Back to Journals » Infection and Drug Resistance » Volume 15
Cerebrospinal Fluid Changes and Clinical Features of Neurosyphilis Compared with Latent Syphilis Infection in the Central Nervous System: A Cross-Sectional Study
Authors Ge Y, Gou X, Dong X, Peng Y, Yang F
Received 14 May 2022
Accepted for publication 5 September 2022
Published 9 September 2022 Volume 2022:15 Pages 5377—5385
DOI https://doi.org/10.2147/IDR.S371446
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Yumei Ge,1,* Xiaoyu Gou,1,* Xiaoyan Dong,1,2 Yumeng Peng,1,3 Fangfang Yang4
1Department of Laboratory Medicine Center, Zhejiang Provincial People’s Hospital, Affiliated People’s Hospital, Hangzhou Medical College, Hangzhou, 310014, People’s Republic of China; 2The Second Medical College, Zhejiang Chinese Medical University, Hangzhou, 310014, People’s Republic of China; 3Department of Clinical Laboratory Diagnostics, Bengbu Medical College, Bengbu, 233030, People’s Republic of China; 4Department of Clinical Laboratory, The Third Hospital of Mianyang (Sichuan Mental Health Center), Mianyang, 621000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Fangfang Yang, Department of Clinical Laboratory, The Third Hospital of Mianyang (Sichuan mental health center), Mianyang, Sichuan, 621000, People’s Republic of China, Email [email protected]
Purpose: At present, there is no gold standard or unified standard for the diagnosis of neurosyphilis, and the rate of misdiagnosis is high. The diagnosis of neurosyphilis is still challenging. This study compared the clinical indicators between neurosyphilis and latent syphilis infection in the central nervous system. The purpose of this study was to provide evidence for the differential diagnosis and prognosis of patients with neurosyphilis and latent syphilis infection of the central nervous system.
Methods: The clinical data of 59 patients with neurosyphilis and 30 patients with latent syphilis infection in the nervous system from 2008 to 2021 were analyzed. The cerebrospinal fluid and serum biochemical markers were evaluated for all patients.
Results: CSF-nucleated cells, CSF-TRUST, CSF-totalprotein and CSF-IgG (P< 0.001) were significantly different between neurosyphilis and latent syphilis infection in the central nervous system. CSF-TRUST titer was positively correlated with D-D concentration (r = 0.274, P < 0.05), sodion (r =0.251, P < 0.05), respectively. Glucose concentration is the most unreliable in the diagnosis of neurosyphilis (AUC=0.445, P=0.395), and TRUST combined with nucleated cells and total protein is the most accurate in the diagnosis of neurosyphilis (AUC=0.989, P< 0.001).
Conclusion: The combination of TRUST, nucleated cell count and totalprotein detection in CSF can distinguish the patients with neurosyphilis and latent syphilis infection in the central nervous system, which has a significant diagnostic value.
Keywords: neurosyphilis, latent syphilis infection, diagnosis, detection index
Introduction
Neurosyphilis is a group of clinical syndromes caused by Treponema pallidum invading the nervous system, which impairs the meninges, brain, blood vessels or spinal cord.1 Neurosyphilis has the characteristics of great damage to the body, strong concealment and poor prognosis that it could occur in all stages of syphilis.2 At present, there is no gold or unified standard for the diagnosis of neurosyphilis as its clinical symptoms are similar to other nervous system diseases.3,4 Hence, the misdiagnosis rate is high, so the diagnosis of neurosyphilis is still challenging.
Generally, clinical criteria for the diagnosis of neurosyphilis include serology, cerebrospinal fluid examination and neurological symptoms. Serological tests include the nontreponema tests and treponema tests. When neurosyphilis is suspected, CSF examination is required additionally. CSF examination included cell counts, protein determination and TRUST. The increase of protein content (> 450 mg/L) or nucleated cells (> 5 cells/L) indicates the destruction of the blood-brain barrier and intracranial inflammatory reaction, which is helpful to the diagnosis of neurosyphilis. It plays an important role in the judgment of curative effect and prognosis of the disease as well.5
Guidelines from different regions of the world indicate that the positive cerebrospinal fluid VDRL/RPR /TRUST can be used as the marker for the diagnosis of neurosyphilis.6,7 Patients with cerebrospinal fluid VDRL positive and accompanied by neurological symptoms and signs, excluding blood contamination of cerebrospinal fluid, can be diagnosed with neurosyphilis. If the patient’s serological test is positive, cerebrospinal fluid VDRL is negative, but the patient’s cerebrospinal fluid cell count or protein determination is abnormal, and accompanied by neurosyphilis symptoms and signs, the diagnosis of neurosyphilis should be considered. Studies have shown that CSF-TRUST has the same diagnostic ability as CSF-VRDL, and can be used as an alternative experiment in areas where it is inconvenient to carry out CSF-VRDL.8
The serological tests of patients with neurosyphilis and latent syphilis infection in the central nervous system are all positive, there is no specific detection index for the differential diagnosis between neurosyphilis and latent syphilis infection in the central nervous system. The clinical data of 59 patients with neurosyphilis and 30 patients with latent syphilis infection in the central nervous system from 2008 to 2021 were statistically analyzed to provide the diagnostic basis for distinguishing neurosyphilis from latent syphilis infection in the central nervous system.
Methods
This study was approved by the ethics committee of the Zhejiang Provincial People’s Hospital. 59 patients with neurosyphilis and 30 patients with latent syphilis infection in the nervous system were analyzed, retrospectively. Neurosyphilis can be classified according to one of the following criteria (1) CSF-TPPA positive and CSF-TRUST positive; (2) CSF-TPPA positive and CSF-TRUST negative, CSF protein increased (> 500 mg/L) or CFS white blood cell count (> 10 cells/L); (3) CSF-TPPA positive, CSF-TRUST negative, there were signs or symptoms of neurosyphilis, and other clinical causes of such symptoms were excluded. The inclusion criteria for patients with latent syphilis infection in the central nervous system were as follows: serum-TPPA positive, CSF-TPPA positive, CSF-TRUST negative, and there were no nucleated cells or protein elevation in CSF, and no neurosyphilis symptoms and signs. All patients were negative for HBV, HCV and HIV. The exclusion criteria included (1) incomplete data of clinical and/ or relevant laboratory examinations, (2) severe liver or kidney diseases, clinical or laboratory data of infection, and autoimmune diseases. The latent subjects came from the following patients who underwent cerebrospinal fluid examination (1) syphilis history of more than 2 years and/or (2) worried about neurosyphilis.
The clinical data and laboratory results of these subjects were collected and recorded: age, gender, CSF parameters including TPPA, TRUST, nucleated cell counts, red blood cell counts, glucose, Pandy’s test, chloride, and lactate dehydrogenase (LDH), totalprotein (TP), microalbumin (mAlb), IgG. The results of TPPA, TRUST, complement C3, complement C4, IgG, IgA, IgM, PT, APTT, TT, Fib, DD, leukocyte, INR, white blood cell, lymphocyte, monocyte, neutrophils, platelets, eosinophils, basophils, NLR, PLR, LDH, TP, IgG, IgM, CRP, ALT, AST, TB, CB, K, Na, Cl, and GFR in peripheral blood were analyzed.
SPSS 24.0 (IBM SPSS software, Armonk, NY, USA) was used to analyze the statistical data, and GraphPad Prism 8.0 (GraphPad Software, La Jolla, CA) was used for plotting. The data were assessed for normality using the Shapiro–Wilk (S-W) test. Normally distributed data were reported by mean and standard deviation. The difference between the two groups was tested by Student’s t-test. Skewed variables were represented by median and interquartile ranges. Mann–Whitney U-test was used to compare the differences between the two groups. The difference in the constituent ratio was tested by χ2. Spearman correlation analysis was used to analyze the correlation between the two parameters. P-value <0.05 was considered significant.
Results
Basic Data of Study Populations
This study included 59 neurosyphilis (42 males and 17 females) aged 51.36± 15.15 years. There were 30 control patients with latent syphilis infection in the central nervous system, including 16 males and 14 females, aged 47.3± 18.54 years. There was no significant difference in age and gender between the two groups. The data of the study populations are shown in Table 1.
 |
Table 1 Basic Parameters and Cerebrospinal Fluid Parameters Between Neurosyphilis and Central Nervous System Latent Syphilis Patients |
Detection results of CSF, Urine, and Peripheral Blood of Patients with Neurosyphilis and Latent Syphilis Infection in the Central Nervous System
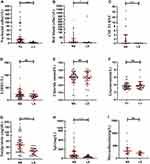
CSF-LDH (P = 0.1772, P > 0.05), CSF-chloride (P = 0.7841, P > 0.05), CSF-glucose (P = 0.3983, P > 0.05), CSF-microalbumin (mAlb) (P = 0.0522, P > 0.05), CSF-red blood cells (P =0.0043, P < 0.01), CSF-nucleated cells, CSF-TRUST, CSF-totalprotein and CSF-IgG (P < 0.001) were detected in patients with neurosyphilis and latent syphilis infection in central nervous system. The detailed results are shown in Figure 1.
Serum IgA, plasma TT and serum conjugated bilirubin (P < 0.05), serum TRUST and serum globulin (P < 0.01), neutrophils in peripheral blood and sodium ion in serum (P < 0.0001) have a significant statistical difference in neurosyphilis and patients of latent syphilis infection in the central nervous system.
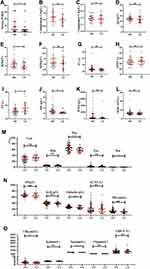
Urine and peripheral blood of patients with neurosyphilis and latent syphilis infection in central nervous system were detected and analyzed. The results showed there was no statistical significance in urine epithelial cells (P = 0.6022), plasma PT (P = 0.1709), plasma APTT (P = 0.6546), plasma Fibrinogen (Fib) (P = 0.4893), plasma D-Dimer (P = 0.1704), WBC (P = 0.4893), hemoglobin (P = 0.9868), platelet (P = 0.4402), lymphocytes (P = 0.0747), monocytes (P = 0.9262), eosinophil (EOS) (P = 0.6186), serum IgG (P = 0.3068), serum IgM (P = 0.4761, serum complement C3 (P = 0.0618), serum complement C4 (P = 0.4522), serum total protein (P = 0.3875), serum albumin (P = 0.8142), serum ALT (P = 0.4968), serum potassium (P = 0.0644), serum chloridion (P = 0.4226), serum ALT (P = 0.4718) between the two groups. The detailed results are shown in Figure 2.
Correlation Analysis Between Urine, Serum, Plasma and Peripheral Blood Test Results and Cerebrospinal Fluid Test Results of Neurosyphilis
We analyzed the relationship between urine, serum, plasma and peripheral blood and CSF-TRUST, cerebrospinal fluid chloride (C-Cl), cerebrospinal fluid glucose (C-GLU), cerebrospinal fluid lactate dehydrogenase (C-LDH), cerebrospinal fluid total protein (C-TP), cerebrospinal fluid IgG (C-IgG), serum TRUST of patients with neurosyphilis. CSF-TRUST titer was positively correlated with D-D concentration (r = 0.274, P < 0.05), sodion (r =0.251, P < 0.05), respectively. CSF Chloride was negatively correlated with urine epithelium (r=−0.393, P<0.05), ALB (r =0.252, P<0.05), respectively. However, it was positively correlated with CRP (r =0.342, P<0.05), Fib (r =0.248, P<0.05), respectively. C-GLU was negatively correlated with PLT (r =−0.285, P<0.05). Nevertheless, it was positively correlated with Hb (r =0.238, P<0.05), ALT (r=0.270, P<0.05), respectively. CSF-LDH was negatively correlated with urine epithelium (r =−0.261, P<0.05), IgG (r =−0.354, P<0.05), TT (r =−0.348, P<0.05), Lym (r =−0.310, P<0.05), TP (r =−0.288, P<0.05), ALB (r=−0.287, P<0.05), respectively. It was positively correlated with Fib (r =0.373, P<0.05), D-D (r =0.248, P<0.05), Neu (r =0.363, P<0.05), CRP (r = 0.514, P<0.05), respectively. CSF-IgG was negatively correlated with Lym (r =−0.343, P<0.05). It was positively correlated with Fib (r =0.373, P<0.05), D-D (r=0.248, P<0.05), Neu(r =0.363, P<0.05), CRP(r =0.514, P<0.05), respectively. CSF-IgG was negatively correlated with Lym (r=−0.343, P<0.05). It was positively correlated with Fib (r =0.302, P<0.05), Neu (r =0.318, P<0.05), G(r =0.314, P<0.05), respectively. Serum-TRUST titer was negatively correlated with Urine epithelium cells (r =−0.247, P<0.05), TT (r =−0.252, P<0.05), LDH (r =−0.252, P< 0.05), respectively. The detailed results are shown in Figure 3. In addition, we provide the r and P value of correlation analysis between parameters in Tables S1 and S2.
 |
Figure 3 Heat map of correlation analysis between urine, serum, plasma, and peripheral blood test results and cerebrospinal fluid test results of neurosyphilis. *, P<0.05. |
Sensitivity and Specificity Analyses of CSF Red Blood Cells, CSF Nucleated Cells, CSF TRUST, CSF Chloride, CSF Glucose, and CSF Total Protein
ROC analysis was used to evaluate CSF red blood cells, CSF nucleated cells, CSF TRUST, CSF chloride, CSF glucose, CSF totalprotein, CSF TRUST combined with CSF nucleated cells, CSF TRUST combined with CSF totalprotein, CSF TRUST combined with CSF totalprotein, CSF TRUST combined with CSF nucleated cells and CSF TRUST combined with CSF totalprotein. ROC analysis showed different sensitivity, specificity and area under curve (AUC) for different cut-off points. Statistics found that the glucose concentration test is the most unreliable in the diagnosis of neurosyphilis (AUC=0.445, P=0.395), and TRUST combined with nucleated cells and totalprotein is the most accurate in the diagnosis of neurosyphilis (AUC=0.989, P<0.001). The results are shown in Table 2 and Figure 4.
 |
Table 2 Evaluation of Cerebrospinal Fluid Indexes of Neurosyphilis |
 |
Figure 4 ROC curve for neurosyphilis diagnosis indicators. |
Discussion
Neurosyphilis is a chronic central nervous system disease that can cause nervous tissue and vascular diseases.9–11 It may occur 5–10 years after the initial infection. About 13.5–30% of untreated syphilis patients eventually develop neurosyphilis.12 At present, there is no gold standard for the diagnosis of neurosyphilis.13
The clinical symptoms of neurosyphilis are various. It is often misdiagnosed because of its similarity with other neurological diseases. Neuroimaging could be used to help neurosyphilis to identify the location of the lesion, reflect the severity of the lesion to a certain extent, and evaluate the prognosis.14 Nevertheless, other central nervous system diseases from virus infection, Alzheimer’s disease, and ischemic stroke may have similar changes, which may have little significance for etiological diagnosis.15 Therefore, the clinical laboratory-related neurosyphilis screening indicators play an irreplaceable role in the diagnosis of neurosyphilis. However, the diagnosis of neurosyphilis is still a great challenge, especially in the differential diagnosis of neurosyphilis and latent syphilis infection in the central nervous system, there is a lack of effective diagnostic markers. Previous studies have shown that increased protein content in cerebrospinal fluid is a marker of blood-brain barrier dysfunction.16 Our results also confirmed that CSF total protein plays a significant role in distinguishing patients with neurosyphilis from patients with latent syphilis infection in the central nervous system. Clinical prediction and diagnosis of neurosyphilis in HIV-negative patients have been reported in the literature, and the author put forward that neurological symptoms, CSF TPPA titer, CSF protein, and CSF WBC were identified as independent predictors of neurosyphilis.17 However, we found that TPPA was positive in both patients with neurosyphilis and latent syphilis in the central nervous system. It is difficult to distinguish the clinical types of these two kinds of syphilis. The results of this study show that CSF-nucleated cells, CSF-TRUST, CSF-TP, CSF-IgG, and sodion play a crucial role in distinguishing patients with neurosyphilis from patients with latent syphilis. IgA, TT, conjugated bilirubin, red blood cells, TRUST, Fib and globulin are of great significance in the diagnosis of neurosyphilis.
There were some potential limitations in our study. First, this study was a retrospective study of the clinical data of neurosyphilis patients and patients with latent syphilis infection in the central nervous system from 2007 to 2021, which might lead to regional limitations in the results of this study as well as lead us to be unable to understand the changes of the disease in time. In addition, Because the diagnosis of neurosyphilis and latent syphilis infection in the central nervous system still lacks the gold standard so far, there may be a certain possibility of misjudgment when diagnosing neurosyphilis.
Conclusion
IgA, TT, conjugated bilirubin, red blood cells, TRUST, Fib and globulin are of great significance in the diagnosis of neurosyphilis. CSF-nucleated cells, CSF-TRUST, CSF-TP, CSF-IgG, neutrophils and sodium ions play a very significant role in distinguishing neurosyphilis from latent syphilis infection in the central nervous system. CSF-TRUST combined with nucleated cells and total protein is the most accurate biomarker for the diagnosis of neurosyphilis.
Abbreviations
IgA, immunoglobulin A; CSF, cerebrospinal fluid; TRUST, toluidine red unheated serum test; VDRL, venereal disease research laboratory test; RPR, rapid plasma reagin test; TPPA, treponema pallidum particle agglutination assay; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; LDH, lactate dehydrogenase; TP, totalprotein; mAlb, microalbumin; IgG, immunoglobulin G; IgM, immunoglobulin M; CRP, C-reactive protein; PT, Prothrombin time; APTT, activated partial thromboplastin time; TT, thrombin time; Fib, fibrinogen; DD, D-dimer; INR, international normalized ratio; WBC, white blood cell; Lym, lymphocyte; Mon, monocyte; Neu, neutrophil; PLT, platelet; Eos, eosinophil; Bas, basophil; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte; HCT, hematocrit; RDW-CT, red cell distribution width coefficient of variation; ALT, alanine aminotransferase; AST, aspartate aminotransferase; mAlb, microalbumin; Hb, hemoglobin; TP, total protein; G, globulin; ALB, albumin; ROC, receiver operating characteristic curve; AUC, area under curve; ALP, alkalinephosphatase; TB, total bilirubin; CB, conjugated; Cr, creatinine; UA, uric acid; TG, triglyceride; TC, total cholesterol; LDLC, low-density lipoprotein cholesterol; HDLC, high-density lipoprotein cholesterol; Glu, glucose. K, potassium ion; Na, sodium ion; Cl, chloride ion. GFR, glomerular filtration rate.
Compliance with Ethical Standards
The authors declare that they have compliance with ethical standards.
Ethical Approval
As the study was performed with retrospective resources and will not have any impact on the diagnosis and treatment of patients, the ethics committee of Zhejiang Provincial People’s hospital has approved the exemption of informed consent (2019KY311). The study has been performed by the ethical standards laid down in the 1964 Declaration of Helsinki.
Acknowledgments
The authors would like to thank the encouragement and support of all the members in the Department of Clinical Laboratory, Zhejiang Provincial People’s Hospital. Yumei Ge and Xiaoyu Gou are co-first authors for this study.
Funding
Zhejiang Provincial Natural Science Fund (LY20H200008). The funding body did not participate in the design of the study; the collection, analysis, and interpretation of the data; or in the writing the manuscript.
Disclosure
The authors declare that they have no competing interests.
References
1. Peermohamed S, Kogilwaimath S, Sanche S. Neurosyphilis. Cmaj. 2020;192(29):E844. doi:10.1503/cmaj.200189
2. Ren M, Dashwood T, Walmsley S. The intersection of HIV and syphilis: update on the key considerations in testing and management. Curr HIV/AIDS Rep. 2021;18(4):280–288. doi:10.1007/s11904-021-00564-z
3. Dersch R, Singh AE. Neurosyphilis and Lyme neuroborreliosis. Curr Opin Neurol. 2021;34(3):403–409. doi:10.1097/wco.0000000000000923
4. Tuddenham S, Katz SS, Ghanem KG. Syphilis laboratory guidelines: performance characteristics of nontreponemal antibody tests. Clin Infect Dis. 2020;71(Suppl1):S21–S42. doi:10.1093/cid/ciaa306
5. Drago F, Ciccarese G, Parodi A. Cerebrospinal fluid tests for neurosyphilis diagnosis. Sex Transm Infect. 2020;96(5):387. doi:10.1136/sextrans-2020-054465
6. Prevention CfDCa. STD surveillance case definitions. Available from: https://www.cdc.gov/std/stats/casedefinitions-2014.pdf.
7. Janier M, Hegyi V, Dupin N, et al. 2014 European guideline on the management of syphilis. J Eur Acad Dermatol Venereol. 2014;28(12):1581–1593. doi:10.1111/jdv.12734
8. Gu W, Yang Y, Wu L, Yang S, Ng LK. Comparing the performance characteristics of CSF-TRUST and CSF-VDRL for syphilis: a cross-sectional study. BMJ Open. 2013;3(2):e002204. doi:10.1136/bmjopen-2012-002204
9. Shi M, Zhou Y, Li Y, et al. Young male with syphilitic cerebral arteritis presents with signs of acute progressive stroke: a case report. Medicine. 2019;98(48):e18147. doi:10.1097/md.0000000000018147
10. Xu L, Han F. Neurosyphilis complicated with pial arteriovenous fistula: a rare case report. Medicine. 2019;98(45):e17770. doi:10.1097/md.0000000000017770
11. Qi S, Xu Y, Luo R, et al. Novel biochemical insights in the cerebrospinal fluid of patients with neurosyphilis based on a metabonomics study. J Mol Neurosci. 2019;69(1):39–48. doi:10.1007/s12031-019-01320-0
12. Friedrich F, Geusau A, Greisenegger S, Ossege M, Aigner M. Manifest psychosis in neurosyphilis. Gen Hosp Psychiatry. 2009;31(4):379–381. doi:10.1016/j.genhosppsych.2008.09.010
13. Skalnaya A, Fominykh V, Ivashchenko R, et al. Neurosyphilis in the modern era: literature review and case series. J Clin Neurosci. 2019;69:67–73. doi:10.1016/j.jocn.2019.08.033
14. Narvaez EO, Ramos MC, Faria Do Amaral LL, Reis F. Neurosyphilis and high-resolution vessel wall imaging: a powerful tool to detect vasculitis and neuritis. Neurol India. 2022;70(1):160–161. doi:10.4103/0028-3886.338673
15. Jum’ah A, Aboul Nour H, Alkhoujah M, Zoghoul S, Eltous L, Miller D. Neurosyphilis in disguise. Neuroradiology. 2022;64(3):433–441. doi:10.1007/s00234-021-02827-3
16. Levchik N, Ponomareva M, Surganova V, Zilberberg N, Kungurov N. Criteria for the diagnosis of neurosyphilis in cerebrospinal fluid: relationships with intrathecal immunoglobulin synthesis and blood-cerebrospinal fluid barrier dysfunction. Sex Transm Dis. 2013;40(12):917–922. doi:10.1097/olq.0000000000000049
17. Lu Y, Ke W, Yang L, et al. Clinical prediction and diagnosis of neurosyphilis in HIV-negative patients: a case-control study. BMC Infect Dis. 2019;19(1):1017. doi:10.1186/s12879-019-4582-2
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.