Back to Journals » Clinical Interventions in Aging » Volume 16
Cervical Ossification of Ligamentum Flavum: Elaborating an Underappreciated but Occasional Contributor to Myeloradiculopathy in Aging Population Based on Synthesis of Individual Participant Data
Authors Zhang B , Chen G, Chen X, Sun C, Chen Z
Received 30 March 2021
Accepted for publication 27 April 2021
Published 24 May 2021 Volume 2021:16 Pages 897—908
DOI https://doi.org/10.2147/CIA.S313357
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zhi-Ying Wu
Baoliang Zhang,1– 3 Guanghui Chen,1– 3 Xi Chen,1– 3 Chuiguo Sun,1– 3 Zhongqiang Chen1– 3
1Peking University Third Hospital, Department of Orthopaedics, Beijing, 100191, People’s Republic of China; 2Engineering Research Center of Bone and Joint Precision Medicine, Beijing, 100191, People’s Republic of China; 3Beijing Key Laboratory of Spinal Disease Research, Beijing, 100191, People’s Republic of China
Correspondence: Zhongqiang Chen
Orthopaedic Department, Peking University Third Hospital, No. 49 North Garden Road, Haidian District, Beijing, 100191, People’s Republic of China
Tel +86 10 13801132346
Fax +86 10 82267368
Email [email protected]
Purpose: Cervical ossification of ligamentum flavum (COLF) is a rare clinical entity which can occasionally contribute to severe myeloradiculopathy. Many orthopedists are unfamiliar with or underestimate this pathology. Therefore, a comprehensive research is obligatory to reappraise the epidemiological, radiological, clinical and histopathological characteristics of COLF-myeloradiculopathy based on synthesis of individual patient data.
Methods: Following Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines, PubMed, EMBASE, Scopus and Web of Science databases were searched for studies discussing COLF-myeloradiculopathy from the inception to December 2020.
Results: A total of 94 cases from 54 studies were identified. The annual publications demonstrated a steady increase, and most reports were from Japan and China. The mean age was 58.76± 13.39 years and nearly 60% of cases occurred in the 55– 64 and 65– 74 years age group. The male-female ratio was 1.4:1. Most cases belonged to East Asian population (60.64%). COLF predominately appeared in the lower cervical and cervicothoracic spine (76.60%) and mainly affected C4-5 (23.29%) and C5-6 (21.23%). Single-segment type ossification accounted for 62.76 and 45.45% of ossification lesions distributed bilaterally. The majority of COLF (81.1%) were spontaneous, and motor disturbance (76.4%), spinal ataxia (62.5%) and sensory disturbance (58.9%) were the most common manifestations. Histopathologically, it’s a metaplastic process of endochondral ossification with the formation of mature lamellar bone which was distinguished from calcification of ligamentum flavum. About 21.28% of concurrent COLF and COPLL cases were identified as a separated group, with unique characteristics.
Conclusion: COLF is an underappreciated but potentially growing pathogeny of myeloradiculopathy in aging population, though its distinct epidemiological, radiological, clinical and histopathological features are not fully supported by current evidence. However, our findings will provide several referential data for future researches to shed light on COLF.
Keywords: ossification of ligamentum flavum, cervical myelopathy, semeiology, epidemiology, radiography, histopathology
Introduction
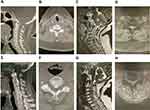
Ossification of the ligamentum flavum (OLF) is a relatively rare clinical entity secondary to a combination of intrinsic and extrinsic factors, which can occur in all parts of the spine, particularly in the thoracic spine but rarely in the cervical region.1,2 In 1962, Koizumi et al first reported a 55-year-old male patient with cervical OLF (COLF) at C6-7 presenting with progressive quadriparesis (Figure 1A–D).3 Subsequently, advanced imaging modalities, especially computed tomography (CT) and magnetic resonance imaging (MRI), increased the detectable rate of asymptomatic COLF.4 Meanwhile, several symptomatic cases who required surgical intervention have been taken seriously and increasingly studied since 1980s as a cause of acquired cervical myelopathy.5–8 Thence, this pathology was no longer overlooked in clinical practice, though its etiology and pathogenesis have not been conclusively established.
The prevalence and demographic characteristics of COLF remains obscure due to insufficient controlled epidemiological data in current literature. A Japanese scholar reported the relative incidence of asymptomatic OLF in the cervical spine was approximately 0.9%.9 Al-Jarallah et al found the prevalence of COLF was 10.8% among a consecutive series of 102 Arab hospitalized patients, and the male-female ratio was 2.7:1.10 Recently, Liang et al conducted an whole-spine CT-based epidemiological survey involving 2000 Chinese population, and detected only 5 COLF subjects (0.3%).11 Additionally, whether geographical and ethnic distribution of COLF are unique or consistent with TOLF remains unknown.
Progressive COLF can contribute to chronic myeloradiculopathy and an evolving quadriparesis.12 As other myelopathies derived from cervical spondylosis or OPLL, it is refractory to conservative management, and surgical decompression is recommended.13 Moreover, the coexistence of OLF and OPLL in the cervical spine, another extremely rare phenomenon, was scatteredly reported (Figure 1E–H).14–16 The prevalence of this phenomenon in the latest epidemiological study could be calculated as only 0.2%.17 Different ossification lesions have common pathologic features, while simultaneously holding unique traits specific to each entity. Therefore, it is also essential to elucidate its epidemiological and clinical characteristics for targeted surgical strategies.
Due to its rarity, the majority of published studies on COLF were case reports or case series, underpowered to make a comprehensive understanding and appraisal of this entity so many physicians are unfamiliar with or overlook it. To the best of our knowledge, there has been no systematic research on this issue. Therefore, we considered it of interest to conduct a comprehensive reappraisal through synthesizing all published individual patient data of COLF in the current literature, aimed to thoroughly summarize its epidemiological, radiological, clinical and histopathological characteristics.
Materials and Methods
Search Strategy
This systematic research was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.18 Potentially relevant literature was identified through searching PubMed, EMBASE, Cochrane library and Web of Science databases from the inception to December 2020. The search strategy included combinations of the terms “cervical ossification of ligamentum flavum” “cervical ossification of yellow ligament” “ossified ligamentum flavum AND cervical” and “ossified yellow ligament AND cervical” either as free words, keywords or as Medical Subject Heading (MeSH) terms. The reference lists of retrieved articles were manually searched for relevant articles.
Inclusion and Exclusion Criteria
Articles considered for full review met the following inclusion criteria: (i) examined adults with a clinically defined diagnosis of COLF based on preoperative imageological findings, intraoperative identification and/or postoperative histopathological examinations, (ii) reported epidemiological, radiological, clinical or histopathological data, (iii) Prospective clinical trials, retrospective studies, case series and case reports, and (iv) were published in English in a peer-reviewed journal. Cadaver studies, animal studies and reviews, studies with no abstract or full-text available, those considered irrelevant for the topic of this article, those written in other languages were excluded.
Data Extraction
The following data at individual patient level from eligible studies were extracted: (i) literature characteristics (eg country, publication year, study design), (ii) demographic characteristics (eg sex, age, ethnicity), (iii) radiological characteristics (eg affected segments, ossification type, concomitant OPLL), (iv) clinical characteristics (presenting symptoms, disease duration, management), and (v) histopathological characteristics (HE staining, Masson staining).
Statistic Analysis
A systematic, narrative approach was utilized for this research. Continuous variables were presented as means with standard deviations and categorical variables as frequencies and percentages (%). t-test or chi-square test was used to compare different groups, and p values <0.05 were considered statistically significant. All analyses were performed using IBM SPSS (v22.0, Armonk, NY: IBM Corp).
Results
Literature Characteristics
The successive steps of the literature search are summarized in Figure 2. A total of 94 cases of COLF were finally identified in 54 eligible studies, including 48 case reports, 5 case series and 2 clinical studies. The number of patients in reported studies ranged from one to fifteen. The articles were published between 1962 and 2020. The number of published articles, when grouped by publication year into 10-year intervals from 1960 to 2020, demonstrated a steady increase from 1960~1969 (1 article, describing 1 case) to 2010~2020 (21 articles, describing 49 cases) except for that of 1990–1999 (Figure 3A). Most articles were published by Asian institutions (n=37, describing 77 cases), followed by European (n=5, 13 cases), North America (n=3, 3 cases), South American (n=1, 1 case) centers. All papers were published in 13 countries and the top 4 were Japan, China, India and the USA (Figure 3B). The regional distribution of cases reported was plotted on a world map (Figure 3C).
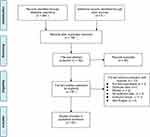 |
Figure 2 Flow chart of the selection of studies included in this systematic review. |
Epidemiological Characteristics
The mean age of patients (data available in 94 cases) was 58.76±13.39 years (range, 27–84 years). The mean age of female (60.00±14.13 years) was older than that of female (57.87±12.90 years), although this difference was not significant (P= 0.224) (Figure 4A). In the age interval 25–34, 35–44, 45–54, 55–64, 65–74, and ≥75 year, COLF was present in 5 cases (5.32%), 9 cases (9.57%), 16 cases (17.02%), 29 cases (30.85%), 26 cases (27.66%), 9 cases (10.64%) (Figure 4B). Gender was specified in 94 cases, 55 individuals (58.51%) were men and 39 were women, at a ratio of 1.4:1 (Figure 4C). According to the ethnic distribution, most cases belonged to East Asian population (60.64%), followed by Caucasians (12.77%) and African descents (12.77%), Arab (11.71%) and Indian (2.13%) (Figure 4D).
Radiological Characteristics
Like TOLF, COLF can also be manifested as single-segment distribution (SSD, Figure 5A and D), two-segment distribution (TSD, Figure 5B and E) and multiple-segment distribution (MSD, Figure 5C and F) on T2-weighted sagittal MRI. In addition, according to the location of ossification lesions in the lamina, COLF could be observed in the central site (CSL, Figure 6A and E), right side (RSL, Figure 6B and F), left side (LSL, Figure 6C and G) and bilateral side of the lamina (BSL, Figure 6D and H) on axial CT images. A total of 146 affected segments in all 94 cases were documented, in which SSD, TSD and MSD ossification accounted for 62.76%, 26.60% and 10.64%, respectively (Figure 7A). Ossification sites on axial CT images were described in 58 cases with 88 levels. CSL, RSL, LSL and BSL ossification accounted for 15.91%, 19.32%, 45.45% and 19.32%, respectively (Figure 7B). The average number of affected interlaminar segments was 1.6. No difference in the number of affected interlaminar segment was observed with respect to gender (P=0.075). Ossification lesions involving the lower cervical (C4-C7) and cervicothoracic region (C7-T1) accounted for 76.6% while those affecting upper cervical spine (C1-C4) accounted for 39.36%. Of all 146 affected segments, the most common level was C4-5 (23.29%, n = 34), followed by C5-6 (21.23%, n = 31), C6-7 (16.44%, n = 24), and C3-4 (15.07%, n = 22) (Figure 7C). On T2-weighted sagittal MRI, the signal of ossified focus may be isointense, hypointense, or both, and an accompanying intramedullary hyperintense signal could be observed in about 10% of cases (9/94).
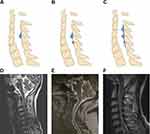 |
Figure 5 COLF distribution on T2-weighted sagittal MRI. (A and D) Single-segment distribution; (B and E) two-segment distribution; (C and F) multiple-segment distribution. |
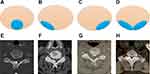 |
Figure 6 COLF location on Axial CT views. (A and E) Central site of the lamina; (B and F) left side of the lamina; (C and G) right side of the lamina; (D and H) bilateral side of the lamina. |
Concurrent Characteristics of COLF and COPLL
The cohort of 20 cases with the coexistence of OLF and OPLL was analyzed as a separate group with the prevalence of 21.28%. The mean ± SD of patients’ age was 59.35±11.01 years, and the male-to-female ratio was 4:1. The mean age of female (45.50±7.92 years) was significantly younger than in that of male (62.81±8.71 years). Moreover, nine (45%) patients were Japanese, five Kuwaitians (25%), two Indians (10%), two Chinese (10%), one South Korean (5%) and one African American (5%). In this group, OPLL was mainly located in C3/4, followed by C4/5 and C5/6 while OLF was mostly involved in C2/3 and C3/4 (Figure 7D). In addition, the number of affected segments of OPLL was significantly greater than that of OLF. Furthermore, COLF tends to occur at the same or neighboring segment of the OPLL margin.
Clinical Characteristics
The majority (81.1%) were spontaneous COLF. Only 4 patients had a previous history of trauma (37 cases available). Moreover, 2 cases were triggered by hypercalcemia and one case by skeletal fluorosis. Average symptomatology duration was 20 months (range, 1–90 months) in reported 55 patients. The 7 clusters of presenting symptoms were summarized in 56 subjects, including motor disturbance (76.4%, n=47), spinal ataxia (62.5%, n=35) and sensory disturbance (58.9%, n=33), bowel and bladder dysfunction (19.6%, n = 11), neck and shoulder discomfort (12.5%, n = 7), radiculopathy and bowel and bladder dysfunction (6.25%, n = 3) (Figure 8). Rare manifestations were also reported such as Brown-Sequard syndrome (2.22%, n = 1) and cough (2.22%, n = 1). The treatment modalities were referred in 64 patients. Only 4 cases received no specific treatment but a wait and watch strategy whereas the other (93.75%) were treated surgically via a posterior approach including laminoplasty, laminectomy, and laminectomy with fusion. Dural adhesion was seen intraoperatively in 22 patients, among which only two were described as dural ossification. Only one patient suffered cerebrospinal fluid leakage (CSF). Follow-up information was available for 50 patients, with an average of 16.4 months (ranged 1–70 months). At the last follow-up, all patients presented with improvement in subjective symptoms or neurological function to a complete or partial degree postoperatively.
 |
Figure 8 The category and frequency of presenting symptoms of COLF patients included. |
Histopathological Characteristics
Histopathologic examinations of surgical specimens were conducted in 24 cases, including Hematoxylin-Eosin staining and Masson staining. The majority of pathologic findings showed metaplastic process of endochondral ossification, in which characteristics of various stages can be observed in adjacent areas. Generally, fiber area showed compact elastic bundles compatible with ligaments at the edge. Fibrocartilage area was identified as well-developed yellow ligament tissue combined in part with chondrocytes and cartilaginous change. Calcified cartilage area referred to large areas of calcified granules or crystalline deposits within degenerative fibrous tissue. Ossified area was characterized by lamellar bone, mature bony trabeculae and bone marrow formation within the bony tissue. In addition, nodules of giant cells and granulomatous reaction were observed occasionally.
Discussion
To the best of our knowledge, this is the first systematic review based on synthesis of published individual patient data for comprehensive reappraisal of COLF, including its epidemiological data, imaging findings, clinical features, and histopathological characteristics. In this study, a total of 94 cases diagnosed with COLF could be identified from the inception to 2020 all over the world, meaning that the clinical entity was as rare as previously reported. However, we considered the real number of cases might be grossly underestimated by the current data. It’s worth noting that, when stratified by the 10-year interval, the number of published articles and cases has obviously risen from 1960–1969 interval to 2010–2020 interval. This might be explained by the following reasons: an actual increase in prevalence of COLF; an growing awareness/enthusiasm for research on COLF; an overall expansion in relevant medical publications. However, no sufficient evidence is available to confirm these speculations. We hypothesized that an increase in the number of symptomatic COLF patients might be a real fact not attributable to literature publication bias or research interests alone, considering the global increase in population with obesity, diabetes, metabolic syndrome, and prolonged life expectancy, which were all positively associated with the occurrence and progression of COLF.19 In addition, we found East Asian countries, especially Japan, produced far more articles about COLF than other countries, which might reflect a geographically isolated distribution of COLF, just like OPLL or TOLF with a distinct predisposition in Japanese.20
Epidemiological and etiology characteristics of COLF remain obscure. Although the current literature does not clearly identify the true prevalence of COLF within the population at large, notable studies involving Japanese and Chinese patients reported the prevalence of 0.3%-0.9%.9,11 In addition, the phenomenon that OLF is less prevalent in cervical region than thoracic region may derive from different regional anatomies and mechanical stresses. On the one hand, an absent or lessened capsular portion of the ligamentum flavum in the cervical spine results in the fact that it does not adhere to the bony tissue directly. On the other hand, the mobility of cervical segments is greater than that of thoracic segments, in which the tensile forces are always static, thus appropriate stress stimulation may induce the process of local ossification.21 Moreover, we found more than half of cases were East Asian population, and the remaining distributed sporadically in other ancestral backgrounds, which might potentially be related to genetic factors. However, a recent bioarchaeology study showed that the prevalence of TOLF and LOLF was 83.2% (273/328) and 28.6% (88/308) in a mid-nineteenth-century European population sample, but no cases were observed in any cervical vertebrae.22 They thought that OLF was likely to be an understudied rather than rare condition in European populations, and these findings should be validated in a clinical scenario. When grouped by age, we found nearly 70% of cases were old than 55 years old, and the male-female ratio of COLF patients was 1.4:1, which implied that COLF might be associated with aging degeneration and slight male preponderance.
Representative COLF can be visualized by CT and MRI while radiography lacks the diagnostic sensitivity for OLF and myelography is usually not required in the present era.23 In this study, we found COLF was mostly presented in one-level involvement, which was not consistent with TOLF. Lang et al found 70.7% of 703 Chinese patients with TOLF were multi-segmented.24 Another epidemiology study by Mori et al revealed the proportion of single-level and multi-level involvement was nearly equivalent in 1094 TOLF cases.25 In addition, ossification lesions were most frequent in the lower cervical and cervicothoracic spine, and predominately affected the C4/5 and C5/6, which reflected degenerative factors might be related to the progression of COLF as other degenerative cervical diseases.26,27 Moreover, noncentral type was more common than central type. Histologically, the ligamentum flavum extends laterally from the midline of vertebral laminae to the intervertebral foramen, which can explain the high-frequency of noncentral type.28 However, it was still filled with confusion how the central type arises at the center of laminae where the ligamentum flavum becomes the thinnest and grows restrictedly towards the spinal canal. From my point of view, the alteration of local stress might be a vital causal factor.
The common symptoms of COLF patients were highly consistent with those typical manifestations of cervical myelopathy, including motor disturbance, spinal ataxia and sensory disturbance. Generally, the majority of the patients presented with more than one symptoms. Like other pathology causing cervical myelopathy, COLF patients usually present first with sensory deficits, followed by limb weakness, and later gait disturbance.29 Considering that unilateral type accounted for nearly 30%, radiculopathy caused by COLF, although rarely, should not be ignored in clinical practice. Interestingly, we found three cases with idiopathic hypercalcemia or skeletal fluorosis presenting continuous long-segment ossification, which revealed hypercalcemia and skeletal fluorosis might contribute to the development of diffuse ossification.30–32 Moreover, previous studies found diabetes mellitus, hyperinsulinism, obesity, hemochromatosis, X-linked hypophosphatemia, and hypoparathyroidism were associated with the spinal ligament ossification,33–36 so clinicians should pay more attention to these metabolism-related factors.
Various immunohistochemical examinations were used to shed light on the histopathological features and molecular mechanism of COLF postoperatively. Clinically, calcification of the ligamentum flavum (CLF) is another entity in the cervical spine, easily confused with OLF. Histologically, CLF is characterized by crystals of calcium pyrophosphate dihydrate or calcium hydroxyapatite rather than mature bone formation in the ligamentum flavum, which is distinguished with OLF.5,37 Anatomically, CLF is not contiguous with or only partially in contact with the laminae.38 Epidemiologically, CLF has a female-predisposition and predominantly lies in the cervical spine, whereas OLF often happens in males and mainly occurs in the thoracic regions.39 These distinctions indicated that the formation of OLF and CLF has different etiology and pathogenesis.
The coexistence of COLF and COPLL is so extremely rare that no study has reported its population-based incidence or prevalence. Kawaguchi et al investigated whole-spine CT of 117 COPLL patients, and found 64.6% of all patients had OLF at any levels of the whole spine except for cervical regions.40 Moreover, several unique characteristics were observed in reported cases. First, patients with the coexistence of OLF and OPLL showed a more distinct male-domination; Second, male patients were associated with more advanced age than female patients. Third, the affected locations of COPLL mainly ranged from the C3-4 to C5-6, and COLF tended to occur at the OPLL margin or the segment close to the end of the OPLL. Finally, COPLL affected more segments than COLF. Posterior longitudinal ligaments spread across multiple intervertebral levels but ligamentum flavum spans a single intervertebral level.26 Unfortunately, further statistical analysis failed to be conducted to reveal the correlation among separate variables due to limited data. Anyway, these findings will provide certain references for future researches.
This study has some limitations. First, the retrospective selection of case reports prevented us from investigating the prevalence of COLF. Second, data derived from case reports or case series were usually conducted within specialized center, which inevitably contribute to reporting bias and selection bias. Third, individual case series might not provide the presentation of collective characteristics of the disease with variable impact on the results. Fourthly, the publication time range of the included papers is longer than 60 years. Finally, several analyses were performed by necessity only in subgroups of patients, because of missing data for the specific variables in the respective publications. Therefore, these findings should be interpreted with caution.
Conclusion
This study comprehensively reappraised the state of the art on COLF for the first time, and preliminarily analyzed its epidemiological, radiological, clinical and histopathological characteristics based on the synthesis of individual patient data, which provided momentous referential data for understanding and cognition of this rare condition. Taken together, COLF may be an increasing and underappreciated cause of myeloradiculopathy in aging population. However, current evidence failed to fully lift the veil of COLF, and future large clinical registration and multicenter collaborations offering better quality data might provide additional information about this entity.
Abbreviations
OLF, ossification of ligamentum flavum; COLF, cervical ossification of ligamentum flavum; CT, computed tomography; MRI, magnetic resonance imaging; OPLL, ossification of posterior longitudinal ligament; TOLF, thoracic ossification of ligamentum flavum; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; MeSH, Medical Subject Heading; CSF, cerebrospinal fluid leakage; CLF, calcification of ligamentum flavum.
Acknowledgments
There were no other contributors to the article than the Authors as well as there was no writing assistance required.
Funding
Our study was supported by National Natural Science Foundation of China (82072479, 81772381).
Disclosure
The authors have declared no conflicts of interest.
References
1. Kim SI, Ha KY, Lee JW, et al. Prevalence and related clinical factors of thoracic ossification of the ligamentum flavum-a computed tomography-based cross-sectional study. Spine J. 2018;18(4):551–557. doi:10.1016/j.spinee.2017.08.240
2. Shepard NA, Shenoy K, Cho W, et al. Extensive ossification of the ligamentum flavum treated with triple stage decompression: a case report. Spine J. 2015;15(4):e9–e14. doi:10.1016/j.spinee.2014.12.011
3. Koizumi M. Three cases of spinal cord paralysis proved by ligamenta flava ossification. Rinsho-Geka (Clin Surg). 1962;17:1181–1188. Japanese.
4. Kubota M, Baba I, Sumida T. Myelopathy due to ossification of the ligamentum flavum of the cervical spine. A report of two cases. Spine. 1981;6(6):553–559. doi:10.1097/00007632-198111000-00005
5. Mak KH, Mak KL, Gwi-Mak E. Ossification of the ligamentum flavum in the cervicothoracic junction: case report on ossification found on both sides of the lamina. Spine. 2002;27(1):E11–E14. doi:10.1097/00007632-200201010-00027
6. Chou YC, Lee CC, Yen PS, et al. Cough induced by ossification of the ligamentum flavum in the high cervical spine. Case report. J Neurosurg. 2004;100(4 Suppl):364–366. doi:10.3171/spi.2004.100.4.0364
7. Inoue H, Seichi A, Kimura A, et al. Multiple-level ossification of the ligamentum flavum in the cervical spine combined with calcification of the cervical ligamentum flavum and posterior atlanto-axial membrane. Eur Spine J. 2013;22(Suppl 3):S416–S420. doi:10.1007/s00586-012-2521-7
8. He R, Fang H. Ossification of the ligamentum flavum in the upper cervical spine: a report of two cases and literature review. Exp Ther Med. 2020;20(2):1734–1738. doi:10.3892/etm.2020.8834
9. Hasue M, Kikuchi S, Fujiwara M, et al. Roentgenographic analysis of ossification of the spinal ligament with special reference to the finding of the whole spine. Seikei Geka. 1980;31:1179–1186.
10. Al-Jarallah K, Al-Saeed O, Shehab D, et al. Ossification of ligamentum flavum in Middle East Arabs: a hospital-based study. Med Princ Pract. 2012;21(6):529–533. doi:10.1159/000339120
11. Liang H, Liu G, Lu S, et al. Epidemiology of ossification of the spinal ligaments and associated factors in the Chinese population: a cross-sectional study of 2000 consecutive individuals. BMC Musculoskelet Disord. 2019;20(1):253. doi:10.1186/s12891-019-2569-1
12. Sonntag VK. Ossification of the ligamentum flavum (OLF): an increasing cause of cervical myelopathy. World Neurosurg. 2011;75(3–4):445–446. doi:10.1016/j.wneu.2010.12.022
13. Guo JJ, Luk KD, Karppinen J, et al. Prevalence, distribution, and morphology of ossification of the ligamentum flavum: a population study of one thousand seven hundred thirty-six magnetic resonance imaging scans. Spine. 2010;35(1):51–56. doi:10.1097/BRS.0b013e3181b3f779
14. Kotani Y, Takahata M, Abumi K, et al. Cervical myelopathy resulting from combined ossification of the ligamentum flavum and posterior longitudinal ligament: report of two cases and literature review. Spine J. 2013;13(1):e1–e6. doi:10.1016/j.spinee.2012.10.038
15. Chachan S, Kasat NS, Keng PTL. Cervical myelopathy secondary to combined ossification of ligamentum flavum and posterior longitudinal ligament-A case report. Int J Spine Surg. 2018;12(2):121–125. doi:10.14444/5018
16. Ishikawa Y, Miyakoshi N, Hongo M, et al. Thin cervical spinal cord between ossifications of the ligamentum flavum and the posterior longitudinal ligament: case report and literature review. World Neurosurg. 2021;145:83–88. doi:10.1016/j.wneu.2020.09.033
17. Bakhsh W, Saleh A, Yokogawa N, et al. Cervical ossification of the posterior longitudinal ligament: a computed tomography-based epidemiological study of 2917 patients. Global Spine J. 2019;9(8):820–825. doi:10.1177/2192568219833658
18. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi:10.1136/bmj.b2535
19. Ono K, Yonenobu K, Miyamoto S, et al. Pathology of ossification of the posterior longitudinal ligament and ligamentum flavum. Clin Orthop Relat Res. 1999;359:18–26. doi:10.1097/00003086-199902000-00003
20. Nouri A, Tetreault L, Singh A, et al. Degenerative cervical myelopathy: epidemiology, genetics, and pathogenesis. Spine. 2015;40(12):E675–E693. doi:10.1097/BRS.0000000000000913
21. Yang J, Ni B, Xie N, et al. Surgical treatments of myelopathy caused by cervical ligamentum flavum ossification. World Neurosurg. 2011;75(3–4):546–550. doi:10.1016/j.wneu.2010.10.041
22. Geber J, Hammer N. Ossification of the ligamentum flavum in a nineteenth-century skeletal population sample from Ireland: using bioarchaeology to reveal a neglected spine pathology. Sci Rep. 2018;8(1):9313. doi:10.1038/s41598-018-27522-x
23. Miyazawa N, Akiyama I. Ossification of the ligamentum flavum of the cervical spine. J Neurosurg Sci. 2007;51(3):139–144.
24. Lang N, Yuan HS, Wang HL, et al. Epidemiological survey of ossification of the ligamentum flavum in thoracic spine: CT imaging observation of 993 cases. Eur Spine J. 2013;22(4):857–862. doi:10.1007/s00586-012-2492-8
25. Mori K, Kasahara T, Mimura T, et al. Prevalence, distribution, and morphology of thoracic ossification of the yellow ligament in Japanese: results of CT-based cross-sectional study. Spine. 2013;38(19):E1216–E1222. doi:10.1097/BRS.0b013e31829e018b
26. Ohara Y. Ossification of the ligaments in the cervical spine, including ossification of the anterior longitudinal ligament, ossification of the posterior longitudinal ligament, and Ossification of the ligamentum flavum. Neurosurg Clin N Am. 2018;29(1):63–68. doi:10.1016/j.nec.2017.09.018
27. Tetreault L, Goldstein CL, Arnold P, et al. Degenerative cervical myelopathy: a spectrum of related disorders affecting the aging spine. Neurosurgery. 2015;77(Suppl 4):S51–S67. doi:10.1227/NEU.0000000000000951
28. Takahashi T, Hanakita J, Minami M. Pathophysiology of calcification and ossification of the ligamentum flavum in the cervical spine. Neurosurg Clin N Am. 2018;29(1):47–54. doi:10.1016/j.nec.2017.09.016
29. Baron EM, Young WF. Cervical spondylotic myelopathy: a brief review of its pathophysiology, clinical course, and diagnosis. Neurosurgery. 2007;60(Supp1):S35–S41. doi:10.1227/01.NEU.0000215383.64386.82
30. Christiano LD, Assina R, Goldstein IM. Ossification of the ligamentum flavum: a unique report of a Hispanic woman. Neurosurg Focus. 2011;30(3):E15. doi:10.3171/2011.1.FOCUS10266
31. Wang W, Kong L, Zhao H, et al. Thoracic ossification of ligamentum flavum caused by skeletal fluorosis. Eur Spine J. 2007;16(8):1119–1128. doi:10.1007/s00586-006-0242-5
32. Kumar H, Boban M, Tiwari M. Skeletal fluorosis causing high cervical myelopathy. J Clin Neurosci. 2009;16(6):828–830. doi:10.1016/j.jocn.2008.08.028
33. Li H, Jiang LS, Dai LY. Hormones and growth factors in the pathogenesis of spinal ligament ossification. Eur Spine J. 2007;16(8):1075–1084. doi:10.1007/s00586-007-0356-4
34. Kobashi G, Washio M, Okamoto K, et al. High body mass index after age 20 and diabetes mellitus are independent risk factors for ossification of the posterior longitudinal ligament of the spine in Japanese subjects: a case-control study in multiple hospitals. Spine. 2004;29(9):1006–1010. doi:10.1097/00007632-200405010-00011
35. Chaput CD, Siddiqui M, Rahm MD. Obesity and calcification of the ligaments of the spine: a comprehensive CT analysis of the entire spine in a random trauma population. Spine J. 2019;19(8):1346–1353. doi:10.1016/j.spinee.2019.03.003
36. Vera CL, Cure JK, Naso WB, et al. Paraplegia due to ossification of ligamenta flava in X-linked hypophosphatemia. A case report. Spine. 1997;22(6):710–715. doi:10.1097/00007632-199703150-00027
37. Miyasaka K, Kaneda K, Sato S, et al. Myelopathy due to ossification or calcification of the ligamentum flavum: radiologic and histologic evaluations. AJNR Am J Neuroradiol. 1983;4(3):629–632.
38. Khan MH, Smith PN, Donaldson WF. Acute quadriparesis caused by calcification of the entire cervical ligamentum flavum in a white female–report of an unusual case and a brief review of the literature: case report. Spine. 2005;30(22):E687–E691. doi:10.1097/01.brs.0000186472.88141.38
39. Xu R, Sciubba DM, Gokaslan ZL, et al. Ossification of the ligamentum flavum in a Caucasian man. J Neurosurg Spine. 2008;9(5):427–437. doi:10.3171/SPI.2008.9.11.427
40. Kawaguchi Y, Nakano M, Yasuda T, et al. Characteristics of ossification of the spinal ligament; incidence of ossification of the ligamentum flavum in patients with cervical ossification of the posterior longitudinal ligament - Analysis of the whole spine using multidetector CT. J Orthop Sci. 2016;21(4):439–445. doi:10.1016/j.jos.2016.04.009
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.