Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 12
Change in Viral Load Count and Its Predictors Among Unsuppressed Viral Load Patients Receiving an Enhanced Adherence Counseling Intervention at Three Hospitals in Northern Ethiopia: An Exploratory Retrospective Follow-Up Study
Received 25 September 2020
Accepted for publication 23 November 2020
Published 7 December 2020 Volume 2020:12 Pages 869—877
DOI https://doi.org/10.2147/HIV.S283917
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Bassel Sawaya
Gedefaw Diress,1 Melese Linger2
1Department of Public Health, College of Health Sciences, Woldia University, Woldia, Ethiopia; 2Department of Midwifery, College of Health Sciences, Woldia University, Woldia, Ethiopia
Correspondence: Gedefaw Diress Department of Public Health
College of Health Sciences, Woldia University, Woldia 400, Ethiopia
Tel +251913756945
Email [email protected]
Background: Enhanced adherence counseling (EAC) is an interventional program that provides targeted adherence counseling for unsuppressed viral load people living with HIV who are receiving antiretroviral therapy before diagnosing treatment failure. However, there is a lack of evidence on change in viral load count among patients receiving EAC intervention. Therefore, this study aimed to assess change in viral load count and its predictors among people living with HIV (PLHIV) in northeast Ethiopia.
Methods: A hospital-based retrospective follow-up study was conducted on 235 randomly selected patients with unsuppressed viral load who started EAC sessions between 2016 and 2019 at three governmental hospitals in the northern part of Ethiopia. Viral load count and patient individual factors were assessed at EAC program enrollment and viral load counts repeated at the end of EAC session. The main outcome variable was a change in viral load count during the EAC session period. A paired sample t-test was used to determine the mean difference in viral load count before and after EAC intervention. Linear mixed-effects models were used to assess the effect of selected factors on viral load count change.
Results: Based on the paired sample t-test, there was a significant mean difference in viral load count before and after EAC intervention (mean difference=16,904, (95% CI: 9986– 23,821; p-value< 0.001). The multivariable linear mixed-effects regression analysis showed that young age (β= 0.03; 95% CI: 0.01, 0.14), urban residence (β= − 0.55; 95% CI: − 0.63, − 0.34), CD4 count of 201– 500 cells/mm3 (β= − 0.67; 95% CI: − 0.87, − 0.43) and long duration on ART (β= − 0.01; 95% CI: − 0.01, − 0.02) were associated with the decline in viral load count.
Conclusion: We detected a substantial decline in viral load count among patients receiving an EAC intervention. Young age, urban residence, CD4 count of 201– 500 cells/mm3 and long duration on ART were the positive predictors of viral load suppression.
Keywords: adherence, anti-retroviral therapy, HIV, viral load
Background
Viral load (VL) determination is the best standard to assess people living with HIV (PLHIV’s) responses to Anti-Retroviral Therapy (ART).1 According to the Ethiopian national ART guideline, the first VL determination for people living with HIV (PLHIV) must be done after six months of starting antiretroviral drugs and then every year regularly.2,3 Unsuppressed VL count (>1000 copies/mL) in patients on ART occurs when ART treatment fails to suppress a person’s VL and is associated with decreased survival and increased HIV transmission. It suggests the virus is not controlled by the existing antiretroviral drugs regimen. As a result, patients with unsuppressed VL count had an increased risk of morbidity and mortality.4
In response to the challenge of achieving the third 90 of the UNAIDS 90–90-90 targets (at least 90% of PLHIV on ART to have viral suppression by 2020),5 the Ethiopian Ministry of Health implemented a routine viral load monitoring program in Ethiopia. To achieve this goal, the barriers to viral suppression should be identified and intervened. Poor adherence, which is associated with low CD4 count and high viral load, leads to clinical HIV disease progression to AIDS or death.6,7 Since many studies identified poor adherence as the commonest risk factor for unsuppressed VL count,8–10 structured enhanced adherence counseling (EAC) is a vital intervention before diagnosing treatment failure mainly in a resource-limited setting. EAC is an interventional program which provides targeted adherence counseling to patients with unsuppressed viral load (VL>1000copies/mL) and who have poor treatment adherence, to help them improve their adherence. EAC is a repeated process that includes exploring the barriers of adherence, supporting clients to find solutions for non-adherence, and developing a plan which improves VL suppression and reduces subsequent treatment failure.11
World Health Organization (WHO) recommended that repeated VL testing for people with unsuppressed VL after 3–6 EAC monthly sessions each one month apart.3 Accordingly, the Ethiopian Federal Ministry of Health (FMoH) introduced repeat VL determination for unsuppressed VL patients at the first test.3 Based on recent national evidence, nearly 27% of pediatrics and 17% of adults reported having an unsuppressed VL which requires EAC and VL determination.12 The Ethiopian national HIV treatment guidelines recommend the management of patients with high viral load through EAC and followed by a repeat viral load test after 3–6 EAC counseling sessions.3
Previous studies revealed that nearly 71% of patients with high initial viral load count (>1000 copies/mL) have a re-suppressed viral load after enhanced adherence counseling intervention.13,14 Similarly, a recent study done in Zimbabwe reported that 31.2% of patients had suppressed viral load after 3 months of EAC session.15 In addition, in Ethiopia, a study was conducted in a similar setting (Hospitals of North Wollo Zone, Ethiopia) among patients with high viral load count, and the study revealed that nearly 66% of patients had viral load suppression after EAC sessions.16 But, a cluster-randomized study on the effectiveness of EAC in South Africa revealed that enrolling unsuppressed viral load patients in the EAC program for 12 months has no benefit on viral load suppression.17
In resource-limited settings like Ethiopia, monitoring the change in viral load count among patients who enrolled in the EAC program is useful for clinicians and program designers to examine the effect of EAC on viral load suppression. Information is needed to decide whether a significant viral load suppression before switching to a second-line regimen. Besides, there is a lack of evidence on the sociodemographic and clinical determinants of change in viral load count after EAC sessions among patients who enrolled in the EAC program (PLHIV with high viral load count). Therefore, the current study aimed to evaluate the change in VL count during EAC sessions and its determinants among PLHIV with unsuppressed VL count in northeast Ethiopia.
Methods
Study Design, Setting, and Period
A hospital-based retrospective longitudinal study was done at three governmental hospitals that implement enhanced adherence counseling sessions in North Wollo Zone, Northern Ethiopia. The data were collected from March to May 2019. HIV patients with viral load >1000 copies/mL are referred for enrolment in the EAC program. Enhanced adherence counseling consists of three to six sessions done every month. After three to six EAC sessions, each client is assessed for adherence and a repeat viral load test is performed. If the viral load is suppressed (≤ 1000 copies/mL of blood), the client is continued on the same ART regimen. On the other hand, if the repeat viral load is greater than 1000 copies/mL despite good adherence to therapy, the client is switched to the second-line ART regimen.
Study Population
All PLHIV who have unsuppressed VL count (VL >1000copies/mL) and who started EAC sessions between 2016 and 2019 at three governmental hospitals in North Wollo Zone, Ethiopia were the study population. Patients who had at least two VL measurements (at the start and end of EAC sessions) were included in the study. Patients who had single VL measurements, those with incomplete records, and patients with a duration on ART < 6 months were excluded from the study.
Sampling Procedure
Initially, we obtained information on the number of PLHIV who underwent VL testing between 2016 and 2019 at three governmental hospitals from the electronic database and VL registration book. And then, we prepared a list of all patients with unsuppressed VLs count (VL ccount>1000copies/mL) for each hospital (589 in Woldia General hospital, 157 in Lalibela General Hospital, and 94 in Meket Primary Hospital). Based on the number of unsuppressed VL patients who enrolled in the EAC session between 2016 and 2019, the total sample size (235) was proportionally allocated to each hospital (165 for Woldia General Hospital, 44 for Lalibela General Hospital, and 26 from Meket Primary Hospital). Finally, using the prepared sampling frame, the study subjects were selected using a random sampling method (computer-generated random number) (Figure 1).
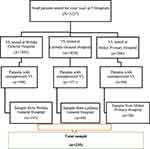 |
Figure 1 Flowchart for the selection process of study participant. |
Data Collection Procedure
A pretested structured checklist was used to extract the data from the patients’ chart, EAC follow-up sheet, viral load registration book, and laboratory result registration book. The checklist was developed using the standardized EAC follow-up sheet and ART follow up form. The patients’ identification numbers were used to extract the necessary data from different data sources in the ART clinics. Data about the baseline socio-demographic, clinical, and treatment-related characteristics of patients were collected from the patients’ chart, EAC follow-up sheet, and the registration card of each patient with HIV. Data about initial and final viral load were collected from the viral load registration book and EAC follow-up sheet.
Variables
The main outcome variable was a longitudinal change in VL count (copies/mL) over the EAC session (3–6 months). Independent variables include age, sex, place of residence, WHO clinical stage, the current ART regimen, baseline CD4 count, nutritional status or body mass index (BMI), opportunistic infections (OIs), functional status (working, ambulatory or bedridden), duration on ART, and level ART adherence.
Laboratory and Clinical Data Measurements
VL Count
VL testing was done at the selected regional laboratory site in the Amhara region using the Abbott RealTime HIV-1 assay. The Abbott RealTime HIV-1 assay is an in vitro reverse transcription-polymerase chain reaction (RT-PCR) assay for the quantitation of Human Immunodeficiency Virus type 1 (HIV-1) on the automated m2000 System in human plasma from HIV-1 infected individuals over the range of 40 to 10,000,000 copies/mL. Anything less than 40 copies/mL is called “undetectable”. In this study, patients with a VL count of ≤1000 copies/mL were classified as suppressed VL.3
Adherence Level
The percentage of ARV drug taken is used as a criterion to classify drug adherence level (Good is defined as ≥ 95% of doses taken as prescribed, Fair defined as 85–94% of doses taken, and Poor defined as <85% of doses taken.3
Duration on ART
Calculated from the date of the first EAC session and the date of ART initiation.
Functional Status
Focus on the patient’s abilities to perform basic activities of daily living, which include basic self-care such as bathing, feeding, toileting, cooking, shopping, and managing one’s own affairs. It was classified as working/ambulatory and bedridden.
Data Analysis Procedure
Baseline characteristics (at the time of high VL count detected) were summarized using frequency and percentages for categorical variables. Continuous type of data with and without normal distribution was described using mean (± SD) and median (IQR), respectively.
We used a paired sample t-test to determine whether there is a significant mean difference in viral load count between two sets of observations (after enhanced adherence counseling sessions and before the initiation of EAC). We examined changes in VL count after the baseline measurement over a follow-up period (EAC session period) in both absolute and transformed form. By taking benefit of the linear mixed model’s flexibility, both the random and fixed effects of predictors on VL counts were determined. Models were fitted to transformed data in order to linearize the relationship with time and make the distribution more symmetric (normal curve). Data transformations ranging from square roots to logarithms form of the VL count were attempted. Lastly, we choose the logarithm form of data because models using the logarithm form were a better fit to the data than those using the square root form.
The Akaike information criteria (AIC) were used to compare the model selection strategy. Regression coefficients with 95% CI were used as measures of association between the independent variables and change in VL count. All analyses were performed using SPSS version 20.
Ethical Consideration
Ethical clearance and letter of cooperation for selected hospitals were obtained from the Institutional Review Committee of Woldia University. Our study was based on a retrospective review of secondary data from medical records of patients and as this was not a primary study the Ethical Review Committee of the Woldia University waived the requirement for informed consent. Data were fully anonymized and no personal identifiers, such as name and private information were not collected. Confidentiality during all phases of research activities was kept and data were held on a secured password protected system. This study was conducted in accordance with the Declaration of Helsinki.
Result
Characteristics of Participants at Baseline
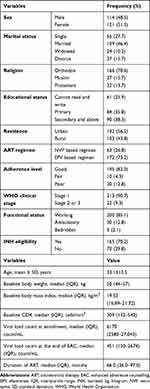
The mean age was 33.1(±12.5 SD) and nearly 36% of patients completed the primary educational level. The median VL count was 6170 copies/mL (IQR 2580–27,043) and the median CD4 count was 309 cells/mm3 (IQR, 132–542) at baseline. At the termination of follow-up (EAC sessions), the median VL count was 451 copies/mL (IQR 0–2674) (Table 1).
Changes in Viral Load Count
Participants in this analysis were followed up for a median of 5.2 months (IQR: 3.4–7.5) during the EAC session period. VL count both at baseline and at the end of EAC sessions had skewed distribution. Thus, reporting the change in VL using the median was better than the mean. As described in Table 1, the median VL count had declined over the EAC session’s period.
However, in this study, we reported also a mean viral load count to make it clear. Based on the paired sample test, there was a significant mean difference between VL at baseline and at the end of EAC sessions (mean difference=16,904, (95% CI: 9986–23,821; p-value<0.001). Table 2 compare the baseline and repeated mean VL count during the follow-up period by different characteristics of participants. A statistically significant difference in changes in VL was found by sex, educational status, residence, baseline CD4 count, baseline adherence level, and WHO clinical stage (Table 2)
 |
Table 2 Comparison of Baseline and Repeated Mean Viral Load Difference by Socio-Demographic and Clinical Characteristics of Participants |
Factors Associated with Viral Load Count Change
Based on the multivariable linear mixed-effects regression; age, residence, baseline CD4 count, and duration on ART were significant predictors of change in VL count. Keeping all the other variables constant, for a 1-year younger in the age of respondents, the VL of the patient decrease by 3% (β= 0.03, p-value ≤0.001). Patients who are from urban residences have a 55% decrement in their VL count (β= −0.55, p-value=0.009) as compared with those from rural ones. Similarly, for a 1-month longer duration on ART, the VL of a patient decreases by 1% (β= −0.01, p-value= 0.004). Patients who had a CD4 count between 201–500 cell/mm3 have a 67% decrement in their VL count (β= −0.67, p-value=0.045) as compared to those having a CD4 count greater than 500 cells/mm3 (Table 3).
 |
Table 3 Baseline Variables Associated with Changes in Viral Load Count |
Discussion
In Ethiopia, the current study is one of the first paper which assessed the effect of the EAC program on change in viral load count. Our finding suggests that the change (suppression) of VL count before and after EAC sessions was significant. Thus, an enhanced adherence counseling program may have a substantial effect on improving VL count among unsuppressed VL patients before switching to a second-line regimen. Mainly, the finding of this study support WHO recommendations. WHO and Ethiopian national HIV care and treatment guidelines recommended enhanced adherence counseling sessions for patients with unsuppressedVL count earlier to switching to a second-line drug.3,5 This finding is supported by previous studies done in different parts of Africa.14–16 But the finding of the current study is opposing to a finding from a study done inSouth Africa17 reported EAC has no effect on viral load suppression.
Age is an important determining factor of virological response after ART initiation. HIV infection progress to AIDS more rapidly in older than in younger individuals. In this study, an increase in the age of HIV patients was associated with an increase in VL count. Similarly, previous studies18,19 have reported that the older age of patients is associated with an extensive increase in VL count. Elderly patients may be at greater risk of high viral load replication and poorer response to treatment due to the high incidence of opportunistic infections and other AIDS-related illnesses. Therefore, regular follow-up may help older HIV patients comply with HIV treatment and achieve viral suppression by providing education and counseling to improve medication adherence. However, a study done in South Africa showed that older patients had a good VL suppression than younger patients.20 Besides a follow-up study in the United State of America revealed that older individuals were more likely to achieve viral suppression than younger persons.21
In this study, the urban residence was associated with better VL count suppression as compared to a rural residence. Former evidence revealed that long travel distance has an effect on ARV drug adherence and chronic care visit in the suggested time. Besides, rural residents with HIV infection often face challenges such as stigma, social isolation, limited transportation, and lack of access to care as compared to urban.22,23 This might result in poor VL suppression in the rural community. Consequently, the healthcare provider must support the rural community to adhere to their follow-up visit and to get better VL suppression.
In line with past evidence, the finding of this study showed that PLHIV with a baseline CD4 count between 201–500 cells/mm3 had significantly lower VL count than those with baseline CD4 count >500 cell/mm3. This might be due to the fact that many PLHIV with CD4 count >500cell/mm3 might have entered the EAC sessions with lower VL count compared to their counterpart. Besides, people with baseline CD4 count 201–500 cell/mm3 are at a more advanced stage of the disease and need more time for a reduction in their VL compared with CD4 count >500cell/mm3. Finally, a longer duration on ART was significantly associated with a decline in VL count. This could be associated with improvement CD4 cells over time after ART initiation which results in a better virological response.3,24
The current study has several limitations. First, the analysis was limited to only those variables that are recorded in the patient charts. Second, due to the lack of a comparison group (patients who did not enroll in the EAC program), we can not assure the change in viral load to attribute only to the EAC intervention. Finally, clients who did not have regular follow-up might not have recorded a second VL test result. Hence, there might be a selection bias.
Conclusion
We detected a substantial decline in VL count among patients with an unsuppressed viral load after who after the EAC session. Younger age, urban residence, baseline CD4 count 201 −500, and longer duration on ART were positively associated with the decline (suppression) in VL count over EAC sessions time. The reasons behind the increment in viral load change in older patients are unclear and should be explored in future studies. In the absence of a comparison group, the role of EAC in achieving viral load suppression appears to be questionable. Therefore, future research is still needed to determine if the viral load count suppression is associated with EAC by including patients who did not undergo EAC.
Abbreviations
ART, antiretroviral therapy; BMI, body mass index; EAC, enhanced adherence counseling; HIV, human immunodeficiency virus; WHO, World Health Organization.
Data Sharing Statement
The data can be available from the corresponding author on a reasonable request.
Ethical Approval
Ethical clearance and letter of cooperation for selected hospitals were obtained from the Institutional Review Committee of Woldia University. Our study was based on a retrospective review of secondary data from medical records of patients and as this was not a primary study the Ethical Review Committee of the Woldia University waived the requirement for informed consent. Data were fully anonymized and no personal identifiers, such as name and private information were not collected. Confidentiality during all phases of research activities was kept and data were held on a secured password protected system. This study was conducted in accordance with the Declaration of Helsinki.
Authors’ Information
GD and ML: Department of Public Health, College of Health Science, Woldia University, Woldia, Ethiopia.
Acknowledgment
The authors wish to thank the academic staff of Woldia University, College of Health Science for suggestions and comments on the proposal and result. We would also like to thank Woldia University for giving us the chance to conduct this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by Woldia University.
Disclosure
The authors report no conflicts of interest for this work.
References
1. World Health Organization. March 2014 Supplement to the 2013 Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. 2014.
2. Barnabas G, Sibhatu MK, Berhane Y. Antiretroviral therapy program in Ethiopia benefits from virology treatment monitoring. Ethiop J Health Sci. 2017;27(1):1–2. doi:10.4314/ejhs.v27i1.1S
3. Ethiopia Federal Ministry of Health. National Guideline for Comprehensive HIV Prevention, Care and Treatment. Addis Ababa, Ethiopia: Ethiopia Federal Ministry of Health; 2017.
4. WHO. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. Geneva, Switherland. Recommendations for a Public Health Approach. 2014.
5. HIV/AIDS JUNPo. HIV/Aids JUNPo. 90-90-90: An Ambitious Treatment Target to Help End the AIDS Epidemic. Geneva: Unaids; 2014.
6. Van Dyke RB, Lee S, Johnson GM, et al. Reported adherence as a determinant of response to highly active antiretroviral therapy in children who have human immunodeficiency virus infection. Pediatrics. 2002;109(4):e61–e61. doi:10.1542/peds.109.4.e61
7. Hogg RS, Heath K, Bangsberg D, et al. Intermittent use of triple-combination therapy is predictive of mortality at baseline and after 1 year of follow-up. AIDS. 2002;16(7):1051–1058. doi:10.1097/00002030-200205030-00012
8. Brooks K, Diero L, DeLong A, et al. Treatment failure and drug resistance in HIV-positive patients on tenofovir-based first-line antiretroviral therapy in western Kenya. J Int AIDS Soc. 2016;19(1):20798. doi:10.7448/IAS.19.1.20798
9. Haile D, Takele A, Gashaw K, Demelash H, Nigatu D. Predictors of treatment failure among adult antiretroviral treatment (ART) clients in bale zone hospitals, South Eastern Ethiopia. PLoS One. 2016;11(10):e0164299. doi:10.1371/journal.pone.0164299
10. Telele NF, Kalu AW, Marrone G, et al. Baseline predictors of antiretroviral treatment failure and lost to follow up in a multicenter countrywide HIV-1 cohort study in Ethiopia. PLoS One. 2018;13(7):e0200505. doi:10.1371/journal.pone.0200505
11. World Health Organization. Adapting and Implementing New Recommendation on HIV Patient Monitoring. 2017.
12. Bonnenfant YT, Hindin MJ, Gillespie D. Couple VCT clients in Ethiopia: a heterogeneous HIV risk group. AIDS Care. 2012;24(7):856–865. doi:10.1080/09540121.2011.648601
13. Bonner K, Mezochow A, Roberts T, Ford N, Cohn J. Viral load monitoring as a tool to reinforce adherence: a systematic review. J Acquir Immune Defic Syndr. 2013;64(1):74–78. doi:10.1097/QAI.0b013e31829f05ac
14. Fox MP, Berhanu R, Steegen K, et al. Intensive adherence counselling for HIV-infected individuals failing second-line antiretroviral therapy in Johannesburg, South Africa. Trop Med Int Health. 2016;21(9):1131–1137. doi:10.1111/tmi.12741
15. Bvochora T, Satyanarayana S, Takarinda KC, et al. Enhanced adherence counselling and viral load suppression in HIV seropositive patients with an initial high viral load in Harare, Zimbabwe: operational issues. PLoS One. 2019;14(2):e0211326.
16. Diress G, Dagne S, Alemnew B, Adane S, Addisu A. Viral load suppression after enhanced adherence counseling and its predictors among high viral load HIV seropositive people in north wollo zone public hospitals, northeast Ethiopia, 2019: retrospective cohort study. AIDS Res Treat. 2020;2020.
17. Fox MP, Pascoe SJ, Huber AN, et al. Effectiveness of interventions for unstable patients on antiretroviral therapy in South Africa: results of a cluster‐randomised evaluation. Trop Med Int Health. 2018;23(12):1314–1325. doi:10.1111/tmi.13152
18. Natural History Project writing Group for COHERE. Factors associated with short-term change in HIV viral load and CD4 cell changes in antiretroviral-naive individuals. AIDS. 2014.
19. Carter M. Older Age Associated with Bigger Increase in Viral Load and Falls in CD4 Count in Patient Not Taking HIV Treatment. 2014.
20. Mutevedzi PC, Lessells RJ, Rodger AJ, M-L. N. Association of age with mortality and virological and immunological response to antiretroviral therapy in rural South Africa adults. PLoS One. 2011;6(7):7.
21. Yehia BR, French B, Fleishman JA, et al. Retention in care is more strongly associated with viral suppression in HIV-infected patients with lower versus higher CD4 counts. J Acquir Immune Defic Syndr. 2014;65(3):333. doi:10.1097/QAI.0000000000000023
22. Azia IN, Mukumbang FC, Van Wyk B. Barriers to adherence to antiretroviral treatment in a regional hospital in Vredenburg, Western Cape, South Africa. South Afr J HIV Med. 2016;17(1):1. doi:10.4102/sajhivmed.v17i1.476
23. Mukumbang FC, Mwale JC, van Wyk B. Conceptualising the factors affecting retention in care of patients on antiretroviral treatment in Kabwe district, Zambia, using the ecological framework. AIDS Res Treat. 2017;2017:11. doi:10.1155/2017/7356362
24. Mutevedzi PC, Lessells RJ, Rodger AJ, Newell M-L. Association of age with mortality and virological and immunological response to antiretroviral therapy in rural South African adults. PLoS One. 2011;6(7):e21795. doi:10.1371/journal.pone.0021795
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.