Back to Journals » Therapeutics and Clinical Risk Management » Volume 19
Changes in Oncological Surgical Principles Driven by Advances in Preoperative Treatments
Authors Horváth ÖP , Bellyei S, Pozsgai É, Vereczkei A
Received 26 April 2023
Accepted for publication 25 June 2023
Published 8 August 2023 Volume 2023:19 Pages 667—674
DOI https://doi.org/10.2147/TCRM.S415860
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Örs Péter Horváth,1 Szabolcs Bellyei,2 Éva Pozsgai,3,4 András Vereczkei1
1Department of Surgery, University of Pécs Clinical Center, Pécs, 7624, Hungary; 2Department of Oncotherapy, University of Pécs Clinical Center, Pécs, 7624, Hungary; 3Department of Public Health, University of Pécs Medical School, Pécs, 7624, Hungary; 4Department of Primary Health Care, University of Pécs Medical School, Pécs, 7623, Hungary
Correspondence: Örs Péter Horváth, Department of Surgery, University of Pécs Clinical Center, Ifjúság Street 13, Pécs, 7624, Hungary, Tel +3672535806, Email [email protected]
Abstract: From a surgical point of view, the development of preoperative oncological treatment has had a profound effect on the surgical treatment trends of cancer as well as on the outcomes of cancer patients. Consequently, these changes have challenged formerly entrenched oncological surgical principles. In our short report, we aimed to summarize the main shifts regarding the surgical principles of cancer treatment due to the development of preoperative oncological therapy in recent years. As a result of successful preoperative treatment, surgeons may perform less radical surgeries, the required free resection margin has been narrowed down to a few millimeters in dimension and preoperative treatment is justified in both definitely resectable tumors and in oligometastatic tumors as well. For prognosis assessment, the post-preoperative oncological treatment stage is now considered decisive, rather than the pretreatment stage as previously thought. Other changes include the introduction of the watch and wait strategy and the reverse order of treatment of the primary tumor and metastasis. Observing the continuously improving outcomes of cancer patients and the developments in oncological treatment modalities, a further expansion of the indication of preoperative treatments is to be expected.
Keywords: preoperative oncological therapy, neoadjuvant, downstaging, complete remission, surgical principles
Background
Oncology has undergone tremendous development in recent decades, leading to increasingly favorable outcomes for cancer patients. Many factors have contributed to these advances, including the increased application of primary and secondary preventive measures, the development of new diagnostic procedures and new approaches for cancer treatment, and the integration of the results gained from basic research (medical genetics, immunology, molecular biology, etc.) into everyday clinical practice. From the point of view of surgery, the improvement of preoperative oncological treatment methods and their extensive application in practice have played the greatest role in this incredible success story.1,2 Thanks to technical developments and the efficiency of new methods, the outcomes in surgical oncology have improved spectacularly. By destroying viable tumor cells, a well-chosen preoperative treatment can lead to a significant reduction in the size of the tumor, thereby improving its surgical management and enable a more favorable prognosis.
Thus significant improvement in survival rates may be attributed to the increased resectability of tumors, the higher number of R0 resections and the reduction of locoregional recurrences, which are mainly due to the significantly higher number of complete pathological remissions.3,4
Gastrointestinal cancers, which are typically known for their poor prognosis, have also shown significant improvements in outcomes. The resectability of esophageal and pancreatic tumors due to the preoperative oncological treatment has increased by approximately 20%.3,4 Significantly increased rates of R0 resections have been reported in advanced gastric5,6 and squamous esophageal cancer,7,8 and significantly improved survival rates have been found in patients with esophageal,9 gastric10,11 and pancreatic cancers.4 Based partly on our own, partly on others’experience, improvement in survival has been demonstrated in almost all gastrointestinal, lung and breast cancers.1,2,12–14
The efficacy of preoperative oncological treatments has led to the development of new principles in surgery, which have continued to change as more and more data and evidence have become available.
The aim of this report is to describe the relatively rapid changes that have taken place in the principles of surgical oncology over the past years based on our own experience2 and the evidence from the literature.
Discussion
- Change in free resection margin: Initially, the main principle of preoperative oncological treatment was that surgery with the same degree of extension should be performed regardless whether preoperative oncological treatment had been carried out or not, because viable cells may remain in the apparently tumor-free area even after preoperative therapy.15 In classical surgery, the required proximal resection margin for gastric tumors was 5–6 cm.16 In esophageal cancer, the recommended proximal resection margin was 2 cm for T2 tumors and 3 cm for T3 tumors.8 In recent years, this recommendation has been changed, with some authors even suggesting that aiming to achieve a certain resection distance should be abandoned. Currently, it is sufficient to declare RO resection if the resection line is tumor-free.17 This is true for the tumors of the esophagus, breast and rectum.18–20
Case 1: In one of our own cases, 20 years ago, preoperative chemotherapy was performed according to the epirubicin, cisplatin, fluorouracil (ECF) regimen for a T4 stage gastric cancer and a complete clinical response was achieved. Total gastrectomy were performed from abdominal approach. Histology confirmed pCR. Today we would perform distal subtotal gastrectomy after carrying out preoperative chemotherapy (Figure 1a–c).
- De-escalating surgery for quality of life improvement: The second important change is that the expected free margin has decreased to one millimeter in breast tumors,19 in liver metastases21 and in the distal free margin of rectal resection.20 The significant reduction in the size of the tumor (downsizing) due to preoperative oncological treatment, and the previously mentioned new rule regarding the free margin have enabled organ-preserving surgeries, which have significantly contributed to the patients’ improved quality of life. Currently, a larynx-preserving esophagus resection can be performed in pharyngoesophageal junction cancer22,23 and similarly, the anal sphincter can be preserved in the case of a lower third rectal cancer.20 Furthermore, breast-conserving surgery can be carried out once significant downsizing had been achieved in breast cancer cases.19,24 In addition many initially node‐positive breast cancer patients achieve complete axillary pathological response after primary systemic therapy. In such cases with 3 or more negative sentinel lymph nodes, axillary dissection might be omitted as a de-escalation of axillary surgery.25
- Post-treatment stage (yTNM) determines the prognosis and extent of surgery: For a long time, the initial clinical stage of the tumor (cTNM) was considered to be the determining factor for predicting prognosis.15 As a result of improving outcomes, it was confirmed that the stage after preoperative oncological treatment (yTNM) should be taken into account when assessing prognosis, because the survival results were identical to patients with tumors of the same stage without neoadjuvant therapy.26 The prognosis is considered particularly favorable if complete pathological remission can be achieved with preoperative oncological treatment.4,6,11,27
- Oncological treatment with curative intent for unresectable disease: Preoperative oncological treatment of unresectable tumors was not recommended at the beginning of the neoadjuvant era, because it had to be verified first that oncological pretreatment of resectable or borderline resectable tumors led to improved survival results.15,28 After improved outcomes following preoperative oncological treatment were reported, first borderline unresectable cases, then patients with oligometastatic disease were being preoperatively treated as well. These included patients with T4 stage and/or metastatic tumors encompassing one or two regions.29 Due to the shift in providing preoperative oncological care for an increasingly growing spectrum of cancer patients, the terminology of preoperative oncological care also changed. Previously, all preoperative oncological treatment was called neoadjuvant treatment. Nowadays, the treatment given to make a surgically irresectable tumor resectable is called conversion treatment. Neoadjuvant treatment is performed when therapy is given to a patient with a tumor that is presumably resectable. Additionally, preoperative oncological treatment may also be justified with the aim to perform less radical surgery and/or to improve prognosis. Patients with oligometastatic cancer make up a heterogeneous patient group, therefore it is not easy to assess treatment outcomes, although encouraging results have been reported recently.12,30 Fukuchi et al reported a 26% resectability and 80% R0 resection rate in oligometastatic gastric cancer patients.31 Even pCR has been found in 5–15% of the oligometastatic tumors of the breast32 and upper GI tract.30 Patients with diffuse metastases are still mostly excluded from preoperative oncological treatment, however cPR is rarely achievable even with diffuse metastases following preoperative treatment. In our practice, for example, we treated a gastric cancer patient -who also had ascites due to carcinosis- with Taxane and Cisplatin preoperative therapy, and achieved complete pathologic remission in the stomach, while the patient’s diffuse carcinosis and ascites also disappeared.33
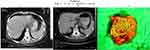 |
Figure 1 (a) T4 gastric cancer demonstrated by CT. (b) CT after chemotherapy shows complete clinical response. (c) Surgical specimen. Normal mucosa can be seen and the histology confirms a pCR. |
Case 2 shows a patient with an oligometastatic cancer (T4N1) in the upper third of the esophagus. After preoperative chemoradiation a complete clinical response was achieved and a larynx preserving partial pharyngo-esophagectomy and free jejunal transfer could be performed (Figure 2a–c).
- Preoperative therapy for lower stage resectable tumors: The most widely used and accepted reason for performing preoperative oncological therapy is advanced cancer with uncertain resectability but without distant metastases.7,11,28 Interestingly, a new trend has begun regarding the treatment of patients with comparatively early-stage cancer; for example, patients with early-stage-cancer, such as T2N0-stage gastric cancer patients have also been treated with neoadjuvant therapy, and pCR rates as high as 50–60% were achieved, although it has not been proven to improve survival.34,35 The question is how long the trend of preoperatively treating patients with increasingly earlier stages of cancer can be continued, in light of the low complication rates associated with oncological treatments. Obviously, further studies will be needed to support the use of preoperative oncological treatment in an increasingly larger spectrum of cancer patients.11
- Surgery can be avoided with “watch and wait” strategy: The best survival outcomes can be gained by achieving complete pathological remission.4,6,27 When establishing the indication for surgery, however, only clinical restaging tests are available. Although, the accuracy of these tests is gradually improving, decision-making cannot be based on them with certainty. It is easier to make the decision to operate or to wait if the operation may significantly impair the quality of life or the surgical risk is high. However, even the most careful restaging carries some uncertainty, so it is very difficult to decide whether to perform resection surgery or to choose the “watch and wait” (W&W) strategy. W&W protocols for patients with complete clinical response have gained increasing popularity since first described by Habr-Gama et al in 2004.36 According to the literature, the expected recurrence rate is around 15–20% in the case of esophageal and rectal tumors37,38 in the first three years after complete clinical response. In addition to the W&W strategy, follow-up visits at 2–3 months are required, which should consist of endoscopy, biopsy, and imaging tests. Intensive surveillance, with the early detection of regrowth lead to a higher rate of successful salvage surgeries, without an increase in the risk of systemic diseases, or adverse survival outcomes. The vast majority (97%) of patients with regrowth after a W&W policy were able to undergo treatment with curative intent for their local regrowth and uncontrolled pelvic disease was very rare.19,37 The cost of control tests can be significant and may impose a burden on the patient.39,40
 |
Figure 2 (a) T4 pharyngo-esophageal junction cancer shown by CT. (b) CT after preoperative chemoradiation, shows complete clinical response. (c) Pharyngo-jejuno-esophagostomy (histology: pCR, T0N0). |
Case 3: Our patient with T3N1 stage rectal cancer underwent preoperative chemoradiation. A clinical complete response could be achieved. The patient did not agree to the planned deep rectal resection with a protective ileostomy. He has been living without complaints for more than 5 years (Figure 3a and b).
- Limiting the treatment of liver metastases of colorectal origin: Apparent or real complete remission following preoperative oncological treatment is a challenging area in the therapy of liver metastases of colorectal tumors.41 Even with the most modern imaging tests determining the stage of the tumor is somewhat more uncertain for liver metastases, than for esophageal and rectal tumors. An incorrectly diagnosed complete remission can be as high as 30–40%,42 and the chance of recurrence is also above 30%.43 Therefore, these are several reasons to decide in favor of surgery and to surgically resect the metastasis. However, surgeons must be prepared for the fact that the place of the tumor cannot always be precisely located, and the resection must be performed according to the results of the imaging, thus leaving the surgeon with a difficult decision. For this reason, the new trend is to give only 3 cycles of chemotherapy to a patient with resectable liver metastases and not to strive for complete response. Thus, the remaining, reduced tumor limits the size of the area that must be removed during surgery,44 but in the meantime fewer chemotherapy cycles can reduce the occurrence of toxic side effects, which is less burdensome for both the patient and -economically- for the health care system.
- The order of treatment of the primary tumor and metastasis can be reversed: The question whether the order of treatment of the primary tumor and the metastasis can be changed, has been raised.45 Previously, it was a strict rule that due to its biological characteristics that it can spread further, the primary tumor must be removed first, and the second step is the resection of the metastasis. The only exception were brain metastases with a relatively urgent indication. According to current recommendations, however, the tumor that has a greater impact on the quality of life and lifespan of the patient should be treated first. So, if the primary tumor does not threaten to cause further complications, preoperative oncological treatment and even the removal of the metastasis can be initiated due to the ever-improving therapeutic options.46 Thus, this reverse order of treatment can no longer be considered uncommon.
 |
Figure 3 (a) CT scan of a T3N1 middle third rectal cancer. (b) Complete clinical response is achieved after preoperative chemoradiation. |
The introduction of new therapeutic modalities is expected to lead to further improvements in outcomes. The 5-year survival rate after initially applied epirubicin, cisplatin, fluorouracil (ECF) treatment was 36%, in patients with locally advanced stage gastric cancer, while treatment according to the new fluorouracil, leucovorin, oxaliplatin, docetaxel (FLOT) protocol improved survival to reach 45%,47 The use of combination treatments that take into account cell membrane receptor status, such as HER2 positivity in gastric cancer, has also led to improved outcomes.48 More and more new molecular biological markers (eg microsatellite instability) have been identified, which indicate whether the preoperative oncological treatment of the tumor with a certain marker would be effective or not.49
The new oncological surgical principles listed in this work are valid if the pretreatment results show complete remission. In all other cases, the old principles remain in effect. Another limitation is that oncological treatment fortunately has increasingly rarer severe side effects. Restaging tests are not yet flawless, which may be a significant biasing factor and, as a result, complete remission can only be confirmed by the pathological examination.
Currently, restaging tests can predict complete remission with around 80% certainty,44,50 and as their accuracy improves, it will be possible to use the W&W strategy with more conviction. Metabolic response tests, like PET-CT scans, that justify the early interruption of preoperative therapy can already be successfully used today,50 but further development is expected in this area of diagnostic imaging as well.
Conclusions
According to current estimates, surgery is involved in the treatment of tumors in about 60–70% of the cases. With the development of preoperative oncological treatments, the place and role of surgical treatment in cancer care is being reevaluated. Presumably, fewer radical surgeries will be needed, organ-preserving operations will be preferred, and the emphasis placed on quality of life will be even greater.
Abbreviations
CR, complete response; ECF, epirubicin, cisplatin, fluorouracil; pCR, pathological complete response; W&W, watch and wait.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Ethics Approval and Consent to Participate
Ethical permission from the Regional Ethical Committee of the University of Pécs Medical School was gained under the reference number 8280-PTE2020. The study was performed in accordance with the Declaration of Helsinki.
Consent for Publication
Consent for the publications of accompanying images was not required according to the regulations of the Regional Ethical Committee of the University of Pécs Medical School since no individual identifiable patient data/images were used and/or presented in the current study, thus the need for informed consent for the present study was waived by the Regional Ethical Committee of the University of Pécs Medical School.
Author Contributions
All authors listed on this article (Horváth ÖP, Bellyei S, Pozsgai É, Vereczkei A) meet all of the following criteria:
- Made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas.
- Have drafted or written, or substantially revised or critically reviewed the article.
- Have agreed on the journal to which the article will be submitted.
- Reviewed and agreed on all versions of the article before submission, during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage.
- Agree to take responsibility and be accountable for the contents of the article.
Funding
The authors received no specific funding for this study.
Disclosure
The authors declare that they have no conflicting interests in this work.
References
1. Lordick F. Principles of neoadjuvant therapy. Chirurg. 2009;80:1000–1005. doi:10.1007/s00104-009-1731-y
2. Vereczkei A, Horváth ÖP. Surgical oncology. In: Horváth ÖP, Oláh A, editors. Surgery Medicina. Budapest: Hungarian; 2017.
3. Strobel O, Neoptolemos J, Jäger D, Büchler MW. Optimizing the outcomes of pancreatic cancer surgery. Nat Rev Clin Oncol. 2019;16:11–26. doi:10.1038/s41571-018-0112-1
4. Villano AM, O’Halloran E, Goel N, et al. Total neoadjuvant therapy is associated with improved overall survival and pathologic response in pancreatic adenocarcinoma. J Surg Oncol. 2022;126(3):502–512. doi:10.1002/jso.26906
5. Kim A, Kim BS, Yook JH, Kim BS. Optimal proximal resection margin distance for gastrectomy in advanced gastric cancer. World J Gastroenterol. 2020;26:2232–2246. doi:10.3748/wjg.v26.i18.2232
6. Kaltenmeier C, Althans A, Mascara M, et al. Pathologic complete response following neoadjuvant therapy for gastric adenocarcinoma: a national cancer database analysis on incidence, predictors, and outcomes. Ann Surg Oncol. 2022;29(5):3096–3108. doi:10.1245/s10434-021-11010-0
7. Yang H, Liu H, Chen Y, et al; AME Thoracic Surgery Collaborative Group. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a Phase III multicenter, randomized, open-label clinical t rial. J Clin Oncol. 2018;36:2796–2803. doi:10.1200/JCO.2018.79.1483
8. Papp A, Cseke L, Farkas R, et al. Chemo-radiotherapy in locally advanced squamous cell oesophageal cancer--are upper third tumours more responsive? Pathol Oncol Res. 2010;16:193–200. doi:10.1007/s12253-009-9206-5
9. Hou S, Pan Z, Hao X, Hang Q, Ding Y. Recent progress in the neoadjuvant treatment strategy for locally advanced esophageal cancer. Cancers. 2021;13(3):5162–5168. doi:10.3390/cancers13205162
10. Capovilla G, Froiio C, Lang H, Berlth F, Grimminger PP. Complete response after neoadjuvant therapy for gastric cancer: implications for surgery. Der Chirurg. 2022;93:138–143.
11. Stark AP, Ikoma N, Chiang YJ, et al. Characteristics and survival of gastric cancer patients with pathologic complete response to preoperative therapy. Ann Surg Oncol. 2019;26:3602–3610. doi:10.1245/s10434-019-07638-8
12. Jung MK, Ott K, Chevallay M, Mönig SP. Treatment options for oligometastatic gastric cancer. Chirurg. 2021;92:515–521. doi:10.1007/s00104-021-01353-5
13. Zapf I, Tizedes G, Pavlovics G, et al. Primer szisztémás terápia mellrákos betegeknél (2007-2010) [Primary systemic therapy in breast cancer patients (2007–2010)]. Magy Seb. 2011;64(2):223–228. Hungarian.
14. Rápolti E, Szigeti A, Farkas R, et al. Neoadjuváns radiokemoterápia lokálisan előrehaladott végbéldaganatok kezelésében [Neoadjuvant radiochemotherapy in the treatment of locally advanced rectal tumors]. Magy Onkol. 2009;53:34–38. Hungarian.
15. Siewert JR, Stein HJ, Fink U. Multimodality therapy for esophageal cancer. Oncologist. 1996;1:210–218. doi:10.1634/theoncologist.1-4-210
16. Kumazu Y, Hayashi T, Yoshikawa T, et al. Resection margin in gastric cancer patients. BMC Surg. 2020;20(1):95. doi:10.1186/s12893-020-00744
17. Maspero M, Sposito C, Benedetti A, et al. Impact of surgical margins on overall survival after gastrectomy for gastric cancer: a validation of Japanese gastric cancer association guidelines on a western series. Ann Surg Oncol. 2022;29:3096–3108. doi:10.1245/s10434
18. van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2013;366(22):2074–2084. doi:10.1056/NEJMoa1112088
19. Choi J, Laws A, Hu J, Barry W, Golshan M, King T. Margins in breast-conserving surgery after neoadjuvant therapy. Ann Surg Oncol. 2018;25(12):3541–3547. doi:10.1245/s10434-018-6702-4
20. Zeng WG, Liu MJ, Zhou ZX, Wang ZJ. A distal resection margin of 1 mm and rectal cancer recurrence after sphincter-preserving surgery: the role of a positive distal margin in rectal cancer surgery. Dis Colon Rectum. 2017;60:1175–1183. doi:10.1097/DCR.0000000000000900
21. Xu D, Wang HW, Yan XL, Li J, Wang K, Xing BC. Sub-millimeter surgical margin is acceptable in patients with good tumor biology after liver resection for colorectal liver metastases. Eur J Surg Oncol. 2019;45:1551–1558. doi:10.1016/j.ejso.2019.03.010
22. Horváth ÖP, Cseke L, Kalmár K, Varga G, Horváth G. Larynx-preserving pharyngo-esophagectomy after chemoradiation in the treatment of cancer of the pharyngo-esophageal junction. Ann Thorac Surg. 2001;72:2146–2147. doi:10.1016/s0003-4975(01)03167-8
23. Nakajima Y, Tachimori H, Miyawaki Y, et al. A survey of the clinical outcomes of cervical esophageal carcinoma surgery focusing on the presence or absence of laryngectomy using the National clinical database in Japan. Esophagus. 2022;19(4):569–575. doi:10.1007/s10388-022-00944-3
24. Mrdutt M, Heerdt A, Sevilimedu V, Mamtani A, Barrio A, Morrow M. Margin width and local recurrence in patients undergoing breast conservation after neoadjuvant chemotherapy. Ann Surg Oncol. 2022;29:484–492. doi:10.1245/s10434-021-10533-w
25. Song YX, Xu Z, Liang MX, et al. Diagnostic accuracy of de-escalated surgical procedure in axilla for node-positive breast cancer patients treated with neoadjuvant systemic therapy: a systematic review and meta-analysis. Cancer Med. 2022;11(22):4085–4103. doi:10.1002/cam4.4769
26. Davies AR, Gossage JA, Zylstra J, et al. Tumor stage after neoadjuvant chemotherapy determines survival after surgery for adenocarcinoma of the esophagus and esophagogastric junction. J Clin Oncol. 2014;32:2983–2990. doi:10.1200/JCO.2014.55.9070
27. Corso G, Kahler-Ribeiro-Fontana S, Pagan E, et al. Ten-year outcome results of cT4 breast cancer after neoadjuvant treatment. Surg Oncol. 2021;124(8):1242–1250. doi:10.1002/jso.26662
28. Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. New Engl J Med. 2006;355:11–20. doi:10.1056/NEJMoa055531
29. Beckert S, Königsrainer A. Oligometastases in gastric and esophageal cancer: current clinical trials and surgical concepts. Chirurg. 2018;89:505–509. doi:10.1007/s00104-018-0645-y
30. Katayama H, Tsuburaya A, Mizusawa J, et al. An integrated analysis of two Phase II trials (JCOG0001 and JCOG0405) of preoperative chemotherapy followed by D3 gastrectomy for gastric cancer with extensive lymph node metastasis. Gastric Cancer. 2019;22:1301–1307. doi:10.1007/s10120-019-00981-5
31. Fukuchi M, Ishiguro T, Ogata K, et al. Prognostic role of conversion surgery for unresectable gastric cancer. Ann Surg Oncol. 2015;22:3618–3624. doi:10.1245/s10434-015-4422-6
32. Hattori M, Iwata H. Advances in treatment and care in metastatic breast cancer (MBC): are there MBC patients who are curable? Clin Oncol. 2018;7:23. doi:10.21037/cco.2018.05.01
33. Ember A, Yousuf AF, Kalmár K, et al. Komplett remisszió neoadjuváns chemotherápia után lokálisan előrehaadott gyomorrák esetén, ami peritonitis carcinomatosát okozott -case report [Complete regression after neoadjuvant chemotherapy in locally advanced gastric cancer causing peritonitis carcinomatosa--a case report]. Magy Seb. 2006;59:445–447. Hungarian.
34. Gabriel E, Attwood K, Narayanan S, Brady M, Nurkin S, Hochwald S. Does neoadjuvant/perioperative chemotherapy improve overall survival for T2N0 gastric adenocarcinoma? J Surg Oncol. 2018;117(4):659–670. doi. doi:10.1002/jso.24894
35. Perez Holguin RA, Olecki EJ, Stahl KA, et al. Management of clinical T2N0 esophageal and gastroesophageal junction adenocarcinoma: what Is the optimal treatment? J Gastrointest Surg. 2022;26(10):2050–2060. doi:10.1007/s11605-022-05441-7
36. Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240:711–717. doi:10.1097/01.sla.0000141194.27992.32
37. Chadi SA, Malcomson L, Ensor J, et al. Factors affecting local regrowth after watch and wait for patients with a clinical complete response following chemoradiotherapy in rectal cancer (InterCoRe consortium): an individual participant data meta-analysis. Lancet Gastroenterol Hepatol. 2018;3:825–836. doi:10.1016/S2468-1253(18)30301-7
38. Fernandez LM, São Julião GP, Figueiredo NL, et al; International Watch & Wait Database Consortium. Conditional recurrence-free survival of clinical complete responders managed by watch and wait after neoadjuvant chemoradiotherapy for rectal cancer in the International watch & wait database: a retrospective, international, multicentre registry study. Lancet Oncol. 2021;22:43–50. doi:10.1016/S1470-2045(20)30557-X
39. Renehan AG, Malcomson L, Emsley R, et al. Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): a propensity-score matched cohort analysis. Lancet Oncol. 2016;17:174–183. doi:10.1016/S1470-2045(15)00467-2
40. Mullaney TG, Lightner AL, Johnston M, Keck J, Wattchow D. ‘Watch and wait’ after chemoradiotherapy for rectal cancer. ANZ J Surg. 2018;88:836–841. doi:10.1111/ans.14352
41. Kuhlmann K, van Hilst J, Fisher S, Poston G. Management of disappearing colorectal liver metastases. J Surg Oncol. 2016;42:1798–1805. doi:10.1016/j.ejso.2016.05.005
42. Barimani D, Kauppila JH, Sturesson C, Sparrelid E. Imaging in disappearing colorectal liver metastases and their accuracy: a systematic review. World J Surg Oncol. 2020;18:264. doi:10.1186/s12957-020-02037
43. Bischof DA, Clary BM, Maithel SK, et al. Surgical management of disappearing colorectal liver metastases. Br J Surg. 2013;100:1414–1420. doi:10.1002/bjs.9213
44. Chen Q, Mao R, Zhao J, et al. From the completion of neoadjuvant chemotherapy to surgery for colorectal cancer liver metastasis: what is the optimal timing? Cancer Med. 2020;9:7849–7862. doi:10.1002/cam4.3283
45. Jegatheeswaran S, Mason JM, Hancock HC, Siriwardena AK. The liver-first approach to the management of colorectal cancer with synchronous hepatic metastases: a systematic review. JAMA Surg. 2013;148:385–391. doi:10.1001/jamasurg.2013.1216
46. Lillemoe HA, Vauthey JN. Surgical approach to synchronous colorectal liver metastases: staged, combined, or reverse strategy. Hepatobiliary Surg Nutr. 2020;9:25–34. doi:10.21037/hbsn.2019.05.14
47. Al‐Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro‐oesophageal junction adenocarcinoma (FLOT4): a randomised, Phase 2/3 trial. Lancet. 2019;393:1948–1957. doi:10.1016/S0140-6736(18)32557-1
48. Lordick F, Shitara K, Janjigian YY. New agents on the horizon in gastric cancer. Ann Oncol. 2017;28:1767–1775. doi:10.1093/annonc/mdx051
49. Gockel I, Lordick F. Neoadjuvante Chemotherapie beim Magenkarzinom. Vielfach eine Übertherapie oder ein sinnvolles Konzept [Neoadjuvant chemotherapy for gastric cancer. Frequent overtreatment or meaningful concept?]. Chirurg. 2020;91:384–390. German. doi:10.1007/s00104-020-01141-7
50. Lordick F. Optimizing neoadjuvant chemotherapy through the use of early response evaluation by positron emission tomography. Recent Results Cancer Res. 2012;196:201–211.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
