–5
No clinically meaningful differences in baseline demographics or medical and ophthalmic history were apparent between cohorts. The responders had a numerically larger mean (SD) CRT at baseline (441.4 [109.7] µm) than the nonresponders (355.7 [97.1] µm), while baseline VA was slightly lower in responders.
Aflibercept was the most frequently used index treatment in both cohorts followed by ranibizumab and the mean number of anti-VEGF injections administered appeared to be consistent over the 2 years of therapy. The majority of the initial anti-VEGF therapy switches were to aflibercept (from bevacizumab [9.3%] and ranibizumab [4.7%]). At 12 and 18 months, the number of switches was lower in responders (10% each) than in nonresponders (23.2% and 30.4%, respectively). Despite changing therapies, 65% of nonresponders were still nonresponders and did not reap the benefit of anti-VEGF treatment. In fact, other studies indicate that 30–65% of participants are nonresponders to anti-VEGF treatment.1–5 This higher percentage of nonresponders in our study could be due to small sample size, differences in the standard of care across practices, and differences in care outcomes and response assessment in real-world studies compared with randomized controlled trials.
Despite receiving a similar number of injections and requiring an alternate anti-VEGF agent more frequently than responders, nonresponders showed a lack of clinically meaningful change in BVA and CRT over 24 months. In fact, the responder cohort had an approximately four times greater change in BVA than the nonresponder group at 12 months and an approximate three times greater change at 18 months. Considering that the base definition of responder/nonresponder centered on CRT only, vision in responders benefited greatly from anti-VEGF therapy, while nonresponders realized only minimal BVA changes. The reduction in CRT in responders was more than twice that of the nonresponders at 12 and 18 months. However, the responders had a higher CRT at baseline, which may partially explain the large difference in the change from baseline.
In sensitivity analysis 1, lowering the CRT to ≥10% yielded a greater number of responders (N = 65) than nonresponders (N = 21), the opposite of the responder to nonresponder ratio with the base definition of ≥20%. Similarly, sensitivity analysis 3 yielded a greater number of responders while sensitivity analysis 2 with a more stringent threshold (responder of ≥20% reduction in CRT after 6 injections) yielded a greater number of nonresponders indicating the stronger influence of CRT change and a much lesser effect of allowing 6 versus 3 injections on outcome. In current practice, generally 6 injections are administered before a decision is made to choose an alternate therapy.15
Of note, optical coherence tomography angiography (OCT-A) has more recently been suggested as a promising method of predicting diabetic macular edema refractory to anti-VEGF therapy.16 Elnahry et al16 found that, after 3 anti-VEGF injections, eyes with a higher CRT at baseline gained more vision than those eyes that failed to improve, similar to our study. Although starting CRT was the strongest predictor of both anatomic and visual response, OCT-A vascular parameters independently predicted the likelihood that macular edema would improve with anti-VEGF agents indicating that nonresponding eyes may have more macular ischemia at the outset. This may be important because irreversible structural damage may occur with inadequately managed swelling. Although larger studies are needed, OCT-A could assist clinicians in identifying eyes that are unlikely to respond to anti-VEGF therapy and making earlier decisions as to when to switch from anti-VEGF therapies to alternate therapies to treat DME.16
A key limitation of this study was its descriptive nature with a sample size of responders and nonresponders too small/underpowered to assess statistical differences between or within groups. Further, real-world studies with adequate sample size and adjustment for confounders are warranted to assess differences in demographics and clinical characteristics among optimal and suboptimal responders. Other study limitations include the lack of data on the type of anti-VEGF regimen administered and the omission of vitreoretinal interface diseases as an exclusion criterion as they can create a serious nonresponse.17
Conclusions
Our findings from the real-world sample indicated that more than 50% of the participants are nonresponders to anti-VEGF therapy for DME, despite switching between different anti-VEGF agents. This outcome is not surprising given the complex pathophysiology of DME.2,18 Further, other studies have shown that targeting pathways other than VEGF, can be beneficial to anti-VEGF nonresponders.2–4,18–22 Current alternatives to anti-VEGF treatment include the use of corticosteroids, which act by targeting anti-inflammatory pathway along with decreasing VEGF synthesis, focal laser, subthreshold laser therapies, and vitrectomy.5 Finally, emerging pharmacotherapeutics targeting other mediators such as cytokines/chemokines, adhesion molecules, and multiple growth factors also show promise to anti-VEGF nonresponders.5,23
Abbreviations
BVA, best visual acuity; BCVA, best-corrected visual acuity; CADTH, Canadian Agency for Drugs and Technologies in Health; CFB, change from baseline; CRT, central retinal thickness; DME, diabetic macular edema; EHR, electronic health records; ETDRS, Early Treatment Diabetic Retinopathy Study; HbA1c, glycosylated hemoglobin; OCT, optical coherence tomography; SD, standard deviation; VA, visual acuity; VEGF, vascular endothelial growth factor.
Data Sharing Statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (eg, protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time, and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
Ethics Approval and Informed Consent
Approval of the protocol was obtained from a centralized independent institutional review board (Advarra, Columbia, MD). Since the collected data were retrospective and anonymized, written informed patient consent was waived.
Acknowledgments
Allergan, an AbbVie company, and the authors thank the patients, study sites, and investigators who participated in this study. This work was presented in part at the 2021 Canadian Agency for Drugs and Technologies in Health (CADTH) Virtual Symposium, November 2–4, 2021. Medical writing support was provided by Evidence Scientific Solutions, Inc., Philadelphia, PA, and funded by Allergan (an AbbVie company).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Funding
Allergan, an AbbVie company, funded this study and participated in the study design, research, analysis, data collection, interpretation of data, and the review and approval of the manuscript. All authors had access to relevant data and participated in the drafting, review, and approval of the manuscript.
Disclosure
Financial arrangements of the authors with companies whose products may be related to the present report are listed as declared by the authors: SS has served as a speaker for AbbVie, Bayer, and Novartis, and a consultant for Bayer, Novartis, and Ripple Therapeutics. KK has served as a consultant to Alcon, Allergan (an AbbVie company), Bayer, Novartis, and received a research grant from Bayer. BS-M serves as a consultant for AbbVie and is an employee of Noesis Healthcare Technologies. DM is a consultant for AbbVie and is an employee of Genesis Research. TK, CZ, and AA are full-time employees of AbbVie and may own AbbVie stock/share options. The authors report no other conflicts of interest in this work.
References
1. Ratra D. Commentary: switching of anti-vascular endothelial growth factor agents in refractory diabetic macular edema. Indian J Ophthalmol. 2021;69(2):367–368. doi:10.4103/ijo.IJO_2611_20
2. Furino C, Boscia F, Reibaldi M, Alessio G, Mencía-Gutiérrez E. Intravitreal therapy for diabetic macular edema: an update. J Ophthalmol. 2021;2021:6654168. doi:10.1155/2021/6654168
3. Chen YP, Wu AL, Chuang CC, Chen SN. Factors influencing clinical outcomes in patients with diabetic macular edema treated with intravitreal ranibizumab: comparison between responder and non-responder cases. Sci Rep. 2019;9(1):10952. doi:10.1038/s41598-019-47241-1
4. Busch C, Fraser-Bell S, Gilaki M, et al. Real-world outcomes of non-responding diabetic macular edema treated with continued anti-VEGF therapy versus early switch to dexamethasone implant: 2-year results. Acta Diabetic. 2019;56(12):1341–1350. doi:10.1007/s00592-019-01416-4
5. Sorour OA, Levine ES, Baumal CR, et al. Persistent diabetic macular edema: definition, incidence, biomarkers, and treatment methods. Surv Ophthalmol. 2023;68(2):147–174. doi:10.1016/j.survophthal.2022.11.008
6. Gonzalez VH, Campbell J, Holekamp NM, et al. Early and long-term responses to anti-vascular endothelial growth factor therapy in diabetic macular edema: analysis of Protocol I data. Am J Ophthalmol. 2016;172:72–79. doi:10.1016/j.ajo.2016.09.012
7. DRCR Retina Network. Protocol I. Available from: https://public.jaeb.org/drcrnet/stdy/146.
8. Dugel PU, Campbell JH, Kiss S, et al. Association between early anatomic response to anti-vascular endothelial growth factor therapy and long-term outcome in diabetic macular edema: an independent analysis of protocol I study data. Retina. 2019;39(1):88–97. doi:10.1097/IAE.0000000000002110
9. Downey L, Acharya N, Devonport H, et al. Treatment choices for diabetic macular oedema: a guideline for when to consider an intravitreal corticosteroid, including adaptations for the COVID-19 era. BMJ Open Ophthalmol. 2021;6(1):e000696. doi:10.1136/bmjophth-2020-000696
10. Bressler NM, Beaulieu WT, Maguire MG, et al. Early response to anti-vascular endothelial growth factor and two-year outcomes among eyes with diabetic macular edema in Protocol T. Am J Ophthalmol. 2018;195:93–100. doi:10.1016/j.ajo.2018.07.030
11. Salumi A, Vila N, Mo Dabber M, Kapusta M. One-year outcomes of aflibercept for refractory diabetic macular edema in bevacizumab nonresponders. Indian J Ophthalmol. 2021;69(2):360–367. doi:10.4103/ijo.IJO_459_20
12. Lim LS, Ng WY, Mathur R, et al. Conversion to aflibercept for diabetic macular edema unresponsive to ranibizumab or bevacizumab. Clin Ophthalmol. 2015;9:1715–1718. doi:10.2147/opth.S81523
13. Rahimy E, Shahlaee A, Khan MA, et al. Conversion to aflibercept after prior anti-VEGF therapy for persistent diabetic macular edema. Am J Ophthalmol. 2016;164:118–127.e2. doi:10.1016/j.ajo.2015.12.030
14. Elman MJ, Aiello LP, Beck RW; Diabetic Retinopathy Clinical Research Network. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117(6):1064–1077:e35. doi:10.1016/j.ophtha.2010.02.031
15. Regillo CD, Callanan DG, Do DV, et al. Use of corticosteroids in the treatment of patients with diabetic macular edema who have a suboptimal response to anti-VEGF: recommendations of an expert panel. Ophthalmic Surg Lasers Imaging Retina. 2017;48(4):291–301. doi:10.3928/23258160-20170329-03
16. Elnahry AG, Noureldine AM, Abdel-Kader AA, et al. Optical coherence tomography angiography biomarkers predict anatomical response to bevacizumab in diabetic macular edema. Diabetes Metab Syndr Obes. 2022;15:395–405. doi:10.2147/DMSO.S351618
17. Kulikov AN, Sosnovskii SV, Berezin RD, et al. Vitreoretinal interface abnormalities in diabetic macular edema and effectiveness of anti-VEGF therapy: an optical coherence tomography study. Clin Ophthalmol. 2017;11:1995–2002. doi:10.2147/OPTH.S146019
18. Grzybowski A, Markeviciute A, Zemaitiene R. Treatment of macular edema in vascular retinal diseases: a 2021 update. J Clin Med. 2021;10(22):5300. doi:10.3390/jcm10225300
19. Chatziralli I. Editorial - Suboptimal response to intravitreal anti-VEGF treatment for patients with diabetic macular edema: is there any point in switching treatment? Eur Rev Med Pharmacol Sci. 2018;22(15):5047–5050. doi:10.26355/eurrev_201808_15648
20. Lai CT, Hsieh YT, Lin CJ, et al. Age, initial central retinal thickness, and OCT biomarkers have an influence on the outcome of diabetic macular edema treated with ranibizumab- Tri-center 12-Month Treat-and-Extend Study. Front Med. 2021;8:668107. doi:10.3389/fmed.2021.668107
21. Kuroiwa DAK, Malerbi FK, Regatieri CVS. New insights in resistant diabetic macular edema. Ophthalmologica. 2021;244(6):485–494. doi:10.1159/000516614
22. Everett LA, Paulus YM. Laser therapy in the treatment of diabetic retinopathy and diabetic macular edema. Curr Diab Rep. 2021;21(9):35. doi:10.1007/s11892-021-01403-6
23. Chauhan MZ, Rather PA, Samarah SM, Elhusseiny AM, Sallam AB. Current and novel therapeutic approaches for treatment of diabetic macular edema. Cells. 2022;11(12):1950. doi:10.3390/cells11121950
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


