Back to Journals » International Journal of Nanomedicine » Volume 13 » T-NANO 2014 Abstracts
Cellular internalization and antioxidant activity of cerium oxide nanoparticles in human monocytic leukemia cells
Authors Patel P, Kansara K, Singh R, Shukla RK, Singh S, Dhawan A, Kumar A
Received 18 October 2016
Accepted for publication 31 October 2016
Published 15 March 2018 Volume 2018:13(T-NANO 2014 Abstracts) Pages 39—41
DOI https://doi.org/10.2147/IJN.S124996
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lei Yang
Pal Patel, Krupa Kansara, Ragini Singh, Ritesh K Shukla, Sanjay Singh, Alok Dhawan, Ashutosh Kumar
Division of Biological and Life Sciences (Formerly Institute of Life Sciences), School of Arts and Sciences, Ahmedabad University, Ahmedabad, Gujarat, India
Abstract: Overproduction of free radicals contributes to oxidative stress and inflammation leading to various disease conditions. Cerium oxide nanoparticles (nanoceria) have been shown to scavenge free radicals and have the potential for being used as a therapeutic agent in disease conditions. Therefore, in the present study, human monocytic leukemia cells (THP-1) were used as a model to evaluate the uptake and free radical scavenging activity of nanoceria. Our data showed a significant (P<0.05) increase in the internalization of nanoceria in a concentration-dependent (10–100 µg/mL) manner in THP-1 cells. Although no cytotoxicity was observed at these concentrations, nanoceria significantly (P<0.05) reduced the amount of reactive oxygen species. This was evident by a significant (P<0.05) decrease in the 2,7-dichlorofluorescein diacetate fluorescence observed in flow cytometry and fluorescence microscopy. The present study shows that nanoceria have therapeutic potential in diseases such as cancer.
Keywords: nanoceria, reactive oxygen species, inflammation, free radicals, antioxidant
Introduction
Inflammation plays a key role in the development of major diseases including cancer, heart failure, arthritis, and Alzheimer’s disease.1 Overproduction of free radicals contributes to inflammation and oxidative stress. Cerium oxide nanoparticles (nanoceria) exhibit antioxidant properties that could be used to develop a therapeutic agent for inflammation and oxidative stress-mediated diseases in future. Reactive oxygen species (ROS) are by-products of normal oxygen metabolism that can cause oxidative damage to macromolecules in biological cells. Free radical species produced within the cell include superoxide (O2−) and hydrogen peroxide (H2O2) and are neutralized in cells by specific enzymes, primarily superoxide dismutase (SOD) and catalase, respectively.2 Nanoceria can act as a catalyst that mimics the antioxidant enzyme SOD. Nanoceria switch between their Ce+3 and Ce+4 oxidation states and are able to scavenge free radicals or ROS.3 In the present study, an attempt was made to investigate the antioxidant properties of nanoceria in THP-1 cells.
Materials and methods
Synthesis of nanoceria
Nanoceria were synthesized according to the method described by Hirst et al,3 with slight modifications. Briefly, in a reaction volume of 50 mL, 5 mM cerium (III) nitrate hexahydrate was added in Milli-Q water and stirred for 15 minutes. Subsequently, 0.4 M H2O2 was added into the above solution (pH was kept below 3) and stirred until yellow color was observed (cerium [III] ions convert into the cerium [IV] oxide due to oxidation reaction).
Characterization of nanoceria
Hydrodynamic size and zeta potential of nanoceria were determined using Zetasizer Nano-ZS (Malvern Instruments, Malvern, UK), and ultraviolet–visible (UV–Vis) measurement was done using UV spectrophotometer (Synergy HT Multi-Mode Microplate Reader; BioTek, Winooski, VT, USA).
Cellular internalization of nanoceria
The experiments were performed in THP-1 cells, which is a human monocytic cell line derived from an acute monocytic leukemia patient, and were purchased from National Centre for Cell Science, Pune, India. Internalization of nanoceria in THP-1 cells (1×105 cells/mL) was assessed at different concentrations (10–100 μg/mL) according to the method developed by Suzuki et al.4
Cytotoxicity of nanoceria
Cytotoxic potential of nanoceria was assessed by propidium iodide (PI) uptake assay using flow cytometer.5 Cells were seeded at density of 1×105 cells/mL and were exposed to different concentrations of nanoceria (10–100 μg/mL) for 24 hours.
Intracellular ROS measurement
ROS generation was detected by 2,7-dichlorofluorescein diacetate dye using fluorescence microscope and flow cytometer.6
Results and discussion
The mean hydrodynamic size and zeta potential of synthesized nanoceria were found to be 161.8±0.8 nm and 32.0±0.15 mV, respectively. High positive charge suggests that synthesized nanoceria are stable in suspension. Change in the oxidation state of surface atom “Ce” in nanoceria was followed for 2 weeks (0, 7, and 15 days). After 15 days of synthesis, two broad absorbance peaks were observed at 250 and 300 nm, which could be attributed due to the presence of mixed oxidation states (+3 and +4, respectively; Figure 1) of surface atom of “Ce” in nanoceria.
  | Figure 1 Ultraviolet–visible spectrum of nanoceria. |
A concentration-dependent significant (P<0.05) increase in the side scatter intensity of THP-1-treated cells was observed after 24 hours’ exposure of nanoceria in flow cytometer, indicating the internalization of nanoparticles in cells (Figure 2A).
Furthermore, any potential cytotoxicity caused by nanoceria due to active internalization in THP-1 cells was determined by estimation of PI uptake. It is evident from Figure 2B that there is no significant increase in PI uptake suggesting that nanoceria, even up to 100 μg/mL concentration, do not induce any membrane damage and thus cytotoxicity.
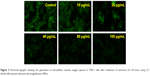
It has been shown earlier that internalization of nanoparticles induces generation of free radicals in cytoplasm, which may be harmful to the cells.7 The present study shows that even though nanoceria particles were internalized in THP-1 cells, they did not induce ROS generation. On the contrary, nanoceria significantly decreased the free radical pool from the THP-1 cells in a concentration-dependent manner (Figure 3). This indicates that unlike other oxide nanoparticles, nanoceria are rapidly internalized by THP-1 cells and retain their antioxidant activity even in the complex pool of cytoplasm.
Conclusion
Our results demonstrate that nanoceria act as an antioxidant as evident by their free radical scavenging activity in mammalian cells and could be a possible therapeutic agent for inflammation and oxidative stress-mediated diseases.
Acknowledgments
The authors acknowledge the funding from the Centre for Nanotechnology Research and Application (CENTRA) by the Gujarat Institute for Chemical Technology (GICT) and the project NanoTOF funded by the Department of Biotechnology, Government of India.
Disclosure
The authors report no conflicts of interest in this work.
References
Killeen MJ, Linder M, Pontoniere P, Crea R. NF-κβ signaling and chronic inflammatory diseases: exploring the potential of natural products to drive new therapeutic opportunities. Drug Discov Today. 2014;19:373–378. | ||
Karakoti AS, Singh S, Dowding JM, Seal S, Self WT. Redox-active radical scavenging nanomaterials. Chem Soc Rev. 2010;39:4422–4432. | ||
Hirst SM, Karakoti AS, Tyler RD, Sriranganathan N, Seal S, Reilly CM. Anti-inflammatory properties of cerium oxide nanoparticles. Small. 2009;5:2848–2856. | ||
Suzuki H, Toyooka T, Ibuki Y. Simple and easy method to evaluate uptake potential of nanoparticle in mammalian cells using a flow cytometric light scatter analysis. Environ Sci Tech. 2007;41:3018–3024. | ||
Dengler WA, Schulte J, Berger DP, Mertelsmann R, Fiebig HH. Development of a propidium iodide fluorescence assay for proliferation and cytotoxicity assays. Anticancer Drug. 1995;6(4):522–532. | ||
Lawler JM, Song W, Demaree SR. Hindlimb unloading increases oxidative stress and disrupts antioxidant capacity in skeletal muscle. Free Radic Biol Med. 2003;35(1):9–16. | ||
Kansara K, Patel P, Shah D, et al. TiO2 nanoparticles induce DNA double strand breaks and cell cycle arrest in human alveolar cells. Environ Mol Mutagen. 2015;56:204–217. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.