Back to Journals » International Medical Case Reports Journal » Volume 15
Cervical Spondylosis as a Hidden Contributing Factor to Fibromyalgia: A Case Report
Received 19 July 2022
Accepted for publication 15 October 2022
Published 8 November 2022 Volume 2022:15 Pages 639—646
DOI https://doi.org/10.2147/IMCRJ.S382872
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
Eric Chun-Pu Chu,1,2,* Linda Yin-King Lee2,*
1New York Chiropractic & Physiotherapy Center, New York Medical Group, Hong Kong, Hong Kong SAR, People’s Republic of China; 2School of Nursing and Health Studies, Hong Kong Metropolitan University, Hong Kong SAR, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Eric Chun-Pu Chu, New York Chiropractic and Physiotherapy Center, 41/F Langham Place Office Tower, 8 Argyle Street, Hong Kong, Hong Kong SAR, People’s Republic of China, Tel +852-3594-7844, Fax +852-3594-6193, Email [email protected]
Abstract: The present case study describes the long-term symptomatic remission in a patient with fibromyalgia (FM) after multimodal spinal manipulation. A 44-year-old woman presented with a chronic headache, severe neck pain, shoulder pain, and back pain lasting for 2 years after experiencing domestic violence. She had sleep disorders, fatigue, and depressive mood. Her primary care physician diagnosed her with FM and comorbid depression. Despite treatment with non-steroidal anti-inflammatory drugs, muscle relaxants, anti-depressants, anti-epileptics, acupuncture, and aqua-therapy, she experienced no appreciable relief from her symptoms. The patient then sought a chiropractic evaluation and potential treatment for her symptoms. At presentation, widespread tenderness was palpable over the neck, shoulder, back, anterior chest, abdominal wall, and buttock. Radiographs showed loss of cervical lordosis, widespread degenerative spondylosis, and osteitis pubis. Surface electromyography (sEMG) revealed neck and thoracic paraspinal muscular spasms. The patient was diagnosed with FM based on the American College of Rheumatology diagnostic criteria and the associated comorbidities. Multimodal chiropractic approaches, which consisted of spinal manipulation, massage, and intermittent motorized cervical traction, were used twice weekly to relieve soft-tissues and intervertebral joints and stretch core musculatures. The patient’s physical and mental complaints were mostly resolved near the end of 9 months of treatment. Her symptom alleviation was associated with corresponding change in normalized sEMG signal and cervical spine realignment at the 16th- and 26th-month follow-ups. Widespread pain in FM can lead to confused thinking and a lack of awareness of cervical spondylosis. In this example, it is assumed that the noxious cervical inputs triggered an ongoing FM process. Chiropractic treatment blocked noxious inputs coming from pain sources, corrected pain thresholds, and lowered excitability, thereby eradicating FM symptoms.
Keywords: chiropractic, degenerative spondylosis, electromyography, fibromyalgia, neck pain
Introduction
Fibromyalgia (FM) is defined by generalized musculoskeletal pain and tenderness persisting for more than 3 months without an apparent physical cause. In the general population, the prevalence of FM is estimated to be 1% to 5%.1 The underlying pathophysiology of FM is largely unknown. An aberration of pain processing pathways is assumed to be a key mechanism behind FM.2 Factors like neuroendocrine disorders, genetic predisposition, oxidative stress, environmental variables, and psychosocial changes may all predispose people to FM.3
There is currently no widely acknowledge test for diagnosing FM. Instead, FM is diagnosed solely based on a common cluster of symptoms reported by patients.3 According to the revised 2016 American College of Rheumatology (ACR) criteria,4 FM may be diagnosed when any 3 of the following criteria are met: 1) Presence of generalized pain in at least 4 of 5 body regions; 2) Symptoms have been present for at least 3 months; 3) The patient has a widespread pain index (WPI) of ≥7 and a Symptom Severity Scale (SSS) score of ≥5, or the patient has a WPI of 4–6 and SSS score ≥9; and 4) A diagnosis of FM is valid irrespective of other diagnoses. FM is currently coded as an inclusion term under MG30.01 chronic widespread pain in ICD-11. ICD-11 also categorizes FM (chronic widespread pain) as a type of chronic primary pain, which is chosen as pain that has persisted for three months with associated distress and/or functional disability but has no clear underlying condition.5 In disorders like FM, “chronic primary pain” may be regarded as a disease in its own right.6
The European League Against Rheumatism (EULAR)7 modified its recommendations for the pharmacological and non-pharmacological management of FM. Widely prescribed medications for pain or depression, such as non-steroidal anti-inflammatory drugs, monoamine oxidase inhibitors, selective serotonin reuptake inhibitors, growth hormone, and corticosteroid, were not recommended due to ineffectiveness.7 The effects of most non-pharmacological approaches (acupuncture, biofeedback, and manual-type therapies) were limited and “weak for” recommendation. The only “strong for” therapy-based recommendation in the guidelines was exercise therapy (aerobic and strengthening exercises). Exercise is useful for increasing anti-nociceptive neurotransmitters and reducing glutamate.8 Initial treatment options, such as patient education, physical exercise, and cognitive-behavioral therapy, focus on modifying a patient’s activities and beliefs that affect the illness.7,9 Once the noxious focus has been established, further regimes for mechanism-based therapy should be customized to the specific needs of the individual.
In this study, we seek to provide contemporary viewpoints on pain processing in FM. The suppression of nociceptive input by neck manipulative intervention alleviated the headache, spinal pain, and FM associated symptoms in this case. It enables a better understanding of the potential effects of noxious inputs on peripheral and central sensitizations, as well as the complex effects they may have on the development of FM. It is possible to speculate that central sensitization, once established, is a dynamic condition responsible for an amplification of ongoing signals originating from a periphery pain source. Widespread pain in FM can lead to confused thinking and a lack of awareness of cervical spondylosis. Therapeutic interventions should address the issues that have been identified. This case report supports the notion that FM symptoms can be alleviated by lowering the noxious input coming from the peripheral pain sources.10
Case Report
A 44-year-old housewife, a mother of two children, presented with occipital headache, severe neck, shoulder pain, and back pain lasting for 2 years. The patient complained continued depression due to incidence of domestic violence by her husband. She suffered from sleep disturbances and frequently woke up tired, with lingering tiredness. She often experienced uncomfortable bowel symptoms such as abdominal pain and constipation. She had no past surgeries and no history of systemic disorders. There was no family history of neurological or mental disease. After screening blood tests were normal, her physician prescribed non-steroidal anti-inflammatory drugs (ibuprofen) and muscle relaxants (eperisone) to manage her pain but had no appreciable relief from her symptoms. The patient repeatedly sought care from various doctors for over 2 years. Her laboratory tests did not reveal any evidence of rheumatologic or metabolic disorders and her complete blood count was in the normal range. She was diagnosed with FM accompanied by depression and was treated with antidepressants (amitriptyline), anti-epileptics (pregabalin), aqua-therapy, and acupuncture. Due to the lack of effectiveness of the treatments after 6 months, her symptoms continued to impact her everyday life. She discontinued the previous regimens of treatment and sought chiropractic care for treatment of the pain.
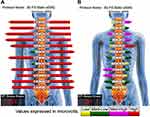
The patient presented with a forward head and rounded-shoulder posture. Physical examination revealed widespread tenderness in 13/18 predefined tender points of FM over the neck, upper chest, shoulder, upper back, buttock, and hip. Muscular hypertonicity was palpable in the bilateral sternocleidomastoid, pectoralis, upper trapezius, supraspinatus, levator scapulae, rhomboid, quadratus lumborum, and gluteal muscles. Intervertebral restriction was palpated at C5-C7, C7-T1, and L4-5 segments. Global range of cervical motion was restricted to 10° in bilateral rotations and extension due to pain. Intensity of the widespread back pain was rated 6 out of 10 on an 11-point numeric pain rating scale, with 0 representing no pain and 10 representing the most severe pain. Manual strength testing in left shoulder abduction, elbow extension, and knee extension was reduced and graded 4/5. Her standing radiographs (Figure 1) showed diffused degenerative spondylosis, reverse cervical lordosis, lumbar hypolordosis, and mild lumbar levoconvexity. Surface electromyography (sEMG) was applied to measure the level of paraspinal muscle tension. In a schematic representation, the myoelectric activity of each measured level is recorded and expressed by color bars.12 sEMG revealed high levels of muscle tension in our patient’s cervical and all spinal segments (Figure 2). The patient was diagnosed with FM with spondylosis, based on the ACR diagnostic criteria4 of pain generalized over multiple body regions (13/18 tender points), severe and long duration of pain, and associated comorbidities. The differential diagnosis of FM includes systemic, rheumatic, and endocrine disorders. The exclusion of other alternative diagnoses can generally be achieved based upon a thorough history, physical examination, and previous laboratory investigations.
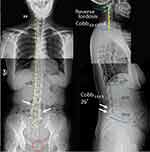 |
Figure 1 Standing plain radiographs at initial presentation. Antero-posterior (left) and lateral neutral (right) views shows disc space narrowing (hollow black arrows), degenerative hypertrophy of facets (white arrows), and anterior marginal lipping of vertebrae that is compatible with degenerative spondylosis. Reverse cervical lordosis and mild lumbar levoconvexity are noted. Joint irregularity, subchondral erosion, and sclerosis of the left pubic symphysis (encircled in red) are likely sequelae of postpartum osteitis pubis. The central sacral line (dashed yellow line) represents the global axis, and the posterior vertebral line (yellow line) highlights the cervical curvature. The reference value for an adult is 38.1°–45.6° for lumbar L1–L5 curve by means of the Cobb method.11 |
Multimodal chiropractic intervention consisted of the following: 1) Spinal manipulation was applied to relieve the restricted vertebral segments of the deformed curvatures, 2) Therapeutic ultrasound and a handheld massage device (Strig®, Korea) were used to relax hypertonic muscles and stabilize the spine, 3) Intermittent motorized cervical traction (MID 4M Series®, WIZ Medical, Korea) was used to decompress the intervertebral spaces by stretching the vertebral segments intermittently, and 4) Strengthening exercises at home. Outpatient visits were conducted 3 times a week for the first 3 months. The patient reported significant relief of headache and neck and back pain, along with improved cervical and lumbar mobility, after the first month of treatment. Her numeric pain scale rating was reduced from 6 to 2 on the 1–10 scale. She described falling asleep more quickly and improved sleep quality. Subsequently, treatment sessions were scheduled twice weekly for an additional 6 months. A personalized home exercise program comprised muscle strengthening, mobility training, and ergonomic advice was provided to avoid triggering the symptoms of FM. The continued treatment also gradually improved her physical symptoms, depressed mood, and lingering tiredness which were then mostly resolved near the end of the 9 months of treatment period. Simultaneously, with the help of a local domestic violence support group, the patient was able to secure a protective order that placed her spouse on a good behavior bond based on her allegations of his abusive behavior.
Thereafter, the patient continued a monthly maintenance care program comprising spinal adjustment, symptom monitoring, home stretching exercise, and ergonomic advice over the next 2 years. Improvements in the patient’s physical and mental functions were associated with corresponding changes on the follow-up radiographs 16 months after the treatment initiation. The patient’s straight neck had been corrected and paraspinal sEMG activity at most spinal levels had returned to baseline myoelectric activities (Figure 2B). A final follow-up visit at 26 months revealed that the corrected effects were maintained (Figure 3) and no treatment-related adverse events were observed. A summary timeline of events for this case is presented in Figure 4.
 |
Figure 3 Comparison of cervical alignment over time in the same patient shown in Figure 1. (A) At the 16th month evaluation, the sagittal Cobb C2-C7 is 3°. The posterior vertebral line (yellow line) highlights the cervical curvature. (B) Repeat radiographs radiograph at the 26th month evaluation demonstrates a regain of cervical lordosis to Cobb 14°. The exostoses in the spinous process C2/C3 interspace and sesamoid ossicles (white arrow) of the nuchal ligament indicate a mechanical stress of the nuchal osteo-ligamentous attachment. |
 |
Figure 4 Clinical timeline of the presenting case. |
Discussion
Fibromyalgia is thought, at least in part, to be a central sensitization at the CNS-level, resulting in a heightened response to normally painful stimuli (hyperalgesia) and a painful response to non-painful stimuli (allodynia).3 Central sensitization has the net effect of recruiting noxious or non-noxious stimuli to nociceptive neurons, resulting in increased or augmented action potential output.13 Functional magnetic resonance imaging (fMRI) of the brain has demonstrated reduced functional connectivity in the descending pain-modulating system and a hyperexcitability in the pain matrix related to central sensitization.14–16 It is believed that maintaining central sensitization requires continual noxious peripheral signals, even in syndromes like fibromyalgia, which are characterized by the absence of identifiable peripheral lesions.17
Over the years, peripheral origins of pain have also been recognized as a possible cause of FM. Approximately 20–35% of the FM patients attribute to peripheral neuropathic origin.18 Primary afferent nociceptors (Aδ and C-fibers) are responsible for conveying the perception of pain to the projection neurons in the dorsal horn of the spinal cord. Following that, subsets of projection neurons in turn transmit the noxious signals up to the brain where a pain perception is generated.19 The dorsal horn of the spinal cord is also responsible for the descending signals from the supraspinal center.19 As a response to tissue injury, chemical inflammatory mediators are produced from the injured tissues. The repetitive exposure to noxious stimuli triggers action potentials that are conveyed bi-directionally, orthodromically (normally) to the spinal cord and antidromically (oppositely) away from the cord to the peripheral terminal.20 Pathologically, an antidromic conduction of noxious signals generates a substantial excitation in the terminal of the C-fiber, inducing terminal neurotransmitters to be released at the injured site and activating the surrounding nociceptors. Thus, antidromic conduction can cause a widespread activation throughout the entire peripheral receptive field of the axon.20 This process is known as peripheral sensitization, which is a reduction in spike threshold and amplification in the responsiveness of peripheral sensory nerve fibers for an external stimulation.13,21 Structural abnormalities of C-fibers and altered C-fiber efferent function might play a role in FM.18
There may be multiple subsets of FM with different etiopathogenesis. Although central sensitization plays an important role in FM, it is even more important to understand the initial cause, that is, the persistent nociceptive input associated with tissue injury, including contributors to inflammatory pain.3 For instance, manipulative intervention improved the physical and mental complaints of this patient, suggesting that her cervical nociceptive aberration bypassed the interconnected pathways in the complex mechanism of FM. Peripheral abnormalities may contribute to increased nociceptive tonic supply in the spinal cord, resulting in central sensitization.3 Nakajima et al reported a 37-year-old man who underwent an open reduction for a left femoral neck fracture that gradually developed laryngopharyngeal paresthesia and severe upper back pain. He was diagnosed with FM according to the ACR diagnostic criteria and underwent medication, rehabilitation, and trigger point lidocaine injections, but his symptoms remained unchanged. One year later, the patient underwent caudal epidural blocks once a month for a total of four blocks. He reported that his back pain and laryngopharyngeal paresthesia were relieved for about 3 weeks after each nerve block. Based on those results, the authors hypothesized that pain sensation is typically associated with an injury of somatic structures; for patients with FM, pain may be felt in uninjured and in unrelated innervated areas.22
According to Vierck, if the peripheral source of pain is successfully blocked, the symptoms of FM will disappear or even not develop in the first place.10 A clinical study was undertaken in 18 patients with chronic whiplash-related disorders to investigate the perceptive change suggestive of sensory hypersensitivity. Following medial branch block procedure of the cervical spine, all patients experienced at least an 80% reduction in the intensity of their familiar neck pain, with significant differences in pressure and cold pain thresholds of all remote sites.23 It was postulated that a nerve block cuts down nociceptive input into the CNS with subsequently reduced excitability of CNS neurons and/or facilitation of inhibitory pathways.23
Chronic pain and psychiatric disorders frequently co-occur. Chronic pain can trigger stress and anxiety for patients. Distress can lead to physical symptoms such as headache, chest pain, upset stomach, and hypertension. Pain and mental health effects are inextricably linked and leave patients trapped in a vicious cycle. A mental health surveillance study24 used face-to-face interviews of 367 Japanese people with chronic pain and found an extremely high rate (95%, n=347) of co-occurring psychiatric disorders according to DSM-IV-TR criteria.25 Among them, psychiatric disorders were found in 96.9% of the patients (n=134) with FM and in 93.5% of the others (n=213) without FM. There was not a statistically significant difference between the two groups using a chi-square test.24 In an fMRI brain study,26 symptoms of depression corresponded to the magnitude of pain-evoked neuronal activations in the amygdala and contralateral anterior insula, which are the areas of the brain associated with affective pain processing.
In addition to pain, sleep disorders, mood issues, and fatigue are described as some of the most bothersome symptoms in FM. Depression itself can cause fatigue and inactivity, weakening the core muscles. A consequent morphological modification of the spine alters the load on the intervertebral joints, discs, and ligaments of the back, resulting in back pain, muscle strains and degenerative changes. Open-ended interviews conducted on 40 adults with FM found that 77.5% (n=31) experienced tiredness/lack of energy/fatigue that did not go away, even after sleep or rest.27 While no evident structural pathology has been found in the muscles of patients with FM, the self-reported feeling is generally thought to be caused by the CNS. According to the mechanism, fatigue is possibly due to metabolic changes in muscle tissue.3 Microdialysis studies of the muscle interstitial (extra cellular) fluid, where nociceptor free nerve endings terminate, have shown increased concentrations of metabolites (glutamate, pyruvate, and lactate) and serotonin, indicative of increased anaerobic muscle metabolism in patients with FM.28,29 Mental stress can increase muscle tone in both healthy individuals and patients with FM.29
Electromyography (EMG), the recording of electrical activity in muscles, is regarded as a functional assessment of specific muscle groups. sEMG captures gross myoelectric activity rather than individual motor units, it aims at assessing functional integrity and performance in both static and dynamic states.30 A sEMG study29 recorded activity of the bilateral upper trapezius, biceps brachii, and erector spinae muscles and showed that patients (n=51) with FM had higher resting muscle tension (EMG amplitude) and shorter EMG rest time values compared to the controls (n=31). The results were indicative of higher anticipatory muscle tension in the patients with FM than in the controls.29 Thus, the inability to relax in patients with FM could be attributed to the higher baseline activity and is consistent with the reduced rest time observed in the sEMG study by Zetterman et al. In the present case, the patient experienced significant relief of headache, neck and back pain, along with improved cervical and lumbar mobility, after the first month of chiropractic care. The patient gradually achieved complete resolution of physical and mental symptoms near the end of 9 months of treatment. Physical function improvements were associated with corresponding changes in follow-up radiography and sEMG. These findings are in concordance with the hypothesis that blocking the ongoing noxious stimulation from injured tissues can alleviate the symptoms of FM.10,22
This study is limited by its clinical diagnosis eminently relying on patient reporting and physician assessment. Radiography and sEMG are ancillary techniques merely for the evaluation of physical changes, but there is a lack of scientific testing for determining the presence of pain and mental symptoms. Furthermore, multimodal interventions were used to treat the patient. Therefore, distinguishing the exact efficacy between chiropractic approaches, massage, and strengthening exercises is impossible. The mechanisms of symptom alleviation merit additional exploration. The strength of this study is to provide evidence that FM is a dynamic condition responsible for an amplification of ongoing stimuli. Alleviating the noxious input coming from the peripheral sources of pain can end the symptoms of FM.
Conclusion
FM is a disorder in pain modulation/processing. Central and peripheral sensitizations are certainly contributing factors to FM. It is more critical to determine the ongoing processing of noxious stimuli in injured tissues that contributes to pain enhancement in central and peripheral sensitizations. Due to the complexity and multifaceted character of FM, a mechanism-based intervention should be tailored to each patient to address the specific issues identified.
Abbreviations
ACR, American College of Rheumatology; CNS, central nervous system; DSM-IV-TR, Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision; EULAR, European League Against Rheumatism; FM, fibromyalgia; fMRI, functional magnetic resonance imaging; IASP, International Association for the Study of Pain; sEMG, surface electromyography; SSS, symptom severity scale; WPI, widespread pain index.
Ethics Approval and Informed Consent
Written informed consent for publication of the clinical details and clinical images was obtained from the patient. Ethical approval for case report is not required as it is not considered a human medical research.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Kang JH, Choi SE, Park DJ, Lee SS. Disentangling diagnosis and management of fibromyalgia. J Rheum Dis. 2022;29(1):4–13. doi:10.4078/jrd.2022.29.1.4
2. Harte SE, Harris RE, Clauw DJ. The neurobiology of central sensitization. J Appl Behav Res. 2018;23(2):e12137. doi:10.1111/jabr.12137
3. Siracusa R, Paola RD, Cuzzocrea S, Impellizzeri D. Fibromyalgia: pathogenesis, mechanisms, diagnosis and treatment options update. Int J Mol Sci. 2021;22(8):3891. doi:10.3390/ijms22083891
4. Wolfe F, Clauw DJ, Fitzcharles MA, et al. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum. 2016;46(3):319–329. doi:10.1016/j.semarthrit.2016.08.012
5. Nicholas M, Vlaeyen JWS, Rief W, et al.; IASP Taskforce for the Classification of Chronic Pain. The IASP classification of chronic pain for ICD-11: chronic primary pain. Pain. 2019;160(1):28–37. doi:10.1097/j.pain.0000000000001390.
6. Treede RD, Rief W, Barke A, et al. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the International Classification of Diseases (ICD-11). Pain. 2019;160:19–27. doi:10.1097/j.pain.0000000000001384
7. Macfarlane GJ, Kronisch C, Dean LE, et al. EULAR revised recommendations for the management of fibromyalgia. Ann Rheum Dis. 2017;76(2):318–328. doi:10.1136/annrheumdis-2016-209724
8. Bobinski F, Ferreira TAA, Cordova MM, et al. Role of brainstem serotonin in analgesia produced by low-intensity exercise on neuropathic pain after sciatic nerve injury in mice. Pain. 2015;156:2595–2606. doi:10.1097/j.pain.0000000000000372
9. Friedberg F, Williams DA, Collinge W. Lifestyle-oriented non-pharmacological treatments for fibromyalgia: a clinical overview and applications with home-based technologies. J Pain Res. 2012;5:425–435. doi:10.2147/JPR.S35199
10. Vierck CJ
11. Furlanetto TS, Sedrez JA, Candotti CT, Loss JF. Reference values for Cobb angles when evaluating the spine in the sagittal plane: a systematic review with meta-analysis. Motricidade. 2018;14(2–3):115–128. doi:10.6063/motricidade.10890
12. Chu ECP. Preventing the progression of text neck in a young man: a case report. Radiol Case Rep. 2022;17(3):978–982. doi:10.1016/j.radcr.2021.12.053
13. Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10(9):895–926. doi:10.1016/j.jpain.2009.06.012
14. Ioachim G, Warren HJM, Powers JM, Staud R, Pukall CF, Stroman PW. Altered pain in the brainstem and spinal cord of fibromyalgia patients during the anticipation and experience of experimental pain. Front Neurol. 2022;13:862976. doi:10.3389/fneur.2022.862976
15. Jensen KB, Loitoile R, Kosek E, et al. Patients with fibromyalgia display less functional connectivity in the brain’s pain inhibitory network. Mol Pain. 2012;8:32. doi:10.1186/1744-8069-8-32
16. Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46(5):1333–1343. doi:10.1002/art.10225
17. Lee YC, Nassikas NJ, Clauw DJ. The role of the central nervous system in the generation and maintenance of chronic pain in rheumatoid arthritis, osteoarthritis and fibromyalgia. Arthritis Res Ther. 2011;13(2):211. doi:10.1186/ar3306
18. Grayston R, Czanner G, Elhadd K, et al. A systematic review and meta-analysis of the prevalence of small fiber pathology in fibromyalgia: implications for a new paradigm in fibromyalgia etiopathogenesis. Semin Arthritis Rheum. 2019;48(5):933–940. doi:10.1016/j.semarthrit.2018.08.003
19. Yam MF, Loh YC, Tan CS, Khadijah Adam S, Abdul Manan N, Basir R. General pathways of pain sensation and the major neurotransmitters involved in pain regulation. Int J Mol Sci. 2018;19(8):2164. doi:10.3390/ijms19082164
20. Sorkin LS, Eddinger KA, Woller SA, Yaksh TL. Origins of antidromic activity in sensory afferent fibers and neurogenic inflammation. Semin Immunopathol. 2018;40(3):237–247. doi:10.1007/s00281-017-0669-2
21. Wei S, Tao Z, Xue Y, Cao D. Peripheral sensitization. In: Turker H, Benavides LG, Gallardo GR, Villar MMD,editors. Peripheral Nerve Disorders and Treatment. London: IntechOpen; 2019. 10.5772/intechopen.90319
22. Nakajima F, Aratani S, Fujita H, et al. A case of fibromyalgia involving pain throughout the body treated with site-specific targeted pain control. SpringerPlus. 2016;5(1):1027. doi:10.1186/s40064-016-2572-z
23. Schneider GM, Smith AD, Hooper A, et al. Minimizing the source of nociception and its concurrent effect on sensory hypersensitivity: an exploratory study in chronic whiplash patients. BMC Musculoskelet Disord. 2010;11:29. doi:10.1186/1471-2474-11-29
24. Miki K, Nakae A, Shi K, et al. Frequency of mental disorders among chronic pain patients with or without fibromyalgia in Japan. Neuropsychopharmacol Rep. 2018;38:167–174. doi:10.1002/npr2.12025
25. Kawa S, Giordano J. A brief historicity of the diagnostic and statistical manual of mental disorders: issues and implications for the future of psychiatric canon and practice. Philos Ethics Humanit Med. 2012;7:2. doi:10.1186/1747-5341-7-2
26. Giesecke T, Gracely RH, Williams DA, Geisser ME, Petzke FW, Clauw DJ. The relationship between depression, clinical pain, and experimental pain in a chronic pain cohort. Arthritis Rheum. 2005;52:1577–1584. doi:10.1002/art.21008
27. Humphrey L, Arbuckle R, Mease P, Williams DA, Samsoe BD, Gilbert C. Fatigue in fibromyalgia: a conceptual model informed by patient interviews. BMC Musculoskelet Disord. 2010;11:216. doi:10.1186/1471-2474-11-216
28. Gerdle B, Ernberg M, Mannerkorpi K, et al. Increased interstitial concentrations of glutamate and pyruvate in vastus lateralis of women with fibromyalgia syndrome are normalized after an exercise intervention - A case-control study. PLoS One. 2016;11(10):e0162010. doi:10.1371/journal.pone.0162010
29. Zetterman T, Markkula R, Partanen JV, Miettinen T, Estlander AM, Kalso E. Muscle activity and acute stress in fibromyalgia. BMC Musculoskelet Disord. 2021;22(1):183. doi:10.1186/s12891-021-04013-1
30. McManus L, De Vito G, Lowery MM. Analysis and biophysics of surface EMG for physiotherapists and kinesiologists: toward a common language with rehabilitation engineers. Front Neurol. 2020;11:576729. doi:10.3389/fneur.2020.576729
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.