Back to Journals » Vascular Health and Risk Management » Volume 18
Cervical Artery Dissections: Etiopathogenesis and Management
Authors Keser Z , Chiang CC , Benson JC, Pezzini A, Lanzino G
Received 2 July 2022
Accepted for publication 19 August 2022
Published 2 September 2022 Volume 2022:18 Pages 685—700
DOI https://doi.org/10.2147/VHRM.S362844
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Pietro Scicchitano
Zafer Keser,1 Chia-Chun Chiang,1 John C Benson,2 Alessandro Pezzini,3 Giuseppe Lanzino4
1Department of Neurology, Mayo Clinic, Rochester, MN, USA; 2Department of Radiology, Mayo Clinic, Rochester, MN, USA; 3Department of Clinical and Experimental Sciences, Neurology Clinic, University of Brescia, Brescia, Italy; 4Department of Neurosurgery, Mayo Clinic, Rochester, MN, USA
Correspondence: Zafer Keser, Department of Neurology – Mayo Clinic, 200 First St SW, Rochester, MN, 55905, USA, Email [email protected]
Abstract: Cervical Artery Dissection (CeAD) is a frequent stroke etiology for patients younger than 50 years old. The most common immediate complications related to CeAD are headache and neck pain (65– 95%), TIA/ischemic stroke (> 50%), and partial Horner’s syndrome (25%). The prevailing hypothesis regarding the pathogenesis of sCeAD is that the underlying constitutional vessel wall weakness of patients with sCeAD is genetically determined and that environmental factors could act as triggers. The stroke prevention treatment of CeAD remains controversial, involving anticoagulation or antiplatelet therapy and potentially emergent stenting and/or thrombectomy or angioplasty for selected cases of carotid artery dissection with occlusion. The treatment of headache associated with CeAD depends on the headache phenotype and comorbidities. Radiographically, more than 75% of CeAD cases present with occlusion or non-occlusive stenosis. Many patients demonstrate partial and complete healing, more commonly in the carotid arteries. One-fifth of the patients develop dissecting pseudoaneurysm, but this is a benign clinical entity with an extremely low rupture and stroke recurrence risk. Good recovery is achieved in many CeAD cases, and mortality remains low. Family history of CeAD, connective tissue disorders like Ehlers-Danlos syndrome type IV, and fibromuscular dysplasia are risk factors for recurrent CeAD, which can occur in 3– 9% of the cases. This review serves as a comprehensive, updated overview of CeAD, emphasizing etiopathogenesis and management.
Keywords: cervical artery dissection, genetics, stroke
Introduction
Cervical artery dissections (CeADs) are uncommon entities of stroke in general; the yearly incidence is estimated to be 2.6–3/100,000.1 In a population-based study from Olmsted County, Minnesota, the annual incidence was 1.72/100,000 for carotid artery dissections and 0.97/100,000 for vertebral artery dissections.2 The actual incidence may be higher because of numerous asymptomatic cases. However, CeADs are relatively common causes of acute ischemic stroke in young and middle-aged groups, accounting for up to 25% of such cases.3 Strokes in the young and middle-aged have a disproportionately more significant economic impact than stroke in the elderly.4 Beyond neurologic ischemic events, CeADs can also cause various immediate complications, such as headache and neck pain, cranial neuropathies, Horner’s syndrome, pulsatile tinnitus, or long-term complications, such as pseudoaneurysm formation.2,5 Many studies investigating CeADs focus on managing immediate complications, particularly ischemic stroke or TIA, with short-term follow-up.6–8 The primary purpose of this review is to provide an in-depth literature review of various other immediate and long-term complications of CeADs and their management. Additionally, we highlight the pathophysiology, etiology, and essential imaging characteristics of CeADs.
Pathophysiology
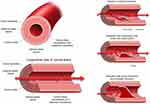
The arterial wall includes five layers; from the lumen outwardly in order; tunica intima (also known as the endothelium), internal elastic lamina, tunica media (muscular layer), external elastic lamina, tunica adventitia (Figure 1).
Bilateral common carotids (and their branches) and bilateral vertebral arteries constitute the cervical arterial vasculature. A tear in the tunica intima of the vessel wall is the most common triggering event in the pathophysiology of CeADs.9 Immediately after the initial tear, blood dissects into the space immediately under the tunica intima (false lumen or pseudolumen), which causes an intramural hematoma10 (Figure 1). In some cases, CeAD can be triggered by a primary intramural hematoma, without an intimal tear.11 In CeADs related to trauma, pathogenesis also potentially includes other factors such as a shear injury in the setting of extreme neck movements, direct vascular injury, intraoral traumas, and direct laceration from fractured bones.12 Once formed, intramural hematomas can become enlarged due to bleeding in the vasovasorum of tunica media.13 The downstream effect of this intramural hematoma depends on its location. If subintimal, the hematoma often leads to arterial stenosis and/or occlusion. If subadventitial, it often leads to the formation of a pseudoaneurysm, often called a dissecting pseudoaneurysm.
The effects of CeADs are often related to mass effect, arterial stenosis, and distal thromboembolism. The mass effect from dissecting pseudoaneurysms can result in Horner’s syndrome (oculosympathetic paresis), pulsatile tinnitus, cranial or cervical root neuropathies, neck pain, or headaches.2,14–17 The dissection site itself is highly thrombogenic. This is related to both turbulence and stagnation of the local blood flow (especially in the case of significant luminal narrowing) and exposure of blood to local subintimal thrombogenic factors.10
Etiology
More than half of the CeAD cases are spontaneous; prior trauma is identified in only up to 40% of cases. Most traumatic events (up to 90%) are mild or trivial insults.18 Cervical chiropractic neck manipulations, heavy lifting, sport associated injuries, and whiplash are the most common etiologies,18,19 Other etiologies like childbirth, coughing, sneezing, vomiting, practicing yoga or vigorous exercise have been implicated.20,21 Of the major traumatic events leading to CeADs, half are due to motor vehicle accidents, followed by assault, falls, and hanging.12 Many other factors have been implicated in increasing a patient’s risk for CeADs, including connective tissue disorders, acquired conditions such as infection, hypertension, and anatomical aberrations such as elongated styloid processes. An observational study of patients with CeAD reported that recent infection was more common in the internal carotid artery while preceding minor neck trauma in vertebral artery dissection.22
Collagen Vascular Disorders and CeAD
Although underlying collagen vascular disorders that meet diagnostic and genetic criteria is uncommonly found in CeADs (1–5% of spontaneous CeADs), isolated mild connective tissue abnormalities in skeletal, ocular, and skin systems (ie, joint hypermobility or multiple dislocations, easy bruising, poor wound healing or easy bruising) are frequently observed in patients with spontaneous CeAD (50–96%)23–25 Patients with subclinically increased vascular tortuosity or enlarged aortic root diameter are also more prone to have CeAD.26,27 Hence, most cases of so-called spontaneous CeAD likely occur in the setting of systemic yet poorly defined mild connective tissue disorders.
Fibromuscular dysplasia (FMD), a non-atherosclerotic and non-inflammatory arteriopathy characterized by multiple focal stenosis and tortuosity and commonly seen in middle-aged women, has a significant association with CeADs (Figure 2). More than 10% of patients with FMD have been found to have CeADs with dissections most commonly involving the cervical carotid arteries; vertebral artery involvement is rarer. More than 90% of dissections in FMD are in the carotid or vertebral arteries.28 Interestingly, patients with FMD are more likely to experience clinically symptomatic vascular events following dissections and tend to undergo endovascular interventions more commonly than patients with dissections but without underlying FMD.29 Close to 40% of patients with spontaneous CeAD were found to have FMD.30 The Italian Project on Stroke in Young Adults Cervical Artery Dissection (IPSYS CeAD) study, a multicenter observational cohort study reported that cerebrovascular FMD was found in 8% of all patients with spontaneous CeAD.31 Additionally, CeAD patients with cerebrovascular FMD, compared to patients without FMD, had more intracranial aneurysms, and more likely to be diagnosed with migraine (any migraine, regardless of subtype). The occurrence of preceding minor trauma was inversely associated with cerebrovascular FMD.31
 |
Figure 2 Prominent tortuosity of bilateral vertebral (A) and carotid arteries (A and B) in a patient with fibromuscular dysplasia (FMD). |
Overall, the majority of the patients with CeAD have either subtle subclinical signs of underlying connective tissue disorder or are diagnosed with multisystemic connective tissue abnormalities that meet clinical, radiographic, and/or genetic criteria.
Anatomical Considerations
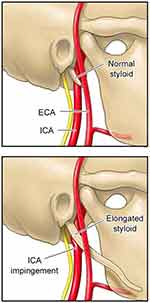
An elongated styloid process is not uncommon in the general population (incidence ranging from 4% to 28%). In rare cases, this anatomic anomaly can cause irritation and compression of the maxillio-vertebral recess, which contains the internal carotid arteries, internal jugular vein, and cranial nerves (Eagle syndrome) (Figure 3).32 In addition, elongated styloid processes have also been implicated in CeADs, likely related to direct mechanical injury.33 As such, few authors have recommended surgical shortening of the styloid process.32 However, given that it is a relatively common anatomical variant, such invasive procedures should only be considered after extensive studies ruling out other etiologies or if patients have recurrent dissections without clear etiologies.
Genetics of CeAD
A positive family history should be a risk factor if a disease is genetically mediated. In other words, the condition would show a clustering pattern within pedigrees. However, the evidence favoring familial aggregation of spontaneous cervical artery dissection (sCeAD) is somewhat limited.34 Previous reports have reported a positive family history in 2–3% of their moderate size cohorts (181–200 patients).35,36 In two large (n = 1934 patients) international multicenter cohort studies recruited in 23 neurologic departments participating in the Cervical Artery Dissection and Ischemic Stroke Patients (CADISP)-plus consortium, only 1.03% of the patients (20 patients, 17 families) had a family history of CeAD.25 Similarly, 16 patients (1.0%) with a first-degree family history of arterial dissection were found among the 1530 patients with sCeAD included in the multicentre IPSYS CeAD.37 It might be that a family history of sCeAD was overestimated in the early studies due to recruitment and selection biases.36 Still, the rate of asymptomatic cases poses a problem for understanding the actual incidence of familial CeAD. Overall, it is hard to estimate the prevalence of familial cases accurately. It is even more challenging to study the observed and expected prevalences of familial sCeAD based on mere coincidence and gauge the contributory role of genetic factors in the development of sCeAD. Notwithstanding, the available evidence suggests that 1) the involvement of the same artery within the families, 2) younger age at the first dissection (mean age of familial sCeAD is 38.7 vs 42–44 years of age in the general population),38,39 and 3) increased risk of recurrent or multivessel sCeAD.36 These findings potentially indicate that the familial occurrence of sCeAD is not entirely coincidental. A higher incidence of familial dissections in other locations, such as renal or intracranial arteries, or aorta, also suggests a genetic predisposition to sCeAD.36 Not infrequently, patients with CeAD have comorbid vascular conditions, such as FMD,40 aortic root dilation,41 hyperdistensibility of the arterial wall,42 or endothelial dysfunction,43 and an association with intracranial aneurysms44 for which an underlying genetic predisposition is assumed. Finally, various skin biopsy studies reporting a high prevalence (over 50%, most manifested as composite collagen fibrils and fragmentation of elastic fibers) of connective tissue abnormalities45–47 in cases with sporadic CeAD also support the role of genetic predisposition in CeAD. These connective tissue aberrations are likely transmitted in an autosomal-dominant fashion48,49 without meeting the diagnostic criteria for known monogenic connective tissue disorders.
Overall, there are solid arguments supporting the hypothesis that genetic factors play an essential role in the pathophysiology of sCeAD, in rare cases as part of a single gene disorder and more commonly as part of a multifactorial predisposition.50 The prevailing hypothesis regarding the pathogenesis of sCeAD is that the underlying constitutional weakness of the vessel wall of patients with sCeAD is genetically determined and that environmental factors such as acute infection or minor trauma could trigger it.
Single Gene Disorders Causing sCeAD
When investigating skin biopsies in patients, candidate gene mutations were typically selected based on a presumed association between CeAD and common connective tissue abnormalities in known connective tissue disorders. Genes known to carry mutations responsible for vascular Ehlers-Danlos syndrome (EDS IV, OMIM 13050; COL3A1 gene), Marfan syndrome (MFS, OMIM 154700; FBN1 gene), pseudoxanthoma elasticum (PXE, OMIM 264800; ABCC6 gene), osteogenesis imperfecta, and Loeys-Dietz syndrome, as well as other genes involved in the synthesis of extracellular matrix components were analyzed in series of consecutive patients with sCeAD, with mostly negative results. The reported rate of hereditary connective tissue disorders (HCTD) in sCeAD is, overall, very low (0.5%–2.0% for EDS IV; 0.6%–0.9% for MFS).51 Similarly, with few notable exceptions,52 sCeAD is rare among patients with a proven diagnosis of HCTD.
On the other hand, the main limitation in linkage studies on sCeAD is the small number of large families with only several members affected.53 An alternative approach for linkage studies can be studying families with only one member affected by sCeAD but several others having dermal connective tissue aberrations (intermediate phenotype).47,49 However, none of the analyses performed to date have identified any significant linkage peak.
Interestingly, taking advantage of the potential of the new generation sequencing (NGS) approach, Grond-Ginsbach and co-workers identified pathogenic mutations in different genes related to arterial connective tissue phenotypes in 4 out of 9 Caucasian pedigrees with a family history of sCeAD investigated by a panel of 11 pre-defined candidate genes.54 The same research group, later on, detected CeAD-causing variants in 5 out of 17 pedigrees analyzed by whole-exome sequencing,55 hence, underscoring that the importance of monogenic alterations in disease pathophysiology is especially prominent in patients with familial sCeAD.
sCeAD as a Complex Disease
For most patients with CeAD, there is no evidence of an underlying monogenic disease, and the etiology of CeAD is multifactorial. Early studies conducted based on the candidate gene approach yielded inconsistent results. However, these studies have remarkably small sample size, mainly due to the low incidence of sCeAD, which makes it challenging to have adequate power.50 In 2015, the investigators of the CADISP project created a consortium dedicated to the genetics of sCeAD and reported the first reliable association based on the results of a genome-wide association study (GWAS) in 1393 patients with CeAD and 14,416 controls.56 They revealed that the allele A of a genetic variant (rs9349379) of the phosphatase and actin regulator 1 gene (PHACTR1) was identified as a risk factor for CeAD (OR = 1.33, p = 4.46 × 10−10). One year later, Kiando and co-workers similarly showed that the same rs9349379[A] allele of PHACTR1 was similarly associated with an increased risk for FMD (OR = 1.39, p < 7.36 × 10−10).57 Moreover, a pooled meta-analysis from various GWAS (29 studies, 23,285 patients) showed an association between rs9349379[A] and an increased risk of migraine without aura (p < 2.81 × 10−10),58 which is more commonly seen in both FMD and CeAD. These interesting findings support an overlap between FMD, CeAD and migraine. More intriguingly, another meta-analysis of 4 large GWAS including 15,420 individuals with coronary artery disease and 15,062 controls, showed an association between the same allele, rs9349379[A] and decreased risk of coronary artery disease (p = 5.8 × 10−19).59
Even though rs9349379 is positioned in a PHACTR1 intron, it is located 54 kbp upstream of the PHACTR1 transcription site, which takes a role in building arterial endothelial and smooth cells. This site is also close to (about 265 kbp upstream) another PHACTR1 transcription start site taking a role in macrophages.60 PHACTR1 expression was positively correlated with the homozygous A allele at rs9349379 in healthy subjects’ skin macrophages and fibroblasts.61 The effects of homozygous rs9349379[A] or [G] on gene expression were recently studied with the help of induced pluripotent stem cells and CRISPR-Cas9 modification and found the rs9349379[G] genotype increases the expression of endothelin-1 (ET-1).62 The precursor of ET-1 is encoded by the EDN1 gene, which is located 600 kbp upstream of rs9349379. As a further evidence, higher levels of Big ET-1, a precursor of ET-1, was shown in the plasma of healthy subjects harboring G allele, at least in the homozygous state. ET-1 is a potent vasoconstrictor (through smooth muscle contraction) and also takes a role in the proliferation and migration of smooth muscle cells. Interestingly, it is an essential component in vascular relaxation through endothelial cells.63 Thus, the imbalance of ET-1 appears to be crucial in the association of rs9349379 to various cardiovascular diseases. However, further investigation of the mechanisms involved in non-atherosclerotic disorders like CeAD and FMD is still required.
Recent Infections and CeAD
Recent upper respiratory tract infections, flu, and gastrointestinal infections were more common in patients with spontaneous CeAD, though the underlying mechanism and causal association are unclear. This association was independent of coughing and sneezing that might induce a dissection.64,65
Vascular and Other Risk Factors and CeAD
Vascular risk factors66 for CeAD include typical vascular risk factors such hypertension, smoking,22,67 and elevated homocysteine levels.3,26,68,69 Other related risk factors, such as oral contraceptive use,30 and pregnancy, especially in the postpartum period,70 were also shown to be associated with CeAD.
Observational studies reported an association between migraine and CeAD. One-fifth to one-third of CeAD patients were found to have comorbid migraine. The IPSYS study included 2485 patients aged 18–45 with first-ever acute stroke. 334 (13.4%) of patients had an ischemic stroke because of a CeAD. Migraine was more common in the CeAD group compared to those who did not have CeAD (30.8% vs 24.4%, p = 0.01), and it was mainly due to migraine without aura (24.0% vs 15.6%, p < 0.001). A previous systematic review and meta-analysis reported that migraine is associated with a twofold increased risk of CeAD.71 Genetic studies have also identified shared single nucleotide polymorphisms (SNPs) between CeAD and migraine.56
In sum, most CeAD cases are either spontaneous or due to mild trauma. There is a strong association between CeAD and FMD and EDS type IV. It is plausible that CeAD is secondary to clinical or subclinical systemic connective tissue disorder in many cases. Elongated styloid processes are relatively common anatomic variants and should only be considered as the culprit of dissection after extensive work up has been done ruling out other etiologies. The exact genetic underpinnings of dissections are still not exactly clear at this point. The causal relationship with other comorbid conditions like recent infections and vascular risk factors is not well characterized. Like many other cerebrovascular pathologies, many cases suffer from CeAD due to the compound or additive effect of different elements. It is reasonable speculation that underlying connective tissue disorder leads to a baseline vessel wall weakness in cervical arteries. Other factors such as trivial trauma, recent infection, and pregnancy become triggering factors of acute CeAD in those cases.
Imaging
The American Heart Association (AHA) recommends computerized tomography angiography (CTA) or magnetic resonance imaging (MRI) with fat suppression/MRA as the first-line non-invasive imaging techniques for CeAD72 (Figure 4). Diagnostic angiography (DSA) is the gold standard for diagnosis when in doubt or when an intervention such as stenting is needed.73 The most common signs are the flame-shaped tapering of the contrast within the vessel, and false lumen formation.
In general, compared to MRA, CTA 1) has a better spatial resolution (potentially better at visualizing dissections in a small-caliber vertebral artery), 2) can be acquired faster, 3) is more readily available in most healthcare settings, and 4) is superior at diagnosing pseudoaneurysms and intimal flaps.73 Compared to DSA, the gold standard, sensitivity, and specificity for diagnosing CeAD ranges between 65% and 100%.73,74 Classic signs of CeAD in dissection in DSA are 1) flame-shaped “contrast tapering off” luminal stenosis or occlusion, 2) intimal flaps 3) dissecting aneurysm or pseudoaneurysm.75
MRA/MRI with fat suppression is superior to CTA at identifying small intramural hematomas and provides better vessel wall resolution.11 MRI also provides for sensitive detection of ischemic strokes. Classically, dissections on MRA appear as a region of crescentic hyperintensity on T1 fat-saturated images, representing the intramural hematoma. This can be seen without or with associated luminal narrowing and enlargement of the vessel wall diameter.76 MRI-based technologies are also open to advances more than CTA and more advanced sequences like double inversion-recovery,77 diffusion-based multisection motion-sensitized driven equilibrium imaging.78 Compared to DSA, MRA has 50–100% sensitivity and 30–100% specificity for detecting CeAD.74
Duplex sonography can also be used to diagnose CeAD, though this tends to be infrequently employed. One benefit of duplex over MR/CT-based imaging is that the flow dynamics can be assessed. On duplex imaging, CeADs may be recognized by the finding of a double lumen or hyperechoic intramural hematoma.75
In sum, CTA or MRA/MRI with fat saturation is an appropriate initial diagnostic imaging tool to detect CeAD. Although CTA has widespread availability and faster acquisition times, advances in MR-based technologies with higher resolution and field of strength features might become a preferable technique to study dissections further. DSA remains the gold standard for CeAD diagnosis.
Immediate Complications
The most common immediate complications related to CeAD are head and neck pain, TIA/ischemic stroke, and partial Horner’s syndrome.2,5,22
TIA/Ischemic Stroke
More than half of the cases with CeAD experience TIA or ischemic stroke2 (Figure 5). An interesting study utilizing nationwide inpatient data revealed that the prevalence of dissection among stroke hospitalizations was around 7% between age 18–40.79
 |
Figure 5 Right cervical internal carotid artery (ICA) dissecting pseudoaneurysm (white arrow) (reconstructed images from MRA) (figure on the left) and ipsilateral stroke on the ICA territory. |
As with other ischemic strokes, the management of neurologic ischemic due to CeAD has two components: hyperacute treatment and secondary stroke prevention. The degree of luminal stenosis, presence of occlusion, intraluminal thrombus formation on the dissection sites, the amount of tissue at risk, and the intracranial extension of CeAD are essential factors.
Hyperacute Treatments
Many studies have investigated the safety and efficacy of thrombolytics in hyperacute ischemic stroke and CeAD. Although the rate of favorable outcomes in ischemic strokes due to CeAD after thrombolytics is lower compared to stroke due to other etiologies,80,81 the safety profile of thrombolytics is similar. Thus, despite the theoretical risk of intramural hematoma expansion, thrombolytics should never be held due to the presence of CeAD.82
For anterior circulation intracranial large vessel occlusions (LVO) secondary to CeAD, mechanical thrombectomy (MT) is safe and efficacious.83–86 Therefore, like any other etiologies, MT should be offered to patients with anterior and posterior circulation LVO and CeAD. For the cases with occlusive CeAD and intracranial LVO (tandem occlusion), emergent stenting of the internal carotid artery is safe and leads to more successful reperfusion. However, 90-day functional outcomes were similar to patients with angioplasty or aspiration only.87
Stroke Prevention
In addition to aggressive vascular risk factor, antithrombotic strategy with either antiplatelet or anticoagulant use should be implemented as primary or secondary stroke prevention in CeAD for at least three months.88–90 The main decisions to be made are 1) between antiplatelet and anticoagulation therapy and 2) therapy duration. To date, there are two multi-center, open-label, assessor-blind international trials: CADISS7,8 and TREAT-CAD,6 investigating the safety and efficacy of anticoagulation and antiplatelet therapies for stroke prevention in CeAD. Due to the low incidence of CeAD, the sample size (250 in CADISS and 194 in TREAT-CAD) in both trials was lower than other large stroke trials. Also, stroke risk within the first 12 months after CeAD is 2.4%, which is remarkably lower than other stroke etiologies. All taken into consideration, power analysis in CADISS trials showed that the sample size needed to show a meaningful difference between stroke prevention strategies in CeAD is around 10,000 patients.
Both trials included acutely symptomatic patients (<7–14 days) with either vascular events or local symptoms like pain and Horner’s syndrome; most included participants experienced either TIA or stroke. Treatment protocols in CADISS were more liberal than TREAT-CAD. They allowed treating clinicians to decide on an antiplatelet regimen (~45% received dual antiplatelet therapy) and an anticoagulation regimen (90% heparin to warfarin bridge vs 10% warfarin alone). In TREAT-CAD, the antiplatelet group received 300 mg aspirin, and anticoagulant group vitamin K antagonists (VKA) (target international normalized ratio (INR) 2–3); dual antiplatelet therapy used as off-label in clinical practice was not allowed. Another treatment regime – direct oral anticoagulants – heavily used as off-label in real-life clinical practice with likely better safety profile was not used in these trials. Both trials provided follow-up outcomes within three months, but CADISS trial investigators later published one-year follow-up results.7
Ipsilateral stroke occurred in 2% of the patients in the antiplatelet group and 1% in the anticoagulant group with one serious hemorrhagic complication (subarachnoid hemorrhage) in the anticoagulant group.8 Ipsilateral stroke or TIA was 2.4% in both groups in 12-month follow-up.7
TREAT-CAD trial was conducted to show non-inferiority of aspirin to VKA. The primary endpoint was a composite outcome that included ischemic or hemorrhagic stroke, death, or new subclinical ischemic or hemorrhagic lesion on brain MRI. The primary endpoint occurred more commonly in the aspirin group (23% vs 15%). Only one major bleed event (gastrointestinal bleed) happened in the VKA group. The aspirin group experienced more strokes or transient ischemic attack (8%) than the VKA group (2%). The composite outcome failed to show that aspirin was non-inferior to VKA. Although comparisons across trials should be made cautiously, a higher rate of stroke in the aspirin group of TREAT-CAD compared to antiplatelet therapy in CADISS may potentially be explained by the lack of dual antiplatelet therapy in TREAT-CAD.
Although there is no clear consensus on an antithrombotic treatment regimen for stroke prevention in CeAD, the opinion of our group, as well as certain experts in the field,91 favors anticoagulation then transitioning to antiplatelet therapy after stable imaging and clinical course.92 This is identified as a knowledge gap in the current guidelines.90 Especially, if there is intraluminal thrombus and significant luminal narrowing, or recurrent TIA/strokes, anticoagulation might be preferred choice. Data for DOACs in CeAD are limited, but it can be considered in selected cases.93,94 Caution is advised with anticoagulation use in patients with large ischemic stroke, ischemic stroke with hemorrhagic conversion, and CeAD with intracranial extension. In patients with CeAD and recurrent TIA or strokes, endovascular therapy can be considered for stroke prevention.90 The benefit of long-term antithrombotic is unclear, especially in cases who have full healing and a recent study showed that it may be reasonable to discontinue after the acute period.95
Headache and Neck Pain
Headache or neck pain are amongst the most common symptoms after CeAD (65–95% of cases).2,14 In a cohort of 161 patients, headache was the initial manifestation of CeAD in 47% patients with internal carotid dissection and 33% patients with vertebral artery dissection.96 In the Cervical Artery Dissection and Ischemic Stroke Patients observational study, 982 patients with either internal carotid artery (n = 619) or vertebral artery (n = 327) dissection were included. Compared to patients with vertebral artery dissection, patients with internal carotid artery dissection reported headache upon presentation more frequently, but less likely to have neck pain or ischemic stroke.22
Headache associated with CeAD is typically severe. Migraine-like headache is common, though cluster headache and thunderclap headache have also been reported.97,98
A recent and relatively sudden (thunderclap at times) onset of intractable severe head or neck pain, especially when accompanied by other neurological deficits, should raise suspicion for CeAD.97
Typically, carotid dissections present pain in the ipsilateral temporal area and vertebral dissections in the ipsilateral occipital area99 (Figure 6). Also, isolated neck pain tends to be milder than isolated headaches.99
No studies specifically tested pharmacological treatments for dissection-induced headache. In real-life practice, migraine preventive and acute treatments are commonly used, though in patients with ischemic stroke or arterial stenosis, whether associated with CeAD or not, triptans should be avoided.
Horner’s Syndrome
One-fourth of patients with CeAD present with partial Horner’s syndrome (miosis, ptosis, and but not anhidrosis) (Figure 7), which is the third most common symptom of CeAD. Post-ganglionic third-order oculosympathetic nerve fibers are located in the carotid sheath adjacent to the internal carotid and can be compressed by enlarging vessel wall secondary to intramural hematoma or a dissecting pseudoaneurysm.100 Horner’s syndrome can also occur after a brainstem stroke secondary to vertebral artery dissection. Nerve fibers controlling facial sweating are located adjacent to the external carotid and are spared by internal artery dissections.
 |
Figure 7 Partial Horner’s syndrome of the left eye; ptosis and miosis. Note: Used with permission of Mayo Foundation for Medical Education and Research, all rights reserved. |
Pulsatile Tinnitus
8% of patients with CeAD reported pulsatile tinnitus, which is more often associated with carotid dissections. This could be related to the fact that the petrous segment of the internal carotid artery is located near the tympanic cavity. Interestingly, patients who experience pulsatile tinnitus tend to have a more favorable clinical course and experience ischemic stroke less commonly.101
Cranial Neuropathies
Cranial neuropathies typically occur with carotid artery dissections and 12% of spontaneous carotid dissections can have isolated or multiple cranial nerve palsies.15 Half of the cases experience lower cranial neuropathies due to the local mass effect from hematoma (IX, X, XI, XII). Figure 8A demonstrates that lower cranial nerves travel adjacent to the internal carotid artery. Patients can also experience cranial nerve III, IV, V, VI palsies.15 The hypoglossal nerve is the most commonly involved nerve palsy.102
Cervical Radiculopathy/Myelopathy
Compressive cervical radiculopathy, most commonly at C5-C6 level (Figure 8B) or myelopathy secondary to compression or spinal cord ischemic stroke or subarachnoid hemorrhage, can rarely occur secondary to vertebral artery dissection.103,104
Patients with CeADs may experience ischemic damage to the brain or spinal cord due to distal embolization or vessel occlusion, and may also have local compressive symptoms such as head/neck pain, partial Horner syndrome, pulsatile tinnitus, and/or cranial or cervical neuropathies. However, patients with CeAD could also remain asymptomatic, which leads to underdiagnosis of the condition present as an incidental finding when head imaging is arranged.
Long-Term Outcomes
There are limited data available for long-term recovery and outcomes of CeAD. Like other cerebrovascular pathologies, the primary functional recovery measure from stroke due to CeAD was the modified Rankin Scale (mRS). Although limited, some studies report long-term evolution of vasculopathy and local compressive symptoms.
Spontaneous Healing of the Vessel
One-fourth of cervical carotid dissections and half of the vertebral artery dissections present with occlusion, whereas 56% of patients of cervical carotid dissections and 39% of vertebral artery dissections have non-occlusive stenosis.2 Radiologically, many patients demonstrate “remodeling” and complete healing can be seen in approximately a third of cases, more commonly in the carotid arteries. Such healing occurs for the most part in the first three months, and 82% of healed CeADs did so within the first year.2 Similarly, after complete occlusion of the carotid artery, the rate of complete recanalization is 16% at one month, 50% at three months, and 60% at 6 and 12 months.105
Dissecting Pseudoaneurysm Formation
Dissecting pseudoaneurysm is a type of aneurysm that occurs secondary to dissection related intramural hematoma growing towards the adventitial layer. In one series, dissecting pseudoaneurysms were seen in 19% of carotid dissection and 11% of vertebral dissections. In another series, dissecting aneurysms were present in up to 49.3% of patients with CeAD, and patients with aneurysm formation were more likely to have multiple CeADs, arterial redundancies, migraines, and symptoms.106 83% of vertebral artery aneurysms and 54% of carotid artery aneurysms either resolved or improved. However, patients with dissecting aneurysms in CeADs tend to remain asymptomatic.106,107
Recurrent Transient Ischemic Attacks/Strokes and Dissections
The risk of recurrent TIA/stroke ranges between 0% and 13%, and most recurrent events occur within the first few weeks after the dissection.3,5 Multiple dissections, worsening stenosis/occlusion, and very rarely chronic dissecting aneurysm along with vascular risk factors such as hypertension, or can be associated risk factors for recurrent TIA/strokes.5,108–111 Patients experiencing recurrent events might benefit from endovascular interventions with good short- and long-term outcomes.
Although recurrent CeAD is rare in most series (<5%), a recent large cohort revealed it could happen in up to 9.2%. Most recurrent events occur within the first two months.3,22,112,113 It is possible that late recurrences are underreported.114 Younger age, family history, connective tissue disorders like EDS type IV, and FMD were risk factors associated with recurrent CeAD.36,113,115,116 The IPSYS CeAD study reported a recurrence rate of 3.3% at a median follow-up of 34 months. Cerebrovascular FMD and a history of migraine were independent predictors of CeAD recurrence.31
Clinical Outcome
Half of the CeAD patients suffer from a reduced quality of life.96,116 Good recovery (mRS 0–2) is achieved in 75–92% of CeAD cases.2,11 Mortality in CeAD is estimated to be low <5%.2,5,111 Functional recovery from ischemic stroke due to CeAD is no different from any other stroke when controlled for age and other factors.117 Presence and severity of the stroke, arterial occlusion, type of vessel (carotid >vertebral), and older age are associated with worse functional outcomes.3,111,118,119
With the healing of carotid dissection, partial Horner’s syndrome usually improves, but the long-term prognosis of Horner’s syndrome in CeAD is not well studied. Typically, pulsatile tinnitus resolves approximately 2–3 months after the initial injury.120,121 Unless there is significant parenchymal damage in the brain or spinal cord, compressive symptomatology improves and resolves over a few months with the resolution of intramural hematoma.2,104,122
Conclusion
Despite being a relatively rare entity, CeAD accounts for approximately 25% of ischemic stroke in patients younger than 50 years old. Most cases are caused by a tear in the intimal layer of the vessel with subsequent intramural hematoma. In the acute setting, CeAD leads to either embolization causing TIA/strokes or local compressive symptoms. Hyperacute treatment of stroke in CeAD mirrors that of other ischemic strokes, which includes thrombolytic and endovascular thrombectomy when indicated. The benefit of emergent stenting in addition to thrombectomy in tandem occlusions, for cases of internal carotid artery dissection, remains unclear. Antiplatelet or anticoagulation therapy is also used though more studies are needed to reach a consensus on secondary stroke prevention. The topic of CeAD would continue to benefit from additional research studies. Specifically, an updated epidemiological study would help determine the true incidence of CeAD, and large-scale studies could help determine the natural history of the disease and develop consensus treatment strategies.
Disclosure
Dr Giuseppe Lanzino reports Consultant: Superior Medical Editors: Nested Knowledge. The authors report no other conflicts of interest in this work.
References
1. Béjot Y, Daubail B, Debette S, Durier J, Giroud M. Incidence and outcome of cerebrovascular events related to cervical artery dissection: the Dijon Stroke Registry. Int J Stroke. 2014;9(7):879–882. doi:10.1111/ijs.12154
2. Lee VH, Brown RD, Mandrekar JN, Mokri B. Incidence and outcome of cervical artery dissection: a population-based study. Neurology. 2006;67(10):1809–1812.
3. Debette S, Leys D. Cervical-artery dissections: predisposing factors, diagnosis, and outcome. Lancet Neurol. 2009;8(7):668–678.
4. Smajlović D. Strokes in young adults: epidemiology and prevention. Vasc Health Risk Manag. 2015;11:157–164.
5. Touzé E, Gauvrit JY, Moulin T, Meder JF, Bracard S, Mas JL. Risk of stroke and recurrent dissection after a cervical artery dissection: a multicenter study. Neurology. 2003;61(10):1347–1351.
6. Engelter ST, Traenka C, Gensicke H, et al. Aspirin versus anticoagulation in cervical artery dissection (TREAT-CAD): an open-label, randomised, non-inferiority trial. Lancet Neurol. 2021;20(5):341–350.
7. Markus HS, Levi C, King A, Madigan J, Norris J. Antiplatelet Therapy vs Anticoagulation Therapy in Cervical Artery Dissection: the Cervical Artery Dissection in Stroke Study (CADISS) Randomized Clinical Trial Final Results. JAMA Neurol. 2019;76(6):657–664.
8. Markus HS, Hayter E, Levi C, Feldman A, Venables G, Norris J. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurol. 2015;14(4):361–367.
9. Keser Z, Meschia JF, Lanzino G. Craniocervical Artery Dissections: a Concise Review for Clinicians. Mayo Clin Proc. 2022;97(4):777–783.
10. Bond KM, Krings T, Lanzino G, Brinjikji W. Intracranial dissections: a pictorial review of pathophysiology, imaging features, and natural history. J Neuroradiol. 2021;48(3):176–188.
11. Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344(12):898–906.
12. Fusco MR, Harrigan MR. Cerebrovascular dissections: a review. Part II: blunt cerebrovascular injury. Neurosurgery. 2011;68(2):517–530.
13. Völker W, Dittrich R, Grewe S, et al. The outer arterial wall layers are primarily affected in spontaneous cervical artery dissection. Neurology. 2011;76(17):1463–1471.
14. Mokri B, Sundt TM, Houser OW, Piepgras DG. Spontaneous dissection of the cervical internal carotid artery. Ann Neurol. 1986;19(2):126–138.
15. Mokri B, Silbert PL, Schievink WI, Piepgras DG. Cranial nerve palsy in spontaneous dissection of the extracranial internal carotid artery. Neurology. 1996;46(2):356–359.
16. Arnold M, Kappeler L, Georgiadis D, et al. Gender differences in spontaneous cervical artery dissection. Neurology. 2006;67(6):1050–1052.
17. Manabe H, Yonezawa K, Kato T, Toyama K, Haraguchi K, Ito T. Incidence of intracranial arterial dissection in non-emergency outpatients complaining of headache: preliminary investigation with MRI/MRA examinations. Acta Neurochir Suppl. 2010;107:41–44.
18. Engelter ST, Grond-Ginsbach C, Metso TM, et al. Cervical artery dissection: trauma and other potential mechanical trigger events. Neurology. 2013;80(21):1950–1957.
19. Biller J, Sacco RL, Albuquerque FC, et al. Cervical arterial dissections and association with cervical manipulative therapy: a statement for healthcare professionals from the American heart association/American stroke association. Stroke. 2014;45(10):3155–3174.
20. Stamboulis E, Raptis G, Andrikopoulou A, et al. Spontaneous internal carotid artery dissection: an uncommon cause of recurrent postpartum headache. J Neuroimaging. 2011;21(1):76–78.
21. Caso V, Paciaroni M, Bogousslavsky J. Environmental factors and cervical artery dissection. Front Neurol Neurosci. 2005;20:44–53.
22. Debette S, Grond-Ginsbach C, Bodenant M, et al. Differential features of carotid and vertebral artery dissections: the CADISP study. Neurology. 2011;77(12):1174–1181.
23. Brandt T, Morcher M, Hausser I. Association of cervical artery dissection with connective tissue abnormalities in skin and arteries. Front Neurol Neurosci. 2005;20:16–29.
24. Giossi A, Ritelli M, Costa P, et al. Connective tissue anomalies in patients with spontaneous cervical artery dissection. Neurology. 2014;83(22):2032–2037.
25. Debette S, Goeggel Simonetti B, Schilling S, et al. Familial occurrence and heritable connective tissue disorders in cervical artery dissection. Neurology. 2014;83(22):2023–2031.
26. Rubinstein SM, Peerdeman SM, van Tulder MW, Riphagen I, Haldeman S. A systematic review of the risk factors for cervical artery dissection. Stroke. 2005;36(7):1575–1580.
27. Kim BJ, Yang E, Kim NY, et al. Vascular Tortuosity May Be Associated With Cervical Artery Dissection. Stroke. 2016;47(10):2548–2552.
28. Olin JW, Froehlich J, Gu X, et al. The United States Registry for Fibromuscular Dysplasia: results in the first 447 patients. Circulation. 2012;125(25):3182–3190.
29. Kadian-Dodov D, Gornik HL, Gu X, et al. Dissection and Aneurysm in Patients With Fibromuscular Dysplasia: findings From the U.S. Registry for FMD. J Am Coll Cardiol. 2016;68(2):176–185.
30. Talarowska P, Dobrowolski P, Klisiewicz A, et al. High incidence and clinical characteristics of fibromuscular dysplasia in patients with spontaneous cervical artery dissection: the ARCADIA-POL study. Vasc Med. 2019;24(2):112–119.
31. Bonacina S, Grassi M, Zedde M, et al. Clinical Features of Patients With Cervical Artery Dissection and Fibromuscular Dysplasia. Stroke. 2021;52(3):821–829.
32. Baldino G, Di Girolamo C, De Blasis G, Gori A. Eagle Syndrome and Internal Carotid Artery Dissection: description of Five Cases Treated in Two Italian Institutions and Review of the Literature. Ann Vasc Surg. 2020;67:
33. Raser JM, Mullen MT, Kasner SE, Cucchiara BL, Messé SR. Cervical carotid artery dissection is associated with styloid process length. Neurology. 2011;77(23):2061–2066.
34. Floßmann E, Schulz UG, Rothwell PM. Systematic review of methods and results of studies of the genetic epidemiology of ischemic stroke. Stroke. 2004;35(1):212–227.
35. Baumgartner R, Arnold M, Baumgartner I, et al. Carotid dissection with and without ischemic events: local symptoms and cerebral artery findings. Neurology. 2001;57(5):827–832.
36. Schievink WI, Mokri B, Piepgras DG, Kuiper JD. Recurrent spontaneous arterial dissections: risk in familial versus nonfamilial disease. Stroke. 1996;27(4):622–624.
37. Bonacina S, Grassi M, Zedde M, et al. Long-term outcome of cervical artery dissection. Neurol Sci. 2020;41(11):3265–3272.
38. Schievink W, Mokri B, Whisnant J. Internal carotid artery dissection in a community. Rochester, Minnesota, 1987-1992. Stroke. 1993;24(11):1678–1680.
39. Giroud M, Fayolle H, Andre N, et al. Incidence of internal carotid artery dissection in the community of Dijon. J Neurol Neurosurg Psychiatry. 1994;57(11):1443.
40. De Bray J, Marc G, Pautot V, et al. Fibromuscular dysplasia may herald symptomatic recurrence of cervical artery dissection. Cerebrovascular Dis. 2007;23(5–6):448–452.
41. Tzourio C, Cohen A, Lamisse N, Biousse V, Bousser M-G. Aortic root dilatation in patients with spontaneous cervical artery dissection. Circulation. 1997;95(10):2351–2353.
42. Guillon B, Tzourio C, Biousse V, Adrai V, Bousser M, Touboul P. Arterial wall properties in carotid artery dissection: an ultrasound study. Neurology. 2000;55(5):663–666.
43. Lucas C, Lecroart J, Gautier C, et al. Impairment of endothelial function in patients with spontaneous cervical artery dissection: evidence for a general arterial wall disease. Cerebrovascular Dis. 2004;17(2–3):170–174.
44. Schievink WI, Mokri B, Piepgras DG. Angiographic frequency of saccular intracranial aneurysms in patients with spontaneous cervical artery dissection. J Neurosurg. 1992;76(1):62–66.
45. Brandt T, Hausser I, Orberk E, et al. Ultrastructural connective tissue abnormalities in patients with spontaneous cervicocerebral artery dissections. Ann Neurol. 1998;44(2):281–285.
46. Brandt T, Orberk E, Weber R, et al. Pathogenesis of cervical artery dissections: association with connective tissue abnormalities. Neurology. 2001;57(1):24–30.
47. Ulbricht D, Diederich N, Hermanns-Lê T, Metz R, Macian F, Pierard G. Cervical artery dissection: an atypical presentation with Ehlers–Danlos-like collagen pathology? Neurology. 2004;63(9):1708–1710.
48. Grond‐Ginsbach C, Klima B, Weber R, et al. Exclusion mapping of the genetic predisposition for cervical artery dissections by linkage analysis. Ann Neurol. 2002;52(3):359–364.
49. Wiest T, Hyrenbach S, Bambul P, et al. Genetic analysis of familial connective tissue alterations associated with cervical artery dissections suggests locus heterogeneity. Stroke. 2006;37(7):1697–1702.
50. Grond-Ginsbach G, Debette S, Pezzini A. Genetic approaches in the study of risk factors for cervical artery dissection. Handbook Cerebral Artery Dissection. 2005;20:30–43.
51. Debette S. Pathophysiology and risk factors of cervical artery dissection: what have we learnt from large hospital-based cohorts? Curr Opin Neurol. 2014;27(1):20–28.
52. Adham S, Billon C, Legrand A, et al. Spontaneous Cervical Artery Dissection in Vascular Ehlers-Danlos Syndrome: a Cohort Study. Stroke. 2021;52(5):1628–1635.
53. von Pein F, Välkkilä M, Schwarz R, et al. Analysis of the COL3A1 gene in patients with spontaneous cervical artery dissections. J Neurol. 2002;249(7):862–866.
54. Grond-Ginsbach C, Brandt T, Kloss M, et al. Next generation sequencing analysis of patients with familial cervical artery dissection. Eur Stroke J. 2017;2(2):137–143.
55. Traenka C, Kloss M, Strom T, et al. Rare genetic variants in patients with cervical artery dissection. Eur Stroke j. 2019;4(4):355–362.
56. Debette S, Kamatani Y, Metso TM, et al. Common variation in PHACTR1 is associated with susceptibility to cervical artery dissection. Nat Genet. 2015;47(1):78–83.
57. Kiando SR, Tucker NR, Castro-Vega L-J, et al. PHACTR1 is a genetic susceptibility locus for fibromuscular dysplasia supporting its complex genetic pattern of inheritance. PLoS Genet. 2016;12(10):e1006367.
58. Anttila V, Winsvold BS, Gormley P, et al. Genome-wide meta-analysis identifies new susceptibility loci for migraine. Nat Genet. 2013;45(8):912–917.
59. Genetics S. A genome-wide association study in Europeans and South Asians identifies five new loci for coronary artery disease. Nat Genet. 2011;43(4):339–344.
60. Perdu J, Gimenez-Roqueplo A-P, Boutouyrie P, et al. α1-antitrypsin gene polymorphisms are not associated with renal arterial fibromuscular dysplasia. J Hypertens. 2006;24(4):705–710.
61. Reschen ME, Lin D, Chalisey A, Soilleux EJ, O’Callaghan CA. Genetic and environmental risk factors for atherosclerosis regulate transcription of phosphatase and actin regulating gene PHACTR1. Atherosclerosis. 2016;250:95–105.
62. Gupta RM, Hadaya J, Trehan A, et al. A genetic variant associated with five vascular diseases is a distal regulator of endothelin-1 gene expression. Cell. 2017;170(3):522–533. e515.
63. Ganesh SK, Morissette R, Xu Z, et al. Clinical and biochemical profiles suggest fibromuscular dysplasia is a systemic disease with altered TGF‐β expression and connective tissue features. FASEB J. 2014;28(8):3313–3324.
64. Grau AJ, Brandt T, Buggle F, et al. Association of cervical artery dissection with recent infection. Arch Neurol. 1999;56(7):851–856.
65. Guillon B, Berthet K, Benslamia L, Bertrand M, Bousser MG, Tzourio C. Infection and the risk of spontaneous cervical artery dissection: a case-control study. Stroke. 2003;34(7):e79–81.
66. Debette S, Metso T, Pezzini A, et al. Association of vascular risk factors with cervical artery dissection and ischemic stroke in young adults. Circulation. 2011;123(14):1537–1544.
67. Pezzini A, Caso V, Zanferrari C, et al. Arterial hypertension as risk factor for spontaneous cervical artery dissection. A case-control study. J Neurol Neurosurg Psychiatry. 2006;77(1):95–97.
68. Benninger DH, Herrmann FR, Georgiadis D, et al. Increased prevalence of hyperhomocysteinemia in cervical artery dissection causing stroke: a case-control study. Cerebrovasc Dis. 2009;27(3):241–246.
69. Metso TM, Metso AJ, Salonen O, et al. Adult cervicocerebral artery dissection: a single-center study of 301 Finnish patients. Eur J Neurol. 2009;16(6):656–661.
70. Salehi Omran S, Parikh NS, Poisson S, et al. Association between Pregnancy and Cervical Artery Dissection. Ann Neurol. 2020;88(3):596–602.
71. Rist PM, Diener HC, Kurth T, Schürks M. Migraine, migraine aura, and cervical artery dissection: a systematic review and meta-analysis. Cephalalgia. 2011;31(8):886–896.
72. Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/ SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. Vasc Med. 2011;16(1):35–77.
73. Hakimi R, Sivakumar S. Imaging of Carotid Dissection. Curr Pain Headache Rep. 2019;23(1):2.
74. Provenzale JM, Sarikaya B. Comparison of test performance characteristics of MRI, MR angiography, and CT angiography in the diagnosis of carotid and vertebral artery dissection: a review of the medical literature. AJR Am J Roentgenol. 2009;193(4):1167–1174.
75. Shakir HJ, Davies JM, Shallwani H, Siddiqui AH, Levy EI. Carotid and Vertebral Dissection Imaging. Curr Pain Headache Rep. 2016;20(12):68.
76. Zweifler RM, Silverboard G. Arterial Dissections and Fibromuscular Dysplasia. Stroke. Elsevier; 2011:661–686.
77. Hunter MA, Santosh C, Teasdale E, Forbes KP. High-resolution double inversion recovery black-blood imaging of cervical artery dissection using 3T MR imaging. AJNR Am J Neuroradiol. 2012;33(11):E133–137.
78. Obara M, Kuroda K, Wang J, et al. Comparison between two types of improved motion-sensitized driven-equilibrium (iMSDE) for intracranial black-blood imaging at 3.0 tesla. J Magn Reson Imaging. 2014;40(4):824–831.
79. Atalay YB, Piran P, Chatterjee A, et al. Prevalence of Cervical Artery Dissection Among Hospitalized Patients With Stroke by Age in a Nationally Representative Sample From the United States. Neurology. 2021;96(7):e1005–e1011.
80. Engelter ST, Rutgers MP, Hatz F, et al. Intravenous thrombolysis in stroke attributable to cervical artery dissection. Stroke. 2009;40(12):3772–3776.
81. Qureshi AI, Chaudhry SA, Hassan AE, et al. Thrombolytic treatment of patients with acute ischemic stroke related to underlying arterial dissection in the United States. Arch Neurol. 2011;68(12):1536–1542.
82. Engelter ST, Dallongeville J, Kloss M, et al. Thrombolysis in cervical artery dissection–data from the Cervical Artery Dissection and Ischaemic Stroke Patients (CADISP) database. Eur J Neurol. 2012;19(9):1199–1206.
83. Haussen DC, Jadhav A, Jovin T, et al. Endovascular Management vs Intravenous Thrombolysis for Acute Stroke Secondary to Carotid Artery Dissection: local Experience and Systematic Review. Neurosurgery. 2016;78(5):709–716.
84. Hoving JW, Marquering HA, Majoie C. Endovascular treatment in patients with carotid artery dissection and intracranial occlusion: a systematic review. Neuroradiology. 2017;59(7):641–647.
85. Gory B, Piotin M, Haussen DC, et al. Thrombectomy in Acute Stroke With Tandem Occlusions From Dissection Versus Atherosclerotic Cause. Stroke. 2017;48(11):3145–3148.
86. Schlemm L, von Rennenberg R, Siebert E, et al. Mechanical thrombectomy in patients with cervical artery dissection and stroke in the anterior or posterior circulation - A multicenter analysis from the German Stroke Registry. Neurol Res Pract. 2021;3(1):20.
87. Marnat G, Lapergue B, Sibon I, et al. Safety and Outcome of Carotid Dissection Stenting During the Treatment of Tandem Occlusions: a Pooled Analysis of TITAN and ETIS. Stroke. 2020;51(12):3713–3718.
88. Serkin Z, Le S, Sila C. Treatment of Extracranial Arterial Dissection: the Roles of Antiplatelet Agents, Anticoagulants, and Stenting. Curr Treat Options Neurol. 2019;21(10):48.
89. Rosati LM, Vezzetti A, Redd KT, et al. Early Anticoagulation or Antiplatelet Therapy Is Critical in Craniocervical Artery Dissection: results from the COMPASS Registry. Cerebrovasc Dis. 2020;49(4):369–374.
90. Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: a Guideline From the American Heart Association/American Stroke Association. Stroke. 2021;52(7):e364–e467.
91. Gill R, Biller J. Stroke Prevention in Cervical Artery Dissection. Curr Cardiol Rep. 2021;23(12):182.
92. Liebeskind DS Spontaneous cerebral and cervical artery dissection: treatment and prognosis. UpToDate, Waltham, MA; 2018. Available from: http://www.uptodate.com/contents/spontaneous-cerebral-and-cervical-artery-dissection-clinical-features-and-diagnosis.
93. Caprio FZ, Bernstein RA, Alberts MJ, et al. Efficacy and safety of novel oral anticoagulants in patients with cervical artery dissections. Cerebrovasc Dis. 2014;38(4):247–253.
94. Essibayi MA, Lanzino G, Keser Z. Vitamin K antagonist versus novel oral anticoagulants for management of cervical artery dissection: interactive systematic review and meta-analysis. Eur Stroke J. 2022;1:1–9.
95. Pezzini D, Grassi M, Zedde ML, et al. Antithrombotic therapy in the post acute phase of cervical artery dissection: the Italian Project on Stroke in Young Adults Cervical Artery Dissection. J Neurol Neurosurg Psychiatry. 2022.
96. Silbert PL, Mokri B, Schievink WI. Headache and neck pain in spontaneous internal carotid and vertebral artery dissections. Neurology. 1995;45(8):1517–1522.
97. Gallerini S, Marsili L, Bartalucci M, Marotti C, Chiti A, Marconi R. Headache secondary to cervical artery dissections: practice pointers. Neurol Sci. 2019;40(3):613–615.
98. Lai SL, Chang YY, Liu JS, Chen SS. Cluster-like headache from vertebral artery dissection: angiographic evidence of neurovascular activation. Cephalalgia. 2005;25(8):629–632.
99. Diamanti S, Longoni M, Agostoni EC. Leading symptoms in cerebrovascular diseases: what about headache? Neurol Sci. 2019;40(Suppl 1):147–152.
100. Shankar Kikkeri N, Nagarajan E, Sakuru RC, Bollu PC. Horner Syndrome Due to Spontaneous Internal Carotid Artery Dissection. Cureus. 2018;10(9):e3382.
101. Kellert L, Kloss M, Pezzini A, et al. Prognostic significance of pulsatile tinnitus in cervical artery dissection. Eur J Neurol. 2016;23(7):1183–1187.
102. English SW, Passe TJ, Lindell EP, Klaas JP. Multiple cranial neuropathies as a presentation of spontaneous internal carotid artery dissection: a case report and literature review. J Clin Neurosci. 2018;50:129–131.
103. Crum B, Mokri B, Fulgham J. Spinal manifestations of vertebral artery dissection. Neurology. 2000;55(2):304–306.
104. Eberhardt O, Topka H. Compressive Cervical Radiculopathy due to Vertebral Artery Dissection. J Stroke Cerebrovasc Dis. 2015;24(5):e115–116.
105. Nedeltchev K, Bickel S, Arnold M, et al. R2-recanalization of spontaneous carotid artery dissection. Stroke. 2009;40(2):499–504.
106. Touzé E, Gauvrit J, Meder J, Mas J. Prognosis of cervical artery dissection. Front Neurol Neurosci. 2005;20:129–139.
107. Larsson SC, King A, Madigan J, Levi C, Norris JW, Markus HS. Prognosis of carotid dissecting aneurysms: results from CADISS and a systematic review. Neurology. 2017;88(7):646–652.
108. Mokri B, Piepgras DG, Sundt TM, Pearson BW. Extracranial internal carotid artery aneurysms. Mayo Clin Proc. 1982;57(5):310–321.
109. Kremer C, Mosso M, Georgiadis D, et al. Carotid dissection with permanent and transient occlusion or severe stenosis: long-term outcome. Neurology. 2003;60(2):271–275.
110. Beletsky V, Nadareishvili Z, Lynch J, Shuaib A, Woolfenden A, Norris JW. Cervical arterial dissection: time for a therapeutic trial? Stroke. 2003;34(12):2856–2860.
111. Arauz A, Hoyos L, Espinoza C, Cantú C, Barinagarrementeria F, Román G. Dissection of cervical arteries: long-term follow-up study of 130 consecutive cases. Cerebrovasc Dis. 2006;22(2–3):150–154.
112. Dittrich R, Nassenstein I, Bachmann R, et al. Polyarterial clustered recurrence of cervical artery dissection seems to be the rule. Neurology. 2007;69(2):180–186.
113. Kloss M, Grond-Ginsbach C, Ringleb P, Hausser I, Hacke W, Brandt T. Recurrence of cervical artery dissection: an underestimated risk. Neurology. 2018;90(16):e1372–e1378.
114. Debette S, Markus HS. The genetics of cervical artery dissection: a systematic review. Stroke. 2009;40(6):e459–466.
115. Schievink WI, Mokri B, O’Fallon WM. Recurrent spontaneous cervical-artery dissection. N Engl J Med. 1994;330(6):393–397.
116. Fischer U, Ledermann I, Nedeltchev K, et al. Quality of life in survivors after cervical artery dissection. J Neurol. 2009;256(3):443–449.
117. Leys D, Bandu L, Hénon H, et al. Clinical outcome in 287 consecutive young adults (15 to 45 years) with ischemic stroke. Neurology. 2002;59(1):26–33.
118. Milhaud D, de Freitas GR, van Melle G, Bogousslavsky J. Occlusion due to carotid artery dissection: a more severe disease than previously suggested. Arch Neurol. 2002;59(4):557–561.
119. Arnold M, Bousser MG, Fahrni G, et al. Vertebral artery dissection: presenting findings and predictors of outcome. Stroke. 2006;37(10):2499–2503.
120. Shimizu Y, Yagi M. Pulsatile tinnitus and carotid artery dissection. Auris Nasus Larynx. 2018;45(1):175–177.
121. Pelkonen O, Tikkakoski T, Luotonen J, Sotaniemi K. Pulsatile tinnitus as a symptom of cervicocephalic arterial dissection. J Laryngol Otol. 2004;118(3):193–198.
122. Waespe W, Niesper J, Imhof HG, Valavanis A. Lower cranial nerve palsies due to internal carotid dissection. Stroke. 1988;19(12):1561–1564.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Cerebrovascular Fibromuscular Dysplasia – A Practical Review
Kesav P, Manesh Raj D, John S
Vascular Health and Risk Management 2023, 19:543-556
Published Date: 28 August 2023