Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Changes in Serum Nesfatin-1 After Laparoscopic Sleeve Gastrectomy are Associated with Improvements in Nonalcoholic Fatty Liver Disease
Authors Yang K, Zhang X, Zhou Y, Chen F, Shen M, Wang Y
Received 16 January 2020
Accepted for publication 15 March 2020
Published 1 May 2020 Volume 2020:13 Pages 1459—1464
DOI https://doi.org/10.2147/DMSO.S246281
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Antonio Brunetti
Keyu Yang, Xiaowei Zhang, Yong Zhou, Fu Chen, Mingyang Shen, Yong Wang
Department of General Surgery, Fourth Affiliated Hospital of China Medical University, Shenyang City, Liaoning Province 110032, People’s Republic of China
Correspondence: Yong Wang
Department of General Surgery, Fourth Affiliated Hospital of China Medical University, No. 4, Chongshan East Road, Huanggu District, Shenyang City, Liaoning Province 110032, People’s Republic of China
Tel +8618940259733
Email [email protected]
Background: Nonalcoholic fatty liver disease (NAFLD) is a serious and widespread disease worldwide. Bariatric surgery is one of the treatments for NAFLD. Nesfatin-1 is located in the brain, periphery and plasma. We studied the relationship between nesfatin-1 changes after laparoscopic sleeve gastrectomy (LSG) and NAFLD remission.
Methods: A total of 29 patients participated in the study, which collected clinical information on the patients and indicators of liver function, hepatic steatosis score and nesfatin-1 level before and after LSG.
Results: The average BMI of the patients before surgery was 42.63± 8.91 kg/m2, and the average BMI was 28.54± 5.63 kg/m2 one year after surgery (p < 0.05). One year after LSG, the total weight loss percentage (TWL%) was 32.11± 7.10%. The mean value of nesfatin-1 before surgery was 3.04± 0.81 ng/mL, and the mean value of nesfatin-1 was 5.52± 1.55 ng/mL at one year after surgery (p < 0.05). The average preoperative hepatic steatosis index (HSI) score of the patients was 52.55± 9.17, and the average postoperative HSI score was 38.84± 5.82 (p < 0.05). Before LSG (p < 0.05, r= − 0.81) and 1 year after surgery (p < 0.05, r = − 0.58), HSI and nesfatin-1 were significantly negatively correlated. Percentage of increased nesfatin-1 and percentage of decreased HSI showed positive correlation after LSG.
Conclusion: There was a negative correlation between HSI and nesfatin-1 before and after LSG, which may suggest that nesfatin-1 plays a role in NAFLD.
Keywords: nesfatin-1, NAFLD, bariatric surgery, LSG
Introduction
The prevalence of obesity is increasing rapidly. In the past, obesity and related diseases were considered important problems in Western countries. However, over the past two decades, with the urbanization of Asian countries, sedentary lifestyles and excess nutrition have created a hidden danger for the prevalence of obesity in Asia. Similar to many Western countries, the prevalence of nonalcoholic fatty liver disease (NAFLD) in Asia is approximately 25%.1 Obesity has become a top risk factor for NAFLD.2 The problems caused by NAFLD are not limited to liver diseases such as cirrhosis and liver cancer; NAFLD is also closely related to atherosclerotic cardiovascular events and abnormal glucose metabolism caused by metabolic abnormalities, which require active treatment and intervention.3 NAFLD is also a multiple pathogenesis disease.4 Intestinal hormones, such as glp-1, GIP and leptin, are closely related to the occurrence and development of NAFLD. These intestinal hormones may affect glucose metabolism and insulin resistance and may also directly affect NAFLD by acting on the liver.5,6 Nesfatin-1 is a multifunctional metabolism-related hormone and satiety molecule that is distributed in the central nervous systems and peripheral tissue. Nesfatin-1 is found in the hypothalamus, adipose tissue, stomach, pancreas, and liver. Oh S et al first identified nesfatin-1 in 2006.7 Nesfatin-1 can effectively reduce food intake, cause loss of appetite, relieve hunger, and provide a sense of fullness.8 In addition, nesfatin-1 is involved in glucose regulation and potentiates glucose-induced insulin secretion.9 It is also related to cardiac function regulation, anxiety, depression, anti-inflammation and antiapoptosis.10,11 In recent years, the field of bariatric and metabolic surgery has developed rapidly, becoming important means of treating morbid obesity and related complications.12 Bariatric and metabolic surgery provides a new direction for the treatment of obese patients with NAFLD. At present, laparoscopic sleeve gastrectomy (LSG) is still the most widely performed method, accounting for more than 50% of all operations.13 LSG is a mechanism not only for restricting food intake but also for directly regulating lipid metabolism.14 In this study, we aimed to explore the effect of LSG on NAFLD and serum nesfatin-1 in patients with obesity in order to provide a new possible target for the treatment of NAFLD and determine the mechanism by which LSG exerts its effect on NAFLD.
Patients and Methods
Participants
From January 2018 to June 2018, a total of 68 patients with obesity underwent LSG at the General Surgery of the Fourth Affiliated Hospital of China Medical University. Patients with a diagnosis of NAFLD by semiquantitative ultrasound score (Ballestri’s Ultrasonographic Fatty Liver Indicator (US-FLI) score ≥2 and Hamaguchi Steatosis Score (HSS) ≥1 indicated NAFLD)15 were included. Patient exclusion criteria were as follows: diabetes and impaired glucose tolerance, serious chronic diseases (such as severe autoimmune diseases or cancer), excessive alcohol consumption (higher than 20 g/day for women and 30 g/day for men),16 and hepatitis or other chronic liver diseases. All the patients in the study met the diagnostic criteria for obesity. WHO defines overweight as a BMI (body mass index) equal to or more than 25, and obesity as a BMI equal to or more than 30. A total of 38 patients diagnosed with NAFLD met the criteria, of whom 29 completed a one-year follow-up (dropout rate of 23.7%).
Compliance with Ethical Standards:
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
This study was approved by the institutional research ethics committee of The Fourth Affiliated Hospital of China Medical University. We have provided written informed consent to all patients.
Surgical Method
All operations were performed by the same surgeon at the center. Technical points: (1) Sharp separation of the greater curvature of the stomach was performed starting at the avascular zone of the gastrocolic ligament until the left edge of the esophagus was fully exposed. An ultrasound scalpel was used to free the adhesion of the posterior gastric wall to the pancreas. (2) A 36F bougie was inserted. Using the Endo-GIA Stapler, the incision was started 3–6 cm from the pylorus until the gastric fundus was completely removed. (3) Intermittent suturing of gastric resection margin and omentum with 4–0 absorbable line was performed taking care not to twist the stomach.
Clinical and Laboratory Data
Patients were provided with detailed written materials informing them that their clinical data including age, weight, BMI (body mass index, BMI=body weight (kg)/height squared (m2)), and percent total body weight loss (TWL%) would be collected.
Morning fasting blood samples were collected for testing. The collected blood samples were immediately centrifuged, and then the separated plasma was frozen at −80°C for subsequent purposes. Serum nesfatin-1 concentrations were analyzed with ELISA kits (USCN Life Science, Wuhan, China). In this assay system, the intra-assay and interassay coefficients of variation were always below 10%. A competitive inhibition enzyme immunoassay was used in this study. Monoclonal antibodies against human nesfatin-1 were precoated on the microplate. A competitive inhibition reaction was performed between the biotinylated human nesfatin-1 and unlabeled human nesfatin-1 (standards or samples) with the precoated antibody specific for human nesfatin-1. After incubation, the unbound conjugate was washed off. Next, avidin conjugated to horseradish peroxidase (HRP) was added to each microplate well and incubated. The amount of bound HRP conjugate was inversely proportional to the concentration of nesfatin-1 in the sample. After addition of the substrate solution, the intensity of the color developed was inversely proportional to the concentration of nesfatin-1 in the sample. Testing was performed by a laboratory assistant who did not know the grouping of blood samples. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were detected by an Olympus automatic biochemical analyzer.
Noninvasive Evaluation of NAFLD
Noninvasive evaluation of NAFLD was performed with fully validated indicators. The hepatic steatosis index (HSI) and fatty liver index are commonly used indicators. HSI=8×(ALT/AST)+BMI (+2, if diabetes mellitus; +2, if female). HSI was developed in South Korea.17 Fatty liver index (FLI)=(e0.953*loge (triglycerides) + 0.139*BMI + 0.718*loge(GGT) + 0.053*waist circumference - 15.745)/(1 + e0.953*loge (triglycerides) + 0.139*BMI + 0.718*loge (GGT) + 0.053*waist circumference - 15.745)*100. FLI was developed in Italy.18 Considering that the participants were from Asia, we used HSI as the evaluation method.
Statistical Analysis
SPSS (version 25.0) and GraphPad Prism (version 8.0) were used for data analysis. P values less than 0.05 were considered indicative of statistical significance for all statistical analyses. The data for continuous variables are presented as the mean±SD. Student’s t-test or the Mann–Whitney test were employed to compare the differences between groups. Relationships between variables were evaluated by Pearson’s and Spearman correlation coefficients (data presented as r [95% CI (confidence interval)]).
Results
Impact of LSG on Weight Loss, Nesfatin-1 and Hepatic Steatosis
The mean age of the patients in this study was 33.52±7.52 years. The age range from 22 to 54 years. This study involved 18 women and 11 men. One year after LSG, the TWL% was 32.11±7.10%. The average BMI of the patients before surgery was 42.63±8.91 kg/m2, and the average BMI was 28.54±5.63 kg/m2 one year after surgery (p <0.05). The mean value of nesfatin-1 before surgery was 3.04±0.81 ng/mL, and the mean value of nesfatin-1 was 5.52±1.55 ng/mL one year after surgery (p <0.05) (Figure 1). Before LSG (p < 0.05, r= −0.81;) and 1 year after surgery (p < 0.05, r = −0.58;), HSI and nesfatin-1 were significantly negatively correlated (Figure 2). One year after LSG, the hepatic function indicators ALT, AST and NAFLD scores of the patients improved significantly. ALT and AST levels were significantly lower than those before surgery (p < 0.05). The average preoperative HSI score of the patients was 52.55±9.17, and the average postoperative HSI score was 38.84±5.82 (p < 0.05). One year after LSG, the fasting plasma glucose (FPG) was 4.9±0.3 (mmol/l). The triglyceride was 1.3±0.4 (mmol/l). The basic clinical data, statistical results and NAFLD score results of the patients are summarized in Table 1.
 |
Table 1 Basic Clinical Data and NAFLD Score Results |
 |
Figure 1 Preoperation vs 1 year postoperation in BMI and nesfatin-1. |
 |
Figure 2 Correlation analysis of HSI and nesfatin-1 preoperation vs 1 year. |
Correlation between percentage of decreased HSI and percentage of increased nesfatin-1 after LSG (Figure 3).
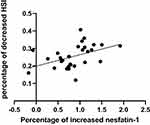 |
Figure 3 Correlation analysis of percentage of increased nesfatin-1 and percentage of decreased HSI after surgery. |
Percentage of increased nesfatin-1 and percentage of decreased HSI were positive correlation after LSG (p < 0.05, r =0.44;).
Discussion
This study found that bariatric surgery has a good therapeutic effect on NAFLD and that the preoperative and postoperative serum nesfatin-1 levels are correlated with the noninvasive HSI score of NAFLD steatosis. This finding may help further study the mechanism of bariatric surgery for NAFLD.
NAFLD is a growing health problem globally, with an estimated global prevalence of approximately 25%.19 NAFLD is strongly associated with obesity, insulin resistance or type 2 diabetes and other metabolic abnormalities. Bariatric surgery is an important treatment for a series of metabolic syndromes, including NAFLD. Nearly 85% of patients with nonalcoholic steatohepatitis (NASH) improved after bariatric surgery; during the 1-year follow-up, bariatric surgery significantly reduced the pathological features of the disease.20 In our study, we also demonstrated that LSG can effectively improve liver function and hepatic fatty degeneration. Although LSG is often classified as restrictive surgery, this is not its only effect. It can directly improve NAFLD by affecting liver glucose metabolism and bile acid metabolism.21 In addition, changes in gastrointestinal hormones, appetite, intestinal flora composition and metabolites have been reported. The nesfatin-1-related changes found in our study following LSG for NAFLD may also be the mechanism.
There are differences in the distribution of nesfatin-1 in different species. Nesfatin-1 is distributed in the central nervous systems and peripheral tissue; it is found in the paraventricular nucleus, arcuate nucleus, supraoptic nucleus and neurons in the lateral hypothalamus of the hypothalamus of rats.7 In mouse and human studies, nesfatin-1 was found to be distributed in subcutaneous fat cells and viscera, and its expression in adipose tissue was higher than that in viscera.22 Nesfatin-1 is also highly expressed in the stomach, pancreas and bladder of dogs.23 These results suggest that nesfatin-1 may play an important role in glucose and lipid metabolism in central and peripheral regions. However, because nesfatin-1 can cross the blood-brain barrier in both directions,24 we have not been able to confirm whether the changes in serum nesfatin-1 are due to increased peripheral or central secretion after LSG.
Nesfatin-1 may be involved in lipid metabolism through a variety of central and peripheral pathways. Continuous peripheral infusion of nesfatin-1 reduced plasma triglyceride and cholesterol levels in mice and significantly improved hepatic steatosis in mice fed a high-fat diet. Processing liver cells using different doses of nesfatin-1 resulted in nesfatin-1 affecting liver cell lipid accumulation via an AMPK-dependent mechanism.25 Nesfatin-1 affects adipose tissue, it can strengthen adipose triglyceride lipase (ATGl) expression and reduce fat formation-related gene acetyl CoA-carboxylase (ACC) expression. Nesfatin-1 may promote brown fat cell differentiation through an mTOR-dependent mechanism.26 In terms of the role of the central system, by knocking out nesfatin-1 in the hypothalamus of rats, significant changes in hepatic glycogen were observed. At the same time, mTOR phosphorylation levels and signal transduction transcriptional activator activity were decreased, followed by suppression of cytokine signaling 3 levels. Nesfatin-1 in the hypothalamus plays a role in glucose homeostasis and hepatic insulin sensitivity by activating the mTOR-STAT3 related signaling pathway.27
Nesfatin-1 is also involved in glucose metabolism, animal studies have found that nesfatin-1 colocalizes with insulin in islet cells and that elevated peripheral blood glucose can promote an increase in nesfatin-1 expression in the islets of rats.28 Nesfain-1 can increase glucose-induced insulin secretion by promoting Ca(2+) influx through L-type Ca(2+) channels in mouse islet β-cells.9 Another study found that nesfatin-1 can enhance glucose-stimulated insulin secretion (GSIS). Using nesfatin-1 to manipulate mouse primary beta cells, we measured changes in the ATP-sensitive K+ (KATP) channel current, the voltage-gated K+ (Kv) channel current, and insulin secretion. Nesfatin-1 inhibited the Kv channel, but KATP channel activity was unaffected. Nesfatin-1 may enhance GSIS through the regulation of ion channels rather than its unidentified receptor.29
In previous studies, the changes of nesfatin-1 after LSG show different results. Lee et al found that levels of nesfatin-1 decreased after LSG. They chose 6 non-morbidly obese (BMI between 25 and 35 kg/m2) undergoing LSG. All the participants involved had poor-controlled T2DM (glycated hemoglobin, HbA1c >7.5%).30 Nesfatin-1 may have a diverse effect on glucose and lipid metabolism, showing different manifestations in obese patients with NAFLD or diabetes. With this in mind, in our study, we included patients with obesity and NAFLD and excluded patients with severe abnormal glucose metabolism. Differences in participant selection principles may be responsible for the different results of these studies.
Nesfatin-1 is an adipokine. Leptin is an adipokine that has a functional similarity to nesfatin-1. The research on Leptin will help us to have a better understanding of nesfatin-1. Leptin is secreted by white fat cells, and serum leptin levels are associated with adipose tissue. Moreover, leptin was first described to act on the satiety center of the hypothalamus through specific receptors (leptin receptors [ObR]), thereby limiting food intake and increasing energy expenditure. It plays an important neuroendocrine role in regulating energy balance, metabolism and reproduction.30 Although leptin has a weight-reducing effect, most obese patients with type 2 diabetes mellitus and NAFLD present with hyperleptinemia, which may be weakened by leptin resistance or tolerance.31 Leptin interacts with the adipoinsular axis.32 Furthermore, leptin inhibits insulin secretion, and desensitization of leptin in the hypothalamus and somatic cells may be an important factor in obesity-related diabetes.33 However, nesfatin-1 was not found to have such characteristics in obese patients with NAFLD or type 2 diabetes.
LSG successfully treats obesity and improves many components of metabolic syndrome, including NAFLD. As bariatric and metabolic surgery for weight loss is invasive and complex, we urgently need to understand the mechanism behind it. Studies have shown that the triglyceride-lowering effects of LSG are far greater than those of weight loss through restricted diets.34 The nesfatin-1 changes found in our study may be one of the mechanisms. Identification of more possible mechanisms will help provide better treatment plans for a wider range of patients at a lower cost with a lower risk of complications.
There are still some limitations in this study: (1) This study is a retrospective study, with a small number of included cases and a relatively short duration, followed up at a single time point after 1 year, and the strength of evidence needs to be further improved. (2) In this study, liver function indicators and a hepatic steatosis scale were used as the evaluation criteria for the effect of LSG on NAFLD, and pathological evidence was lacking.
Acknowledgments
This study was supported by the 2019 Discipline Cultivation Plan of China Medical University, and the 2019 “Double First-Class” Project of Liaoning province. Keyu Yang and Xiaowei Zhang are co-first authors for this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Fan JG, Kim SU, Wong VWS. New trends on obesity and NAFLD in Asia[J]. J Hepatol. 2017;67(4):862–873. doi:10.1016/j.jhep.2017.06.003
2. Younossi ZM, Loomba R, Rinella ME, et al. Current and future therapeutic regimens for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis[J]. Hepatology. 2018;68(1):361–371. doi:10.1002/hep.29724
3. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American association for the study of liver diseases[J]. Hepatology. 2018;67(1):328–357.
4. Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD)[J]. Metabolism. 2016;65(8):1038–1048. doi:10.1016/j.metabol.2015.12.012
5. Marra F, Svegliati-Baroni G. Lipotoxicity and the gut-liver axis in NASH pathogenesis[J]. J Hepatol. 2018;68(2):280–295. doi:10.1016/j.jhep.2017.11.014
6. Cernea S, Roiban AL, Both E, et al. Serum leptin and leptin resistance correlations with NAFLD in patients with type 2 diabetes[J]. Diabetes Metab Res Rev. 2018;34(8):e3050. doi:10.1002/dmrr.3050
7. Oh S, Shimizu H, Satoh T, et al. Identification of nesfatin-1 as a satiety molecule in the hypothalamus[J]. Nature. 2006;443(7112):709. doi:10.1038/nature05162
8. Stengel A, Taché Y. Nesfatin-1—role as possible new potent regulator of food intake[J]. Regul Pept. 2010;163(1–3):18–23. doi:10.1016/j.regpep.2010.05.002
9. Nakata M, Manaka K, Yamamoto S, et al. Nesfatin-1 enhances glucose-induced insulin secretion by promoting Ca2+ influx through L-type channels in mouse islet β-cells[J]. Endocr J. 2011;1102080532.
10. Özsavcí D, Erşahin M, Şener A, et al. The novel function of nesfatin-1 as an anti-inflammatory and antiapoptotic peptide in subarachnoid hemorrhage–induced oxidative brain damage in rats[J]. Neurosurgery. 2011;68(6):1699–1708. doi:10.1227/NEU.0b013e318210f258
11. Merali Z, Cayer C, Kent P, et al. Nesfatin-1 increases anxiety-and fear-related behaviors in the rat[J]. Psychopharmacology. 2008;201(1):115. doi:10.1007/s00213-008-1252-2
12. Jakobsen GS, Småstuen MC, Sandbu R, et al. Association of bariatric surgery vs medical obesity treatment with long-term medical complications and obesity-related comorbidities[J]. JAMA. 2018;319(3):291–301. doi:10.1001/jama.2017.21055
13. Angrisani L, Santonicola A, Iovino P, et al. IFSO worldwide survey 2016: primary, endoluminal, and revisional procedures[J]. Obes Surg. 2018;28(12):3783–3794. doi:10.1007/s11695-018-3450-2
14. Ding L, Sousa KM, Jin L, et al. Vertical sleeve gastrectomy activates GPBAR‐1/TGR5 to sustain weight loss, improve fatty liver, and remit insulin resistance in mice[J]. Hepatology. 2016;64(3):760–773. doi:10.1002/hep.28689
15. Ballestri S, Romagnoli D, Nascimbeni F, et al. Role of ultrasound in the diagnosis and treatment of nonalcoholic fatty liver disease and its complications[J]. Expert Rev Gastroenterol Hepatol. 2015;9(5):603–627. doi:10.1586/17474124.2015.1007955
16. Sberna AL, Bouillet B, Rouland A, et al. European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD) and European Association for the Study of Obesity (EASO) clinical practice recommendations for the management of non‐alcoholic fatty liver disease: evaluation of their application in people with Type 2 diabetes[J]. Diabetic Med. 2018;35(3):368–375. doi:10.1111/dme.13565
17. Lee JH, Kim D, Kim HJ, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease[J]. Digestive Liver Dis. 2010;42(7):503–508. doi:10.1016/j.dld.2009.08.002
18. Bedogni G, Bellentani S, Miglioli L, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population[J]. BMC Gastroenterol. 2006;6(1):33. doi:10.1186/1471-230X-6-33
19. Satapathy SK, Sanyal AJ. Epidemiology and natural history of nonalcoholic fatty liver disease[C]//Seminars in liver disease. Thieme Med Publishers. 2015;35(03):221–235.
20. Lassailly G, Caiazzo R, Buob D, et al. Bariatric surgery reduces features of nonalcoholic steatohepatitis in morbidly obese patients[J]. Gastroenterology. 2015;149(2):379–388. doi:10.1053/j.gastro.2015.04.014
21. Myronovych A, Kirby M, Ryan KK, et al. Vertical sleeve gastrectomy reduces hepatic steatosis while increasing serum bile acids in a weight‐loss‐independent manner[J]. Obesity. 2014;22(2):390–400. doi:10.1002/oby.20548
22. Ramanjaneya M, Chen J, Brown JE, et al. Identification of nesfatin-1 in human and murine adipose tissue: a novel depot-specific adipokine with increased levels in obesity[J]. Endocrinology. 2010;151(7):3169–3180. doi:10.1210/en.2009-1358
23. Prinz P, Stengel A. Expression and regulation of peripheral NUCB2/nesfatin-1. Curr Opin Pharmacol. 2016;31:25–30. doi:10.1016/j.coph.2016.08.012
24. Pan W, Hsuchou H, Kastin AJ. Nesfatin-1 crosses the blood–brain barrier without saturation[J]. peptides. 2007;28(11):2223–2228. doi:10.1016/j.peptides.2007.09.005
25. Yin Y, Li Z, Gao L, et al. AMPK-dependent modulation of hepatic lipid metabolism by nesfatin-1[J]. Mol Cell Endocrinol. 2015;417:20–26. doi:10.1016/j.mce.2015.09.006
26. Wang Y, Li Z, Zhang X, et al. Nesfatin-1 promotes brown adipocyte phenotype[J]. Sci Rep. 2016;6(1):34747. doi:10.1038/srep34747
27. Wu D, Yang M, Chen Y, et al. Hypothalamic nesfatin-1/NUCB2 knockdown augments hepatic gluconeogenesis that is correlated with inhibition of mTOR-STAT3 signaling pathway in rats[J]. Diabetes. 2014;63(4):1234–1247. doi:10.2337/db13-0899
28. Foo KS, Brauner H, Östenson CG, et al. Nucleobindin-2/nesfatin in the endocrine pancreas: distribution and relationship to glycaemic state[J]. J Endocrinol. 2010;204(3):255–263. doi:10.1677/JOE-09-0254
29. Maejima Y, Horita S, Kobayashi D, et al. Nesfatin-1 inhibits voltage gated K+ channels in pancreatic beta cells[J]. Peptides. 2017;95:10–15. doi:10.1016/j.peptides.2017.07.001
30. Lee WJ, Chen CY, Ser KH, et al. Differential influences of gastric bypass and sleeve gastrectomy on plasma nesfatin-1 and obestatin levels in patients with type 2 diabetes mellitus[J]. Curr Pharm Des. 2013;19(32):5830–5835. doi:10.2174/13816128113198880010
31. Moon HS, Dalamaga M, Kim SY, et al. Leptin’s role in lipodystrophic and nonlipodystrophic insulin-resistant and diabetic individuals[J]. Endocr Rev. 2013;34(3):377–412. doi:10.1210/er.2012-1053
32. Dalamaga M, Chou SH, Shields K, et al. Leptin at the intersection of neuroendocrinology and metabolism: current evidence and therapeutic perspectives[J]. Cell Metab. 2013;18(1):29–42. doi:10.1016/j.cmet.2013.05.010
33. Anini Y, Brubaker PL. Role of leptin in the regulation of glucagon-like peptide-1 secretion[J]. Diabetes. 2003;52(2):252–259. doi:10.2337/diabetes.52.2.252
34. Kieffer TJ, Habener JF. The adipoinsular axis: effects of leptin on pancreatic β-cells[J]. Am J Physiol Endocrinol Metab. 2000;278(1):E1–E14. doi:10.1152/ajpendo.2000.278.1.E1
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
