Back to Journals » International Journal of Nanomedicine » Volume 18
Cell Membrane-Camouflaged Nanoparticles Mediated Nucleic Acids Delivery
Authors Lin Y, Guan X, Su J, Chen S, Fu X, Xu X, Deng X, Chang J, Qin A, Shen A , Zhang L
Received 3 August 2023
Accepted for publication 16 December 2023
Published 28 December 2023 Volume 2023:18 Pages 8001—8021
DOI https://doi.org/10.2147/IJN.S433737
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor R.D.K. Misra
Yinshan Lin,1,2,* Xiaoling Guan,1,2,* Jianfen Su,1,2,* Sheng Chen,2 Xihua Fu,1 Xiaowei Xu,2 Xiaohua Deng,2 Jishuo Chang,2 Aiping Qin,2,* Ao Shen,2,* Lingmin Zhang1,2,*
1Pharmacy Department & Panyu Institute of Infectious Diseases, Guangzhou Panyu Central Hospital, Guangzhou, Guangdong, 511400, People’s Republic of China; 2Guangdong Provincial Key Laboratory of Molecular Target & Clinical Pharmacology, the NMPA and State Key Laboratory of Respiratory Disease, School of Pharmaceutical Sciences and the Fifth Affiliated Hospital, Guangzhou Medical University, Guangzhou, Guangdong, 511436, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lingmin Zhang, Guangzhou Panyu Central Hospital, No. 8 Fuyu East Road, Guangzhou, Guangdong, 511400, People’s Republic of China, Email [email protected] Ao Shen; Aiping Qin, Guangzhou Medical University, No. 1 Xinzao Road, Guangzhou, Guangdong, 511426, People’s Republic of China, Email [email protected]; [email protected]
Abstract: Nucleic acids have emerged as promising therapeutic agents for many diseases because of their potential in modulating gene expression. However, the delivery of nucleic acids remains a significant challenge in gene therapy. Although viral vectors have shown high transfection efficiency, concerns regarding teratogenicity or carcinogenicity have been raised. Non-viral vehicles, including cationic polymers, liposomes, and inorganic materials possess advantages in terms of safety, ease of preparation, and low cost. Nevertheless, they also face limitations related to immunogenicity, quick clearance in vivo, and lack of targeting specificity. On the other hand, bioinspired strategies have shown increasing potential in the field of drug delivery, yet there is a lack of comprehensive reviews summarizing the rapid development of bioinspired nanoparticles based on the cell membrane camouflage to construct the nucleic acids vehicles. Herein, we enumerated the current difficulties in nucleic acid delivery with various non-viral vehicles and provided an overview of bioinspired strategies for nucleic acid delivery.
Keywords: nucleic acids, bioinspired nanoparticles, non-viral vehicles, gene therapy, cell membranes
Graphical Abstract:
Introduction
Nucleic acids have promising therapeutic potential for the treatment of various diseases including genetic disorders,1 cancer,2 cardiovascular diseases,3 and infectious diseases,4 as they can target specific genes and modulate their expression. However, the delivery of nucleic acids, such as plasmid DNA (pDNA), small interfering RNA (siRNA), self-amplifying RNA (saRNA), messenger RNA (mRNA), aptamer, and antisense oligonucleotides (ASO) presents significant challenges in gene therapy. Several obstacles need to be overcome before nucleic acids become an effective therapy. First of all, nucleic acids are susceptible to degradation by nucleases in the extracellular environment, which limits their stability and activity. Besides, the lack of tissue or cell selectivity may lead to off-target and other side effects. The negative charge and hydrophilic nature of nucleic acids also pose impediments to crossing cell membranes. And lastly, even if they manage to enter the cells, nucleic acids are hard to escape from lysosomes, which also hamper therapy efficiency.5,6
There are three main approaches for delivering nucleic acids: biological methods, physical methods, and chemical methods. Biological methods mainly rely on viral vectors which are the very first used in nucleic acids delivery due to their high efficiency. Some common examples of viral vectors include retrovirus, adeno-associated virus, adenovirus, lentivirus, and virus-like particles.7 However, the complex compositions of viral vectors remain a problem. Techniques using physical methods then have been developed to deliver nucleic acids into cells both in vitro and in vivo. Electroporation and ultrasonic perforation can transfect nucleic acids into cells by perforating cell membranes.8,9 Such methods apply mechanical force, which may disrupt the structural integrity and lead to cell death. Microinjection is injecting nucleic acids into cells with the assistance of a micropipette and microscope.10 But it needs specialized instruments and can only operate one cell at a time. Chemical methods involve the use of composite nanoparticles, including liposomes,11 polymer,12 peptides,13 gold nanoparticle,14 mesoporous silica,15 and so on. Compared to viral vectors, nanoparticles offer relatively better biocompatibility, structural stability, and simpler preparation scheme.16 Nevertheless, nanoparticles also have certain limitations in terms of immunogenicity, quick clearance in vivo, and lack of targeting specificity.17
To address these limitations, the bioinspired strategy by producing non-viral vehicles with biomimetic structures and functions has raised the attention of potential clinical applications in the field of drug delivery. One of the most powerful approaches to produce bioinspired nanoparticles was cell membrane coating nanotechnology, which was performed by coating synthetic nanoparticles with natural cell membranes. This technology has been extended to other biomembranes, such as the membranes derived from organelles or extracellular vesicles. The cell membrane camouflaged nanoparticles (CMCNPs) have been considered novel nucleic acid vehicles, which possess biocompatibility, low immunogenicity, and efficient targeting capabilities. The cell membrane coating also acts as a protective layer for the nanoparticles and nucleic acids, promoting gene therapy efficiency.18–20 The use of CMCNPs is a promising strategy for gene therapy. Herein, we summarized the difficulties in nucleic acid delivery with nanoparticles at present and provided an overview of the applications of CMCNPs in nucleic acid delivery.
Difficulties in Nucleic Acid Delivery with Nanoparticles at Present
The delivery of nucleic acids with nanoparticles is a complex process that involves several challenges (Figure 1). One of the main challenges is the dissociation of nucleic acids from nanoparticles while circulating. Free nucleic acids are susceptible to nucleases in the blood and extracellular environment, limiting their stabilities and therapeutic potential. To address this issue, CMCNPs provide enhanced protection for nucleic acids, which can reduce dissociation and degradation during delivery.
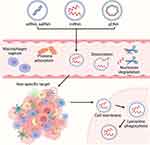 |
Figure 1 Difficulties in nucleic acid delivery with nanoparticles. Created with BioRender.com. |
 |
Figure 4 The sources and functions of cell membranes. Created with BioRender.com. |
Another challenge is the immunogenicity of both free nucleic acids and encapsulated nucleic acids by nanoparticles. They can be detected and phagocytosed by the reticuloendothelial system, leading to a short circulation time. While PEGylation can make nanoparticles “stealth”, it may also lead to potential tissue toxicity and adverse reactions.21 CMCNPs, on the other hand, demonstrate good biocompatibility, reduced immune recognition, and prolonged half-life.
The third challenge is the off-target effects of nucleic acid delivery, potentially causing unintended gene silencing or activation. This is often due to the non-specific binding of nanoparticles to non-target tissues or cells. Nanoparticles need to possess certain sizes and specific ligands to achieve targeting, but modifying nanoparticles through complicated chemical reactions may affect their structures and properties. In contrast, CMCNPs facilitate targeting without chemical conjugations. For example, cancer cell membranes camouflaged nanoparticles improved the recognition to the tumor tissues by homologous targeting, while stem cell membranes camouflaged nanoparticles naturally show tumor tropism.
To exert their therapeutic effect, nanoparticles must enter target cells through processes such as pinocytosis or membrane insertion. The cell membranes, which are lipid bilayers, act as barriers that distinguish the intracellular environment from the extracellular environment. CMCNPs often show enhanced cellular uptake through alternative endocytic pathways such as membrane fusion or receptor-mediated endocytosis. Escaping from endo/lysosomes is a prerequisite for successful gene therapy, and CMCNPs may facilitate this process by fusing with lysosomal membranes.
Preparation of Cell Membranes Camouflaged Nanoparticles for Nucleic Acid Delivery
Cell membranes camouflaged nanoparticles (CMCNPs) have emerged as a promising strategy for the delivery of nucleic acids, offering enhanced targeting ability and efficient intracellular delivery. The preparations of CMCNPs typically involve three key steps: cell membranes extraction, fabrication of nanoparticles for nucleic acids loading, and CMCNPs construction.
Extraction of Cell Membranes
Different methods had been applied to extract cell membranes, including hypotonic lysis, freeze-thaw, ultrasonication, and homogenization. Hypotonic lysis is to swell cells under low osmotic pressure. While this method is widely used in erythrocyte membrane preparation, it is not suitable for other cell types due to relatively low efficiency.22,23 Freeze-thaw is a universal method where cells are repeatedly frozen at low temperatures and thawed at room temperature.24,25 During this process, cell membranes are prone to rupture in the presence of ice crystals. Ultrasonication is another efficient method to isolate cancer cell membranes. The shock waves and shear forces generated by the ultrasonic apparatus disrupt cell membranes. Homogenization has been used for a broad scope of applications. Regardless of the method chosen, it is crucial to maintain the integrity of cell membranes and the activity of proteins on cell membranes. The addition of proteinase inhibitors and conducting the procedures at low temperatures are prerequisites for preserving the functionality of cell membranes. We compared the advantages and disadvantages of cell membranes extraction methods in Table 1.
 |
Table 1 Comparation of Cell Membranes Extraction Methods |
Fabrication of Nanoparticles for Nucleic Acid Loading
The second step is fabricating the nanoparticles to load nucleic acids. Various materials, such as liposome,35 metal-organic frameworks (MOF),36 poly(β-amino ester) (PBAE),37 poly(ethylene imine) (PEI),38 chitosan,39 poly(lactic-co-glycolic acid) (PLGA),40 mesoporous silica nanoparticles41 have been used for gene delivery. Lipids like N, N, N-trimethyl-2,3-bis (octadec-9-en-1-yloxy) propan-1-aminium chloride (DOTMA),42 2,3-Dioleoyloxy-propyl-trimethylammonium-chloride (DOTAP),43 1, 2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE)44 are commonly used in cationic liposome, in which nucleic acid encapsulated in the inner hydrophilic phase or complexed on the surface because of the electrostatic adsorption (Figure 2). Composed by ionizable or cationic lipids, phospholipids, and cholesterol, the lipid nanoparticles (LNP) exhibit better stability than liposomes. PLGA, a biodegradable polymer approved by Food and Drug Administration, shows low nucleic acid delivery capacity due to its negative charge. Zhang et al developed a PLGA/DOTAP core to ensure high loading efficiency of siRNA.45 DOTAP was positively charged and thus can interact with the negatively charged siRNA inside the aqueous core and enhance encapsulation. Moreover, DOTAP improves the endo/lysosomal escape by its fusogenic property or proton sponge effect. Nucleic acid loading can be performed during or after the nanoparticle fabrication. For example, Qiu et al used a microfluidic system to mix synthetic active lipidoid, cholesterol, 1.2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), and 1.2-dimyristoyl-rac-glycero-3-methoxypolyethylene glycol-2000 (DMG-PEG2000) (50: 38.5: 10: 1.5) in ethanol solution with sodium acetate buffer containing mRNA.46 By doing so, mRNA was encapsulated in the internal hydrophilic cavity. Polymers such as PEI, PBAE, and chitosan which are positively charged, can form complexes with negatively charged nucleic acids spontaneously (Figure 2). Calcium phosphate nanoparticles possess biocompatibility and biodegradability, which load nucleic acids by charge neutralization. Carbon nanocages and mesoporous silicas demonstrate specific surface area, which load nucleic acids by absorbing. Metallic nanoparticles, such as gold nanoparticles, metal-organic frameworks (MOF), and Iron nanoparticles benefit from the advantages of small size and stable morphologies. The optimized materials can not only complex the nucleic acids, but possess the characteristics, such as biodegradability, selective distribution, environmental responsiveness, and controlled release within the cells.
Construction of CMCNPs
The camouflage with cell membranes is one of the most important procedures that significantly affects the fabrication and performance of CMCNPs. The source of cell membranes can vary, such as erythrocytes,47 platelets,48 stem cells,49 macrophage,50 or cancer cells51 depending on the desired properties and targeting ability. The approaches mainly include vortex, extrusion, sonication, and microfluidic electroporation. Cell membranes with negative charge because of the glycosylation domain of membrane protein and phospholipids can roughly coat positively charged nanoparticles such as PBAE and MOF through the mutual attraction of positive and negative charges by means of vortex. The extrusion of cell membranes and nanoparticles through polycarbonate film in different apertures are commonly employed to consolidate the core-shell CMCNPs and standardize the particle size based on the mobility of cell membranes (Figure 3).52 Sonication also has been used in several works to construct CMCNPs.53,54 Unlike extrusion, sonication minimizes material loss during the process and gets CMCNPs in good dispersion. The power, frequency, and duration of sonication influence the coating efficiency (Figure 3). Both extrusion and sonication are by virtue of external force. However, it should be noted that sonication can disrupt electrostatic interaction and thus may not be suitable for nanoparticles complexed with nucleic acids on their surface. Microfluidic electroporation is a newly emerging technology by generating temporary hydrophilic holes through the cell membranes using quick high-voltage electric field pulses (Figure 3), which offers the advantages of high throughput and quantitative format.55,56
Applications for Nucleic Acid Delivery by Cell Membranes Camouflaged Nanoparticles
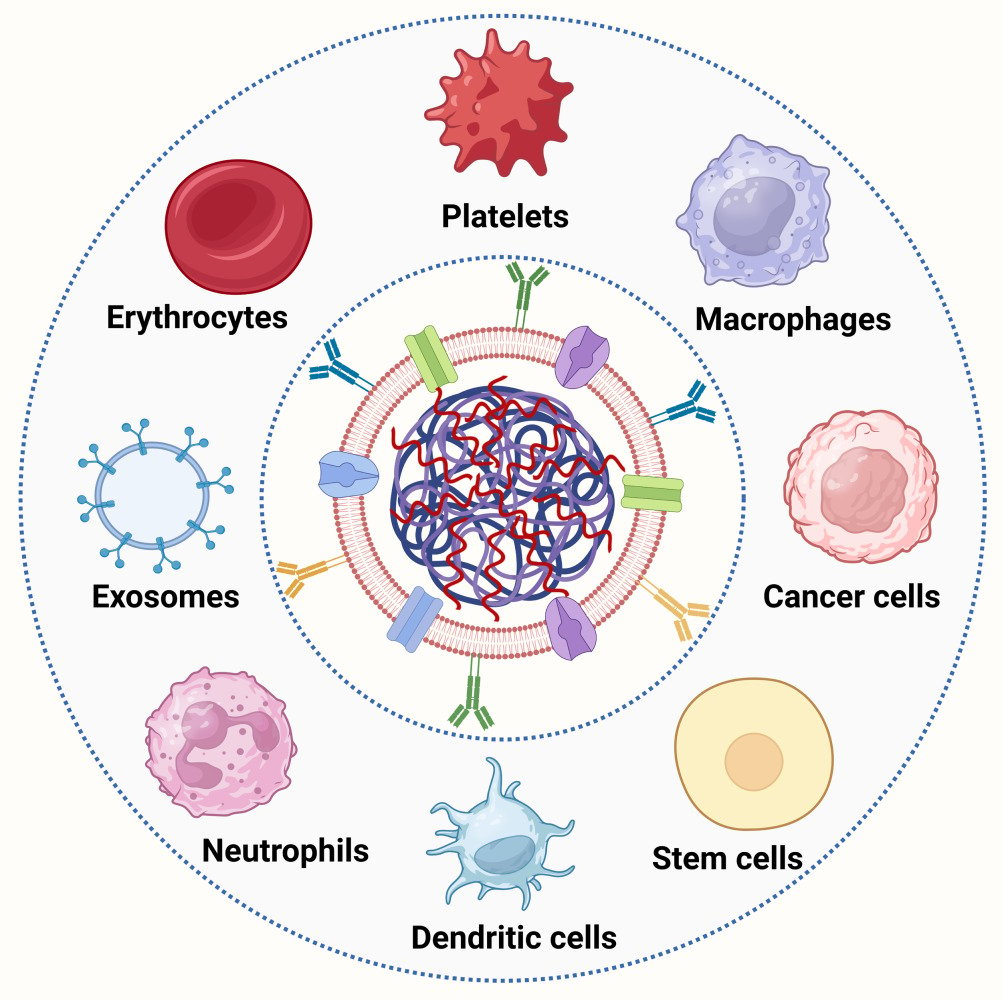
Nanoparticles can be camouflaged with different cell membranes from erythrocytes,57–60 platelets,61–63 macrophages,64–67 cancer cells,68–74 stem cells,75–78 dendritic cells,79–81 neutrophil,82–84 exosomes,85–87 or other membranes depend on desired applications (Figure 4). Blood cells likes erythrocytes and platelets could reduce endothelial reticular system clearance and prolong half-time. Immune cells such as macrophages and neutrophil as well as stem cells are capable of targeting to inflammatory site. Cancer cells camouflaged nanoparticles already used in cancer gene therapy for homologous targeting ability.
Erythrocytes Membranes Camouflaged Nanoparticles (EMCNPs)
Erythrocytes, also known as red blood cells, are the most abundant cell type in mammals, circulating in the bloodstream for approximately 120 days.88 Erythrocytes membranes express markers such as CD47, CD59, and complement factor 1, which send out a “don’t eat me” signal to evade clearance by the reticuloendothelial system.89–91 Mature erythrocytes have no nucleus and organelles, which make membrane extraction relatively straightforward. Erythrocyte membranes demonstrate excellent endurance and tensile strength, allowing them to coat anisotropic nanoparticles.92 Erythrocytes membranes camouflaged nanoparticles (EMCNPs) exhibit good biocompatibility, low immunogenicity, favorable biodegradability, and prolonged circulation time.93–95 Hu et al prepared erythrocyte membranes camouflaged PLGA nanoparticles which demonstrated a longer elimination half-life of 39.6 h compared to 15.8 h of PEG-coated nanoparticles.96 Liang et al used erythrocytes membranes to camouflage matrix metalloproteinase 2 (MMP2)-activated nanoparticles to prepared EMCNPs named REMAIN by incubation after sonication (Figure 5A).97 REMAIN showed a ~20 nm increase in the size than uncoated nanoparticles (Figure 5B). Moreover, REMAIN possessed protein components similar to the ones on the erythrocyte membranes, indicating the successfully camouflage with erythrocytes membranes (Figure 5C). REMAIN remained about 50% in blood after 24 h, exhibiting extended circulation time and effectively inhibiting lung cancer growth in vivo (Figure 5D and E). However, erythrocyte membranes lack adhesion peptides, which limits their targeting capabilities. To address this, several studies have modified erythrocyte membranes to achieve different affinities.98–100 Gao et al camouflaged siRNA-loaded nanogel with erythrocyte membranes and modified them with M2pep and HA2 peptides (Vir-gel).101 The Vir-gel not only had a longer half-life than nanogel alone but also showed stronger fluorescence intensity in the brain, colocalizing with macrophages with the assistance of M2pep and HA2.
 |
Figure 5 Preparation, characteristics and biodistribution of REMAIN. (A) Preparation scheme of REMAIN preparation. (B) Size distribution of MAIN and RAMAIN. (C) Coomassie blue staining of REAMIN. (D) Circulation lifetime of REMAIN in vivo. ns, no significance; ***, P < 0.001. (E) Tumor weight of different treatment. Adapted with permission from Liang L, Cen H, Huang J et al. The reversion of DNA methylation-induced miRNA silence via biomimetic nanoparticles-mediated gene delivery for efficient lung adenocarcinoma therapy. Mol Cancer. 2022;21(1):186.97 |
Platelet Membranes Camouflaged Nanoparticles (PMCNPs)
Platelets are the smallest cells in the bloodstream, with an average lifespan of 7~10 days. Platelet membranes camouflaged nanoparticles (PMCMPs) can serve as a biomimetic delivery system, mimicking platelets in the blood.102 Similar to EMCNPs, PMCNPs demonstrate excellent biocompatibility, reduced immunogenicity, and prolonged circulation time.103,104 Hu et al reported a type of PMCNPs called PNP by enclosing polymeric nanoparticles with human platelets membranes through sonication.105 PNP featured right-side-out unilamellar membrane coating functionalized with immunomodulatory and adhesion antigens associated with platelets. The platelet membranes camouflaging reduced the uptake of polymeric nanoparticles by macrophages and prevented particle-induced complement activation in autologous human plasma. Likewise, our previous work found that platelet membranes camouflaged PLAG/DOTAP nanoparticles showed a much longer half-life in mice (36.44 ± 4.33 h) compared to the non-camouflaged nanoparticles (11 ± 4.65 h).45 The camouflage with platelet membranes ameliorated the surface functions significantly, which reduced immunogenicity and enhanced the circulation lifetime. This approach is beneficial for the nucleic acids to accumulate in the targeted sites.
In addition, platelet membranes possess specific receptors such as P-selectin and CLEC-2, which specifically recognize the CD44 and podoplanin on the cancer cell membranes, respectively.106,107 PMCNPs also can be applied to cancer gene therapy. Zhuang et al reported platelet membranes camouflaged metal-organic framework nanoplatform (P-MOF-siRNA) for the targeted delivery of siRNA in vivo.108 P-MOF-siRNA expressed CD41, CD61 and P-selectin as platelet membranes (Figure 6A). A layer film could be seen outside the P-MOF-siRNA nanoparticles (Figure 6B). The above results indicated that successfully platelet membranes camouflage on MOF. Zhuang also compared P-MOF-siRNA with erythrocytes membrane-camouflaged MOF (R-MOF-siRNA) and found similar gene down-regulation (Figure 6C), as well as reduced binding to macrophages in vitro (Figure 6D). Interestingly, P-MOF-siRNA showed higher accumulation in tumors than R-MOF-siRNA, resulting better tumor inhibition capability and longer lifetime when encapsulated siRNASur (Figure 6D and F). Receptors on platelet membranes like integrin α2β1 and Glycoprotein VI have an affinity for collagen IV and GPIa-IIa, respectively.109,110 PMCNPs can also be utilized in the treatment of autoimmune diseases and atherosclerosis.111,112
 |
Figure 6 Characteristics and siRNA deliver efficiency of P-MOF-siRNA. (A) Western blot of CD41, CD61, and P-selectin of P-MOF-siRNA. (B) TEM of P-MOF-siRNA. (C) Visualization of gene knockdown in GFP-transduced cells after incubation with P-MOF, P-MOF-siRNANC, P-MOF-siRNAGFP, or R-MOF-siRNAGFP for 48 h. (D) Uptake of MOF-siRNA, P-MOF-siRNA, or R-MOF-siRNA by macrophages after 24 h of incubation. (E) Fluorescent of P-MOF-siRNA and R-MOF-siRNA within the tumor 1 hour after intravenous administration. (F) Growth kinetics of tumors implanted subcutaneously into nu/nu mice and treated intravenously with P-MOF-siRNASur or R-MOF-siRNASur. Adapted with permission from Zhuang J, Gong H, Zhou J et al. Targeted gene silencing in vivo by platelet membrane-coated metal-organic framework nanoparticles. Sci Adv. 2020;6(13):eaaz6108.108 Copyright 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. |
Macrophages Membranes Camouflaged Nanoparticles (MMCNPs)
Macrophages, as natural immune and antigen presenting cells, have long half-life. Macrophages membranes camouflaged nanoparticles (MMCNPs) not only can evade the clearance of reticuloendothelial system and prolong the circulation time in vivo, but also actively identify to inflammation tissue and penetrate in solid tumors.113,114 MMCNPs are capable to homologous target to macrophages in inflammation tissue to treat atherosclerosis,115,116 arthritis,117,118 colitis,65,119 hepatitis,120 and other inflammatory disease. Cao et al developed a bone marrow mesenchymal macrophage membrane camouflaged metal-organic framework (MOF) system for plasmid DNA delivery (Macrophages membranes/MOF/pDNA, abbreviated as MMD) in the treatment of sepsis.121 The membranes coating conferred negative charge (Figure 7A) and increased the size of MD, with an average size of 160 ± 5 nm (Figure 7B). MMD remained key membrane antigens including TLR4, TNFR2, CD36, CCR2 and CD47, implying MOF/pDNA nanoparticles were camouflaged by macrophage membranes (Figure 7C). Both confocal laser scanning microscopy, flow cytometry, and quantitative analysis of zinc that revealed MMD uptake by RAW 264.7 cells more than in other cell lines indicating good homotypic targeting (Figure 7D-7F). Macrophages are abundant in tumor microenvironment and closely associated with tumor development, also called tumor associated macrophages.122
 |
Figure 7 Characteristics and cellular uptake of MMD. (A) Zeta potential of MOF, MD, MMD, and MM. (B) Size of MOF, MD, MMD, and MM. (C) Western blot analysis of the expression of characteristic proteins in macrophages, MM, and MMD. (D) CLSM images of RAW 264.7, L929, 4T1, and A549 cells after MMD treatment for 4 h. MMD, red; phalloidin immunostaining, green; DAPI, blue. (E) Flow cytometric profiles of the MMD-treated cells for 4 h. (F) ICP-MS analysis of zinc (Zn) after MMD or MD co-incubation with RAW 264.7, L929, 4T1, or A549 cells for 4 h. MM, Macrophages membranes; MD, MOF/pDNA; MMD, Macrophages membranes/MOF/pDNA. Reprinted with permission from Cao H, Gao Y, Jia H et al. Macrophage-membrane-camouflaged nonviral gene vectors for the treatment of multidrug-resistant bacterial sepsis. Nano Lett. 2022;22(19):7882–7891.121 Copyright 2022 American Chemical Society. |
Likewise, MMCNPs promote tumor targeting by homing to tumor associated macrophages. Nai et al applied macrophage membranes to camouflaged thermosensitive liposomes (C-siRNA/MTSLR).123 After administration, the fluorescence of C-siRNA/MTSL in tumor site was stronger than nanoparticles without macrophages membranes. The surface receptors like integrin α4, integrin β1 facilitate MMCNPs penetrate in solid tumors by recognizing vascular cell adhesion molecule 1(VCAM-1).113 MMCNPs also can be used for cancer targeting gene therapy. Yang et al applied M1 macrophages membranes to coat nanoparticles encapsulating short-hairpin RNA and doxorubicin.52 The prepared MMCNPs evaded from the reticuloendothelial system and enriched in tumor sites, effectively phagocytosing by B16F10 cells and M1 macrophages. Nevertheless, the cellular uptake of MMD and C-siRNA/MTSL in cancer cells were less than in macrophages (Figure 7D-7F).121,123 Since macrophages membranes coating lacks of tumor targeting peptides and exists repels electricity with cancer cells, prohibiting nanoparticle uptake. With the modification of cRGD on macrophages membranes, C-siRNA/MTSL possessed increased cancer accumulation and inhibition effect.123
Mesenchymal Stem Cells Membranes Camouflaged Nanoparticles (MSCMCNPs)
Mesenchymal stem cells (MSCs) are plastic multifunctional fibroblast-like cells with self-renewal capacity and multi-differentiation potential.124 MSCs derived from sources including bone marrow, umbilical cord, and adipose tissues have been widely utilized in cell membranes camouflaged nanoparticles.125,126 MSCs express a variety of chemokine and cytokine receptors such as CXCR1, CXCR2, CCR1, CCR2, thereby mesenchymal stem cells membranes camouflaged nanoparticles (MSCMCNPs) trend to the inflammatory site.127 Hitherto, MSCMCNPs have been applied in acute myocardial infarction,128 hematological diseases129 and bone tissue repair.130 Yao et al assembled a stem cell membranes camouflaged nanocomplex to deliver miRNA.131 They first loaded miRNA with mesoporous silica nanoparticles and then wrapped them with mesenchymal stem cell membranes. This approach enabled efficient loading of miRNA and protected miRNA from degradation. The MSCMCNPs can evade the clearance of the immunologic system, and target ischemic injured cardiomyocytes as well.
MSCs demonstrate strong affinity for the tumor tissue through the binding of chemokine receptors and endothelial adhesion molecules, such as CXCR4.132 Leveraging this natural tumor-targeting ability, MSCs have been used to fabricate cell membranes camouflaged nanoparticles in cancer gene therapy. Yang et al developed a style of biomimetic zeolitic imidazolate framework-8 based on mesenchymal stem cell membranes camouflaged nanoparticles which can deliver so-called “biological bombs” carrying HSV-I thymidine kinase-encoded plasmids and ganciclovir to treat lung cancer.133 The biomimetic MSCMNPs showed enhanced circulation time and drug accumulation in the tumor tissues thus significantly inhibited the tumors. Inspired by the multifunction of MSCs, MSCMCNPs had deliver nucleic acid for diseases therapy (Table 2).
 |
Table 2 Applications of MSCMCNPs in Nucleic Acid Delivery |
Cancer Cell Membranes Camouflaged Nanoparticles (CCMCNPs)
Tumor cells utilize various adhesion molecules such as N-cadherin, epithelial cell adhesion molecules, and galactoagglutinin-3 to promote cell aggregation.135,136 Tumor cells can proliferate indefinitely, and their biomimetic nanoparticles have homologous targeting.137 Many works proved that the cell membrane derived from the cancer cells could be used to camouflage the nucleic acid-loaded nanoparticles, which improved the stability, circulation lifetime, and specificity of the nanoparticles for the effective and precise delivery of nucleic acids.138–140 Fang et al found that cancer cell camouflaged nanoparticles had approximately 40-fold and 20-fold increases in uptake compared with erythrocytes camouflaged nanoparticles and bare nanoparticles cores, respectively.141 Zhang et al designed cancer cell membranes camouflaged nanoparticles (CCMNPs) for homotypic siRNA targeting delivery.142 In their study, a core composed of polymer poly (β-amino ester) loaded with siRNA targeting PLK1 was camouflaged with NCI-H1299 lung cancer cell membranes by extrusion. Owing to the homologous binding adhesion molecules on cancer cell membranes, the CCMNPs showed effective targeting of cancer cells and knocked down PLK1 expression of cancer cells, thereby inhibiting cancer.
The researchers have also explored modification strategies on cancer cell membranes to achieve co-targeting or better cellular uptake. Ding et al used plasma membrane extracted from the genetically engineered cells overexpressing PD1 to coat siRNA loading lipid nanoparticle for purpose of blocking the PD1/PDL1 immune inhibitory axis.143 Liu et al coated mRNA loading nanoparticles with ApoE modified erythrocyte membranes which target to the endothelial cell of blood-brain barrier and cancer cells after evading immunocapture.144 Park et al coated nanoparticles with engineered B16 cell membranes that express hemagglutinin (HA) protein on the surface (named HA-mRNA-NP, Figure 8A).145 At 24 h incubation, HA-mRNA-NP permeated the cytosol, and escaped from endo/lysosomal indicated by attenuated LysoTracker signal (Figure 8B). HA binded to the sialic acid on the lysosome membranes, facilitating escaping from lysosome through membrane fusion. This approach led to high CLuc fluorescence in cells and tumor-bearing mice (Figure 8C and D). Besides, the camouflage with cancer cell-derived membranes endows the nanoparticles with improved stability and enhanced specificity in vitro and in vivo, which not only protects the nucleic acids, but also improves the cellular uptake in the homogeneous cancer cell lines.
 |
Figure 8 Targeting ability of HA-mRNA-NP in vitro and vivo. (A) Preparation and intracellular activity of HA-mRNA-NP. (B) Fluorescent visualization of B16-WT cells incubated with WT-DiO-NP and HA-DiO-NP for 24 h (blue: nuclei, red: endosomes, green: nanoparticles). (C) CLuc fluorescence of B16-WT cells after incubated with WT-mRNA-NP or HA-mRNA-NP. ****P < 0.0001. (D) Visualization of bioluminescent signal from mice intranasally administered with WT-mRNA-NP or HA-mRNA-NP loaded with CLuc mRNA. Reprinted with permission from Park JH, Mohapatra A, Zhou J et al. Virus-mimicking cell membrane-coated nanoparticles for cytosolic delivery of mRNA. Angew Chem Int Ed. 2022;61(2):e202113671. doi:10.1002/anie.202113671.145 Copyright 2023 John Wiley & Sons, Inc. |
Hybrid Membranes Camouflaged Nanoparticles (HMCNPs)
Most of cell membranes camouflaged nanoparticles have utilized a single type of cell membrane, but sometimes this is challenging to meet the complex therapeutic needs. Researchers have developed innovatively biomimetic nanoparticles by mixing two different types of cell membranes to achieve diverse characteristics (Table 3). Gong et al isolated membranes from RAW264.7 macrophage and 4T1 cancer cells, and then fused them by sonication.146 After further camouflaged with the siRNA-loaded nanoparticles using a water bath sonicator, the hybrid membranes camouflaged nanoparticles (HMCNPs) exhibited multi-targeting capability towards both cancer cells and tumor-associated macrophages in vivo.
 |
Table 3 Hybrid Membranes Camouflaged Nanoparticles Applied for Nucleic Acid Delivery |
Exosome Membranes Camouflaged Nanoparticles (EMCNPs)
Exosomes are extracellular vesicles secreted by cells, existing in various body fluids such as blood, urine, and amniotic fluid.154 Exosomes not only share similar proteins with source cells, but also express markers such as CD9, CD63, TSG101, ALIX.155 Exosome membranes contain a variety of transmembrane proteins, lipid anchoring proteins, and ligands, which promote either adhesion or targeting.156 Besides, exosomes rely on matrix stress and aquaporin-1 to achieve free diffusion and rapid transport in confined environments.157 Exosome membranes camouflaged nanoparticles (EMCNPs) have been extensively studied and emerged as a type of excellent drug carriers. Compared with other types of cell membranes camouflaged nanoparticles, EMCNPs show improved biocompatibility, low immunogenicity, and prolonged circulation time. EMCNPs demonstrate homologous targeting ability depending on source cells and enhanced cellular uptake through receptor-mediated endocytosis, pinocytosis, or membrane fusion. Furthermore, exosomes are convenient to be engineered as expected through the advanced genetic engineering and molecular biology technology, which endows the exosomes with improved surface functions or enhanced specificity. These approaches are reasonable to be considered as the alternatives to deliver nucleic acids.
EMCNPs not only improve the biocompatibility of nanoparticles, but also enlarge the utility of exosomes, showing great potential for nucleic acid delivery. Although exosomes can encapsulate nucleic acid, the low loading efficiency limits the applications. Roerig et al mixed extracellular vesicles with siRNA loaded calcium phosphate nanoparticles to improve both siRNA encapsulation efficiency and loading efficiency.158 Zhupanyn et al used extracellular vesicles to modify the PEI/siRNA complexes prior to incubation and ultrasound bath at room temperature.159 Extracellular vesicles from different cell lines increased the transfection efficiency of modified PEI/siRNA complexes. Interestingly, their cross-over experiments found a more general improvement of complex properties upon modification with certain ECVs rather than cell line specificity targeted delivery. Inspired by this, we developed a type of EMCNPs called internally and externally engineered exosomes (I3E), which was consisted of Poly-β amino ester (PBAE) nanoparticles and camouflaged with HEK 293T exosome membranes engineered to display TAM-specific peptides on their surface (Figure 9A).160 I3E was able to load clustered regularly interspaced short palindromic repeats interference (CRISPRi)-encoded plasmid, selectively home to tumor tissues (Figure 9B), and target M2 tumor-associated macrophages by retinoid X receptor β (Figure 9C).161 This type of engineered exosomes resulted in significant repression of PI3Kγ expression and induced the TAMs polarizing to M1 phenotype both in vitro and in vivo (Figure 9D). Our work developed a type of engineered exosomes based on the CRISPR/Cas9 knock-in technology, which produced the exosomes modified with targeting ligands as expected, and enhanced the specificity and surface functions beneficial for nucleic acid delivery.
 |
Figure 9 Design and biological effects of I3E in vivo. (A) Scheme of I3E preparation. (B) PA image of I3E in tumor. (C) Scheme of tumor associated macrophages reprogramming by I3E in situ. (D) Tumor associated macrophages reprogramming effect of I3E in vivo. Reprinted with permission from Zhang L, Lin Y, Li S, Guan X, Jiang X. In situ reprogramming of tumor-associated macrophages with internally and externally engineered exosomes. Angew Chem Int Ed. 2023;62(11): e202217089.160 Copyright 2023 John Wiley & Sons, Inc. |
EMCNPs also have been used to co-deliver nucleic acid and chemical drugs. Han et al designed a type of cascade EMCNPs (S+G@ELP) by fusing M1 macrophage exosomes with photothermal-sensitive liposomes to efficiently co-deliver inhibitor and siRNA.162 The S+G@ELP nanoparticles are multifunctional membranes camouflaged nanoparticles. They not only retained the advantages of M1 exosomes such as tumor-specific targeting, macrophage polarization, and immune evasion, but also provide cascade targeting to tumors combined with photothermal therapy through stimulating the expression of VCAM-1 in tumor tissue.
The bioinspired nanoparticles constructed with exosome membranes shows great potential in carrying nucleic acids, which opens a new avenue for the design of nucleic acid carriers for the complex environments.
Challenges and Prospects for Nucleic Acid Delivery by Cell Membrane-Camouflaged Nanoparticles
Overall, the approaches for nucleic acid delivery by cell membrane-camouflaged are diverse and versatile and can be tailored for specific therapeutic purposes. This technology holds great promise in the field of gene therapy, and ongoing research is expected to lead to significant advancements in the development of safer and more effective gene therapies. Meanwhile, several challenges need to be addressed to fully harness the potential of cell membrane camouflaged nanoparticles for nucleic acid delivery.
One of these challenges is the variability of the cell membrane source, which can impact the properties of the nanoparticles. The choice of cell type affects the targeting ability, biocompatibility, and immunogenicity of the CMCNPs. Moreover, the variability in membrane protein composition influences the stability and activity of the encapsulated nucleic acids. Thus, methods of membrane extraction should be carefully considered to ensure compatibility with diverse cell types while preserving the activity of membrane proteins. The current technology is primarily suitable for preclinical research, and scaling up for industrial production is still challenging.
Secondly, the present work usually ignored the evaluations on the coating quality and the effects on the nucleic acid induced by the production procedure. The procedures are associated the exact dosage and the integrality of nucleic acids within the nanoparticles, which was critically important for the future applications. The comprehensive analysis of the coating quality and the effects on the nucleic acid will improve the technology on nucleic acid delivery greatly.
Another challenge is the potential immunogenicity of the delivery system. Although all cell membranes consist of lipids and proteins, the exact composition can vary among different cell types. This variation may trigger immune responses and lead to the formation of anti-nanoparticle antibodies, which limit the effectiveness of CMCNPs in subsequent treatments. Additionally, the immune response can cause inflammation and tissue damage, which is detrimental to overall health.
Furthermore, it is crucial to thoroughly evaluate the long-term safety and effects of CMCNPs. Minimizing potential toxicity and off-target effects is essential to mitigate harm to patients. Rigorous evaluation and optimization of CMCNPs are necessary to ensure their safety and effectiveness in any clinical applications in the future.
Despite these challenges, the field of cell membranes camouflaged nanoparticles for nucleic acid delivery is exciting and rapidly evolving which holds great promise for gene therapy. Ongoing research and technological developments are expected to lead to significant breakthroughs in the development of efficient and safe nucleic acid delivery systems. With continued efforts, it is anticipated that these innovative approaches will revolutionize gene therapy and provide effective treatments for various diseases.
Conclusion
The delivery of nucleic acids with non-viral vehicles currently encountered many challenges. Bioinspired technology has emerged as a promising solution to address issues related to circulation lifetime, specificity, and safety. In this review, we summarized the development and applications of cell membrane-camouflaged nanoparticles in the field of nucleic acid delivery and outlooked their potential developments. Natural cell-derived membranes, including those from erythrocytes, platelets, macrophages, mesenchymal stem cells, cancer cells, and exosomes, have been utilized to camouflage the nucleic acid-loaded structures. These cell membranes derived from different cell types offer diverse capabilities, which may be useful for clinical applications.
Abbreviations
siRNA, small interfering RNA; CMCNPs, Cell membranes camouflaged nanoparticles; EMCNPs, Erythrocytes membranes camouflaged nanoparticles; PMCNPs, Platelet membranes camouflaged nanoparticles; MMCNPs, Macrophages membranes camouflaged nanoparticles; MSCs, Mesenchymal stem cells; MSCMCNPs, Mesenchymal stem cells membranes camouflaged nanoparticles; CCMCNPs, Cancer cell membranes camouflaged nanoparticles; HMCNPs, Hybrid cell membranes camouflaged nanoparticles; EMCNPs, Exosome membranes camouflaged nanoparticles.
Acknowledgments
We appreciated the National Natural Science Foundation of China (82070406, 82072047, and 82170008), Research Foundation of Education Bureau of Guangdong Province (2021ZDZX2004), Guangdong Province Postgraduate Education Innovation Plan Project (2022SFKC054), and The Open research funds from The Sixth Affiliated Hospital of Guangzhou Medical University (Qingyuan People's Hospital, 202201-303). We thank for the help from BioRender.com for drawing illustration.
Disclosure
The authors declare that there are no competing interests.
References
1. Chen L, Hong M, Luan C, et al. Adenine transversion editors enable precise, efficient A•T-to-C•G base editing in mammalian cells and embryos. Nat Biotechnol. 2023;10. doi:10.1038/s41587-023-01821-9
2. Jo S, Das S, Williams A, et al. Endowing universal CAR T-cell with immune-evasive properties using TALEN-gene editing. Nat Commun. 2022;13(1):3453. doi:10.1038/s41467-022-30896-2
3. Nishiyama T, Zhang Y, Cui M, et al. Precise genomic editing of pathogenic mutations in RBM20 rescues dilated cardiomyopathy. Sci Transl Med. 2022;14(672):eade1633. doi:10.1126/scitranslmed.ade1633
4. Mancuso P, Chen C, Kaminski R, et al. CRISPR based editing of SIV proviral DNA in ART treated non-human primates. Nat Commun. 2020;11(1):6065. doi:10.1038/s41467-020-19821-7
5. Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG. Non-viral vectors for gene-based therapy. Nat Rev Genet. 2014;15(8):541–555. doi:10.1038/nrg3763
6. Raguram A, Banskota S, Liu DR. Therapeutic in vivo delivery of gene editing agents. Cell. 2022;185(15):2806–2827. doi:10.1016/j.cell.2022.03.045
7. Bulcha JT, Wang Y, Ma H, Tai PWL, Gao G. Viral vector platforms within the gene therapy landscape. Signal Transduct Target Ther. 2021;6(1):53. doi:10.1038/s41392-021-00487-6
8. Ryu JY, Won EJ, Lee HAR, et al. Ultrasound-activated particles as CRISPR/Cas9 delivery system for androgenic alopecia therapy. Biomaterials. 2020;232:119736. doi:10.1016/j.biomaterials.2019.119736
9. Laustsen A, Bak RO. Electroporation-based CRISPR/Cas9 gene editing using Cas9 protein and chemically modified sgRNAs. Methods Mol Biol. 2019;1961:127–134. doi:10.1007/978-1-4939-9170-9_9
10. Layden MJ, Rottinger E, Wolenski FS, Gilmore TD, Martindale MQ. Microinjection of mRNA or morpholinos for reverse genetic analysis in the starlet sea anemone, Nematostella vectensis. Nat Protoc. 2013;8(5):924–934. doi:10.1038/nprot.2013.009
11. Zhang L, Wang P, Feng Q, et al. Lipid nanoparticle-mediated efficient delivery of CRISPR/Cas9 for tumor therapy. NPG Asia Mater. 2017;9(10):e441. doi:10.1038/am.2017.185
12. Gao X, Jin Z, Tan X, et al. Hyperbranched poly(β-amino ester) based polyplex nanoparticles for delivery of CRISPR/Cas9 system and treatment of HPV infection associated cervical cancer. J Control Release. 2020;321:654–668. doi:10.1016/j.jconrel.2020.02.045
13. Zhang L, Wang L, Xie Y, et al. Triple-targeting delivery of CRISPR/Cas9 to reduce the risk of cardiovascular diseases. Angew Chem Int Ed. 2019;58(36):12404–12408. doi:10.1002/anie.201903618
14. Mout R, Rotello VM. Cytosolic and nuclear delivery of CRISPR/Cas9-ribonucleoprotein for gene editing using arginine functionalized gold nanoparticles. Biol Protoc. 2017;7(20):e2586. doi:10.21769/BioProtoc.2586
15. Chen Y, Wang X, Liu T, et al. Highly effective antiangiogenesis via magnetic mesoporous silica-based siRNA vehicle targeting the VEGF gene for orthotopic ovarian cancer therapy. Int J Nanomed. 2015;10:2579–2594. doi:10.2147/IJN.S78774
16. Jiao Y, Xia ZL, Ze LJ, Jing H, Xin B, Fu S. Research Progress of nucleic acid delivery vectors for gene therapy. Biomed Microdevices. 2020;22(1):16. doi:10.1007/s10544-020-0469-7
17. Xu C, Ye P, Zhou Y, He D, Wei H, Yu C. Cell membrane-camouflaged nanoparticles as drug carriers for cancer therapy. Acta Biomater. 2020;105:1–14. doi:10.1016/j.actbio.2020.01.036
18. Luk BT, Zhang L. Cell membrane-camouflaged nanoparticles for drug delivery. J Control Release. 2015;220(Pt B):600–607. doi:10.1016/j.jconrel.2015.07.019
19. Zeng Y, Li S, Zhang S, Wang L, Yuan H, Hu F. Cell membrane coated-nanoparticles for cancer immunotherapy. Acta Pharm Sin B. 2022;12(8):3233–3254. doi:10.1016/j.apsb.2022.02.023
20. Fang R, Gao W, Zhang L. Targeting drugs to tumours using cell membrane-coated nanoparticles. Nat Rev Clin Oncol. 2023;20(1):33–48. doi:10.1038/s41571-022-00699-x
21. Hussain Z, Khan S, Imran M, Sohail M, Shah SWA, de Matas M. PEGylation: a promising strategy to overcome challenges to cancer-targeted nanomedicines: a review of challenges to clinical transition and promising resolution. Drug Deliv Transl Res. 2019;9(3):721–734. doi:10.1007/s13346-019-00631-4
22. Wang Y, Zhang K, Qin X, et al. Biomimetic nanotherapies: red blood cell based core-shell structured nanocomplexes for atherosclerosis management. Adv Sci. 2019;6(12):1900172. doi:10.1002/advs.201900172
23. Li H, Peng Q, Yang L, et al. High-performance dual combination therapy for cancer treatment with hybrid membrane-camouflaged mesoporous silica gold nanorods. ACS Appl Mater Interfaces. 2020;12(52):57732–57745. doi:10.1021/acsami.0c18287
24. Qin A, Chen S, Li S, et al. Artificial stem cells mediated inflammation-tropic delivery of antiviral drugs for pneumonia treatment. J Nanobiotechnol. 2022;20(1):335. doi:10.1186/s12951-022-01547-x
25. Chen M, Chen M, He J. Cancer cell membrane cloaking nanoparticles for targeted co-delivery of doxorubicin and PD-L1 siRNA. Artif Cells Nanomed Biotechnol. 2019;47(1):1635–1641. doi:10.1080/21691401.2019.1608219
26. Srivastava I, Xue R, Jones J, et al. Biomimetic surface-enhanced Raman scattering nanoparticles with improved dispersibility, signal brightness, and tumor targeting functions. ACS Nano. 2022;16(5):8051–8063. doi:10.1021/acsnano.2c01062
27. Cao X, Tan T, Zhu D, et al. Paclitaxel-loaded macrophage membrane camouflaged albumin nanoparticles for targeted cancer therapy. Int J Nanomed. 2020;15:1915–1928. doi:10.2147/IJN.S244849
28. Yang Z, Gao D, Guo X, et al. Fighting immune cold and reprogramming immunosuppressive tumor microenvironment with red blood cell membrane-camouflaged nanobullets. ACS Nano. 2020;14(12):17442–17457. doi:10.1021/acsnano.0c07721
29. Jiang Y, Krishnan N, Zhou J, et al. Engineered cell-membrane-coated nanoparticles directly present tumor antigens to promote anticancer immunity. Adv Mater. 2020;32(30):e2001808. doi:10.1002/adma.202001808
30. Zou J, He J, Wang X, et al. Glycoprotein Ib-regulated micro platelet ghost for biosafe distribution and photothermal oncotherapy. J Control Release. 2022;351:341–360. doi:10.1016/j.jconrel.2022.09.036
31. Cai J, Liu J, Wu J, et al. Hybrid cell Membrane-functionalized biomimetic nanoparticles for targeted therapy of osteosarcoma. Int J Nanomed. 2022;17:837–854. doi:10.2147/IJN.S346685
32. Guo Q, Chen C, Wu Z, et al. Engineered PD-1/TIGIT dual-activating cell-membrane nanoparticles with dexamethasone act synergistically to shape the effector T cell/Treg balance and alleviate systemic lupus erythematosus. Biomaterials. 2022;285:121517. doi:10.1016/j.biomaterials.2022.121517
33. Sun W, Ji P, Zhou T, et al. Ultrasound Responsive Nanovaccine Armed with Engineered Cancer Cell Membrane and RNA to Prevent Foreseeable Metastasis. Adv Sci. 2023;10(19):e2301107. doi:10.1002/advs.202301107
34. Rao L, Wu L, Liu Z, et al. Hybrid cellular membrane nanovesicles amplify macrophage immune responses against cancer recurrence and metastasis. Nat Commun. 2020;11(1):4909. doi:10.1038/s41467-020-18626-y
35. Cong X, Tian H, Liu S, et al. Cationic liposome/DNA complexes mediate antitumor immunotherapy by promoting immunogenic tumor cell death and dendritic cell activation. ACS Appl Mater Interfaces. 2020;12(25):28047–28056. doi:10.1021/acsami.0c08112
36. Zhang J, Chen C, Li A, et al. Immunostimulant hydrogel for the inhibition of malignant glioma relapse post-resection. Nat Nanotechnol. 2021;16(5):538–548. doi:10.1038/s41565-020-00843-7
37. Kim J, Vaughan HJ, Zamboni CG, Sunshine JC, Green JJ. High-throughput evaluation of polymeric nanoparticles for tissue-targeted gene expression using barcoded plasmid DNA. J Control Release. 2021;337:105–116. doi:10.1016/j.jconrel.2021.05.047
38. Kou X, Zhang W, Zhang W. Quantifying the interactions between PEI and double-stranded DNA: toward the understanding of the role of PEI in gene delivery. ACS Appl Mater Interfaces. 2016;8(32):21055–21062. doi:10.1021/acsami.6b06399
39. Malmo J, Sorgard H, Varum KM, Strand SP. siRNA delivery with chitosan nanoparticles: molecular properties favoring efficient gene silencing. J Control Release. 2012;158(2):261–268. doi:10.1016/j.jconrel.2011.11.012
40. Cruz LJ, van Dijk T, Vepris O, et al. PLGA-nanoparticles for intracellular delivery of the CRISPR-complex to elevate fetal globin expression in erythroid cells. Biomaterials. 2021;268:120580. doi:10.1016/j.biomaterials.2020.120580
41. Li Y, Chen X, Jin R, et al. Injectable hydrogel with MSNs/microRNA-21-5p delivery enables both immunomodification and enhanced angiogenesis for myocardial infarction therapy in pigs. Sci Adv. 2021;7(9):eabd6740. doi:10.1126/sciadv.abd6740
42. Fernando O, Tagalakis AD, Awwad S, et al. Development of targeted siRNA nanocomplexes to prevent fibrosis in experimental glaucoma filtration surgery. Mol Ther. 2018;26(12):2812–2822. doi:10.1016/j.ymthe.2018.09.004
43. Mai Y, Guo J, Zhao Y, Ma S, Hou Y, Yang J. Intranasal delivery of cationic liposome-protamine complex mRNA vaccine elicits effective anti-tumor immunity. Cell Immunol. 2020;354:104143. doi:10.1016/j.cellimm.2020.104143
44. Bogaert B, Sauvage F, Guagliardo R, et al. A lipid nanoparticle platform for mRNA delivery through repurposing of cationic amphiphilic drugs. J Control Release. 2022;350:256–270. doi:10.1016/j.jconrel.2022.08.009
45. Zhang Y, Qin Y, Li H, et al. Artificial platelets for efficient siRNA delivery to clear “bad cholesterol”. ACS Appl Mater Interfaces. 2020;12(25):28034–28046. doi:10.1021/acsami.0c07559
46. Qiu M, Tang Y, Chen J, et al. Lung-selective mRNA delivery of synthetic lipid nanoparticles for the treatment of pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci U S A. 2022;119(8):e2116271119. doi:10.1073/pnas.2116271119
47. Xu J, Chen T, Sun T, Yu C, Yan D, Zhu L. Erythrocyte membrane camouflaged siRNA/chemodrug nanoassemblies for cancer combination therapy. Biomater Sci. 2022;10(22):6601–6613. doi:10.1039/d2bm01478e
48. Bahmani B, Gong H, Luk BT, et al. Intratumoral immunotherapy using platelet-cloaked nanoparticles enhances antitumor immunity in solid tumors. Nat Commun. 2021;12(1):1999. doi:10.1038/s41467-021-22311-z
49. Mu X, Zhang M, Wei A, et al. Doxorubicin and PD-L1 siRNA co-delivery with stem cell membrane-coated polydopamine nanoparticles for the targeted chemoimmunotherapy of PCa bone metastases. Nanoscale. 2021;13(19):8998–9008. doi:10.1039/d0nr08024a
50. Li K, Qiu Y, Liu X, Huang F. Biomimetic nanosystems for the synergistic delivery of miR-144/451a for oral squamous cell carcinoma. Balkan Med J. 2022;39(3):178–186. doi:10.4274/balkanmedj.galenos.2022.2021-11-1
51. Qiu C, Han H, Sun J, et al. Regulating intracellular fate of siRNA by endoplasmic reticulum membrane-decorated hybrid nanoplexes. Nat Commun. 2019;10(1):2702. doi:10.1038/s41467-019-10562-w
52. Yang C, Ming Y, Zhou K, et al. Macrophage membrane-camouflaged shRNA and doxorubicin: a pH-dependent release system for melanoma chemo-immunotherapy. Research. 2022;2022:9768687. doi:10.34133/2022/9768687
53. Zhang Y, Yang L, Wang H, et al. Bioinspired metal-organic frameworks mediated efficient delivery of siRNA for cancer therapy. Chem Eng J. 2021;426:131926. doi:10.1016/j.cej.2021.131926
54. Copp JA, Fang RH, Luk BT, et al. Clearance of pathological antibodies using biomimetic nanoparticles. Proc Natl Acad Sci U S A. 2014;111(37):13481–13486. doi:10.1073/pnas.1412420111
55. Rao L, Bu LL, Ma L, et al. Platelet-facilitated photothermal therapy of head and neck squamous cell carcinoma. Angew Chem Int Ed. 2018;57(4):986–991. doi:10.1002/anie.201709457
56. Rao L, Cai B, Bu LL, et al. Microfluidic electroporation-facilitated synthesis of erythrocyte membrane-coated magnetic nanoparticles for enhanced imaging-guided cancer therapy. ACS Nano. 2017;11(4):3496–3505. doi:10.1021/acsnano.7b00133
57. Fan Y, Xu P, Fang Q, et al. Biomimetic nanoparticle with glutathione depletion and amplified ROS generation capabilities for synergistic chemo-sonodynamic therapy in squamous cell carcinomas. ACS Appl Mater Interfaces. 2023;15(22):27183–27194. doi:10.1021/acsami.3c03792
58. Ou W, Byeon JH, Soe ZC, et al. Tailored black phosphorus for erythrocyte membrane nanocloaking with interleukin-1α siRNA and paclitaxel for targeted, durable, and mild combination cancer therapy. Theranostics. 2019;9(23):6780–6796. doi:10.7150/thno.37123
59. Jiang Q, Luo Z, Men Y, et al. Red blood cell membrane-camouflaged melanin nanoparticles for enhanced photothermal therapy. Biomaterials. 2017;143:29–45. doi:10.1016/j.biomaterials.2018.11.021
60. Peng Q, Li H, Deng Q, et al. Hybrid artificial cell-mediated epigenetic inhibition in metastatic lung cancer. J Colloid Interface Sci. 2021;603:319–332. doi:10.1016/j.jcis.2021.06.066
61. Wang S, Wang R, Meng N, et al. Platelet membrane-functionalized nanoparticles with improved targeting ability and lower hemorrhagic risk for thrombolysis therapy. J Control Release. 2020;328:78–86. doi:10.1016/j.jconrel.2020.08.030
62. Zhang K, Ma Z, Li S, et al. Platelet-covered nanocarriers for targeted delivery of hirudin to eliminate thrombotic complication in tumor therapy. ACS Nano. 2022;16(11):18483–18496. doi:10.1021/acsnano.2c06666
63. Hu X, Zhao P, Zhang J, et al. Ultrasound-assisted biomimetic nanobubbles for targeted treatment of atherosclerosis. Nanomedicine. 2023;51:102682. doi:10.1016/j.nano.2023.102682
64. Ning P, Yao H, Du F, et al. Gene reprogramming armed macrophage membrane-camouflaged nanoplatform enhances bionic targeted drug delivery to solid tumor for synergistic therapy. Mol Pharm. 2023;20(5):2362–2375. doi:10.1021/acs.molpharmaceut.2c00929
65. Ma Y, Gao W, Zhang Y, et al. Biomimetic MOF nanoparticles delivery of C-Dot nanozyme and CRISPR/Cas9 system for site-specific treatment of ulcerative colitis. ACS Appl Mater Interfaces. 2022;14(5):6358–6369. doi:10.1021/acsami.1c21700
66. Zhuang J, Duan Y, Zhang Q, et al. Multimodal enzyme delivery and therapy enabled by cell membrane-coated metal-organic framework nanoparticles. Nano Lett. 2020;20(5):4051–4058. doi:10.1021/acs.nanolett.0c01654
67. Huang JH, Huang CJ, Yu LN, et al. Bioinspired PROTAC-induced macrophage fate determination alleviates atherosclerosis. Acta Pharmacol Sin. 2023. doi:10.1038/s41401-023-01088-5
68. Qiao L, Gao M, Yi X, et al. Biomimetic gene editing system for precise tumor cell reprogramming and augmented tumor therapy. J Control Release. 2023;356:663–677. doi:10.1016/j.jconrel.2023.03.020
69. Fang Z, Zhang M, Kang R, Cui M, Song M, Liu K. A cancer cell membrane coated nanoparticles-based gene delivery system for enhancing cancer therapy. Int J Pharm. 2022;629:122415. doi:10.1016/j.ijpharm.2022.122415
70. Xu C, Liu W, Hu Y, Li W, Di W. Bioinspired tumor-homing nanoplatform for co-delivery of paclitaxel and siRNA-E7 to HPV-related cervical malignancies for synergistic therapy. Theranostics. 2020;10(7):3325–3339. doi:10.7150/thno.41228
71. Kroll AV, Fang RH, Jiang Y, et al. Nanoparticulate delivery of cancer cell membrane elicits multiantigenic antitumor immunity. Adv Mater. 2017;29(47):
72. Lin Y, Li S, Xiao Z, et al. Epigenetic inhibition assisted chemotherapeutic treatment of lung cancer based on artificial exosomes. Pharmacol Res. 2021;171:105787. doi:10.1016/j.phrs.2021.105787
73. Zhang HT, Peng R, Chen S, et al. Versatile nano-PROTAC-induced epigenetic reader degradation for efficient lung cancer therapy. Adv Sci. 2022;9(29):e2202039. doi:10.1002/advs.202202039
74. Huang S, Le H, Hong G, et al. An all-in-one biomimetic iron-small interfering RNA nanoplatform induces ferroptosis for cancer therapy. Acta Biomater. 2022;148:244–257. doi:10.1016/j.actbio.2022.06.017
75. Zhao J, Mu X, Hou X, Zhang X, Li P, Jiang J. Synergistic treatment of osteosarcoma with biomimetic nanoparticles transporting doxorubicin and siRNA. Front Oncol. 2023;13:1111855. doi:10.3389/fonc.2023.1111855
76. Kaneti L, Bronshtein T, Malkah Dayan N, et al. Nanoghosts as a novel natural nonviral gene delivery platform safely targeting multiple cancers. Nano Lett. 2016;16(3):1574–1582. doi:10.1021/acs.nanolett.5b04237
77. Fan Z, Li PY, Deng J, Bady SC, Cheng H. Cell membrane coating for reducing nanoparticle-induced inflammatory responses to scaffold constructs. Nano Res. 2018;11(10):5573–5583. doi:10.1007/s12274-018-2084-y
78. Yang N, Ding Y, Zhang Y, et al. Surface functionalization of polymeric nanoparticles with umbilical cord-derived mesenchymal stem cell membrane for tumor-targeted therapy. ACS Appl Mater Interfaces. 2018;10(27):22963–22973. doi:10.1021/acsami.8b05363
79. Zhang J, Fan B, Cao G, et al. Direct presentation of tumor-associated antigens to induce adaptive immunity by personalized dendritic cell-mimicking nanovaccines. Adv Mater. 2022;34(47):e2205950. doi:10.1002/adma.202205950
80. Ma X, Kuang L, Yin Y, et al. Tumor-antigen activated dendritic cell membrane-coated biomimetic nanoparticles with orchestrating immune responses promote therapeutic efficacy against glioma. ACS Nano. 2023;17(3):2341–2355. doi:10.1021/acsnano.2c09033
81. Liu W, Zou M, Liu T, et al. Expandable immunotherapeutic nanoplatforms engineered from cytomembranes of hybrid cells derived from cancer and dendritic cells. Adv Mater. 2019;31(18):e1900499. doi:10.1002/adma.201900499
82. Feng L, Dou C, Xia Y, et al. Neutrophil-like cell-membrane-coated nanozyme therapy for ischemic brain damage and long-term neurological functional recovery. ACS Nano. 2021;15(2):2263–2280. doi:10.1021/acsnano.0c07973
83. Li S, Wang Q, Shen Y, et al. Pseudoneutrophil cytokine sponges disrupt myeloid expansion and tumor trafficking to improve cancer immunotherapy. Nano Lett. 2020;20(1):242–251. doi:10.1021/acs.nanolett.9b03753
84. Liu Y, He M, Yuan Y, et al. Neutrophil-membrane-coated biomineralized metal-organic framework nanoparticles for atherosclerosis treatment by targeting gene silencing. ACS Nano. 2023;17(8):7721–7732. doi:10.1021/acsnano.3c00288
85. Zhao L, Gu C, Gan Y, Shao L, Chen H, Zhu H. Exosome-mediated siRNA delivery to suppress postoperative breast cancer metastasis. J Control Release. 2020;318:1–15. doi:10.1016/j.jconrel.2019.12.005
86. Li H, Li S, Lin Y, et al. Artificial exosomes mediated spatiotemporal-resolved and targeted delivery of epigenetic inhibitors. J Nanobiotechnol. 2021;19(1):364. doi:10.1186/s12951-021-01107-9
87. Evers MJW, van de Wakker SI, de Groot EM, et al. Functional siRNA delivery by extracellular vesicle-liposome hybrid nanoparticles. Adv Healthc Mater. 2022;11(5):e2101202. doi:10.1002/adhm.202101202
88. Bratosin D, Mazurier J, Tissier JP, et al. Cellular and molecular mechanisms of senescent erythrocyte phagocytosis by macrophages. A review. Biochimie. 1998;80(2):173–195. doi:10.1016/s0300-9084(98)80024-2
89. Chambers E, Mitragotri S. Prolonged circulation of large polymeric nanoparticles by non-covalent adsorption on erythrocytes. J Control Release. 2004;100(1):111–119. doi:10.1016/j.jconrel.2004.08.005
90. Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000;288(5473):2051–2054. doi:10.1126/science.288.5473.2051
91. Rao L, Xu JH, Cai B, et al. Synthetic nanoparticles camouflaged with biomimetic erythrocyte membranes for reduced reticuloendothelial system uptake. Nanotechnology. 2016;27(8):085106. doi:10.1088/0957-4484/27/8/085106
92. Ben-Akiva E, Meyer RA, Yu H, Smith JT, Pardoll DM, Green JJ. Biomimetic anisotropic polymeric nanoparticles coated with red blood cell membranes for enhanced circulation and toxin removal. Sci Adv. 2020;6(16):eaay9035. doi:10.1126/sciadv.aay9035
93. Xia Q, Zhang Y, Li Z, Hou X, Feng N. Red blood cell membrane-camouflaged nanoparticles: a novel drug delivery system for antitumor application. Acta Pharm Sin B. 2019;9(4):675–689. doi:10.1016/j.apsb.2019.01.011
94. Zhang S, Fu Q, Zhang YJ, et al. Surface loading of nanoparticles on engineered or natural erythrocytes for prolonged circulation time: strategies and applications. Acta Pharmacol Sin. 2021;42(7):1040–1054. doi:10.1038/s41401-020-00606-z
95. Rao L, Bu L, Xu J, et al. Red blood cell membrane as a biomimetic nanocoating for prolonged circulation time and reduced accelerated blood clearance. Small. 2015;11(46):6225–6236. doi:10.1002/smll.201502388
96. Hu CM, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci U S A. 2011;108(27):10980–10985. doi:10.1073/pnas.1106634108
97. Liang L, Cen H, Huang J, et al. The reversion of DNA methylation-induced miRNA silence via biomimetic nanoparticles-mediated gene delivery for efficient lung adenocarcinoma therapy. Mol Cancer. 2022;21(1):186. doi:10.1186/s12943-022-01651-4
98. Wang Y, Chen X, He D, Zhou Y, Qin L. Surface-modified nanoerythrocyte loading DOX for targeted liver cancer chemotherapy. Mol Pharm. 2018;15(12):5728–5740. doi:10.1021/acs.molpharmaceut.8b00881
99. Lv W, Xu J, Wang X, Li X, Xu Q, Xin H. Bioengineered boronic ester modified dextran polymer nanoparticles as reactive oxygen species responsive nanocarrier for ischemic stroke treatment. ACS Nano. 2018;12(6):5417–5426. doi:10.1021/acsnano.8b00477
100. Zhong Y, Ye M, Huang L, et al. A fibrin site-specific nanoprobe for imaging fibrin-rich thrombi and preventing thrombus formation in venous vessels. Adv Mater. 2022;34(16):e2109955. doi:10.1002/adma.202109955
101. Gao X, Li S, Ding F, et al. A virus-mimicking nucleic acid nanogel reprograms microglia and macrophages for glioblastoma therapy. Adv Mater. 2021;33(9):e2006116. doi:10.1002/adma.202006116
102. Wang S, Duan Y, Zhang Q, et al. Drug Targeting via Platelet Membrane–Coated Nanoparticles. Small Struct. 2020;1(1):2000018. doi:10.1002/sstr.202000018
103. Kunde SS, Wairkar S. Platelet membrane camouflaged nanoparticles: biomimetic architecture for targeted therapy. Int J Pharm. 2021;598:120395. doi:10.1016/j.ijpharm.2021.120395
104. Fang RH, Kroll AV, Gao W, Zhang L. Cell membrane coating nanotechnology. Adv Mater. 2018;30(23):e1706759. doi:10.1002/adma.201706759
105. Hu C-MJ, Fang RH, Wang K-C, et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature. 2015;526(7571):7571):118–121. doi:10.1038/nature15373
106. Stone JP, Wagner DD. P-selectin mediates adhesion of platelets to neuroblastoma and small cell lung cancer. J Clin Invest. 1993;92(2):804–813. doi:10.1172/JCI116654
107. Alves CS, Burdick MM, Thomas SN, Pawar P, Konstantopoulos K. The dual role of CD44 as a functional P-selectin ligand and fibrin receptor in colon carcinoma cell adhesion. Am J Physiol Cell Physiol. 2008;294(4):C907–C916. doi:10.1152/ajpcell.00463.2007
108. Zhuang J, Gong H, Zhou J, et al. Targeted gene silencing in vivo by platelet membrane–coated metal-organic framework nanoparticles. Sci Adv. 2020;6(13):eaaz6108. doi:10.1126/sciadv.aaz6108
109. Perrella G, Nagy M, Watson SP, Heemskerk JWM. Platelet GP (Glycoprotein VI) and thrombotic complications in the venous system. Arterioscler Thromb Vasc Biol. 2021;41(11):2681–2692. doi:10.1161/ATVBAHA.121.316108
110. Kasirer-Friede A, Kahn ML, Shattil SJ. Platelet integrins and immunoreceptors. Immunol Rev. 2007;218:247–264. doi:10.1111/j.1600-065X.2007.00532.x
111. Li X, Liu Y, Qi X, et al. Sensitive activatable nanoprobes for real-time ratiometric magnetic resonance imaging of reactive oxygen species and ameliorating inflammation in vivo. Adv Mater. 2022;34(19):e2109004. doi:10.1002/adma.202109004
112. Chen L, Zhou Z, Hu C, et al. Platelet membrane-coated nanocarriers targeting plaques to deliver anti-CD47 antibody for atherosclerotic therapy. Research. 2022;2022:9845459. doi:10.34133/2022/9845459
113. Lopes J, Lopes D, Pereira-Silva M, et al. Macrophage cell membrane-cloaked nanoplatforms for biomedical applications. Small Methods. 2022;6(8):e2200289. doi:10.1002/smtd.202200289
114. Zhang R, Wu S, Ding Q, et al. Recent advances in cell membrane-camouflaged nanoparticles for inflammation therapy. Drug Deliv. 2021;28(1):1109–1119. doi:10.1080/10717544.2021.1934188
115. Chen W, Schilperoort M, Cao Y, Shi J, Tabas I, Tao W. Macrophage-targeted nanomedicine for the diagnosis and treatment of atherosclerosis. Nat Rev Cardiol. 2022;19(4):228–249. doi:10.1038/s41569-021-00629-x
116. Gao C, Huang Q, Liu C, et al. Treatment of atherosclerosis by macrophage-biomimetic nanoparticles via targeted pharmacotherapy and sequestration of proinflammatory cytokines. Nat Commun. 2020;11(1):2622. doi:10.1038/s41467-020-16439-7
117. Zhou K, Yang C, Shi K, et al. Activated macrophage membrane-coated nanoparticles relieve osteoarthritis-induced synovitis and joint damage. Biomaterials. 2023;295:122036. doi:10.1016/j.biomaterials.2023.122036
118. Song X, Zheng Z, Ouyang S, et al. Biomimetic epigallocatechin gallate-cerium assemblies for the treatment of rheumatoid arthritis. ACS Appl Mater Interfaces. 2023;15(28):33239–33249. doi:10.1021/acsami.3c02768
119. Yan X, Pan Q, Xin H, Chen Y, Ping Y. Genome-editing prodrug: targeted delivery and conditional stabilization of CRISPR-Cas9 for precision therapy of inflammatory disease. Sci Adv. 2021;7(50):eabj0624. doi:10.1126/sciadv.abj0624
120. Xu X, Tang H, Guo J, Xin H, Ping Y. A dual-specific CRISPR-Cas nanosystem for precision therapeutic editing of liver disorders. Signal Transduct Target Ther. 2022;7(1):269. doi:10.1038/s41392-022-01071-2
121. Cao H, Gao Y, Jia H, et al. Macrophage-membrane-camouflaged nonviral gene vectors for the treatment of multidrug-resistant bacterial sepsis. Nano Lett. 2022;22(19):7882–7891. doi:10.1021/acs.nanolett.2c02560
122. Xiang X, Wang J, Lu D, Xu X. Targeting tumor-associated macrophages to synergize tumor immunotherapy. Signal Transduct Target Ther. 2021;6(1):75. doi:10.1038/s41392-021-00484-9
123. Nai J, Zhang J, Li J, et al. Macrophage membrane- and cRGD-functionalized thermosensitive liposomes combined with CPP to realize precise siRNA delivery into tumor cells. Mol Ther Nucleic Acids. 2022;27:349–362. doi:10.1016/j.omtn.2021.12.016
124. Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8(9):726–736. doi:10.1038/nri2395
125. Wu H, Zhou Y, Tabata Y, Gao J. Mesenchymal stem cell-based drug delivery strategy: from cells to biomimetic. J Control Release. 2019;294:102–113. doi:10.1016/j.jconrel.2018.12.019
126. Zhang M, Zhang F, Liu T, et al. Polydopamine nanoparticles camouflaged by stem cell membranes for synergistic chemo-photothermal therapy of malignant bone tumors. Int J Nanomed. 2020;15:10183–10197. doi:10.2147/IJN.S282931
127. Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4(3):206–216. doi:10.1016/j.stem.2009.02.001
128. Luo L, Tang J, Nishi K, et al. Fabrication of synthetic mesenchymal stem cells for the treatment of acute myocardial infarction in mice. Circ Res. 2017;120(11):1768–1775. doi:10.1161/CIRCRESAHA.116.310374
129. Ho TC, Kim HS, Chen Y, et al. Scaffold-mediated CRISPR-Cas9 delivery system for acute myeloid leukemia therapy. Sci Adv. 2021;7(21):eabg3217. doi:10.1126/sciadv.abg3217
130. Cai Y, Wu C, Ou Q, et al. Enhanced osteoarthritis therapy by nanoengineered mesenchymal stem cells using biomimetic CuS nanoparticles loaded with plasmid DNA encoding TGF-β1. Bioact Mater. 2023;19:444–457. doi:10.1016/j.bioactmat.2022.04.021
131. Yao C, Wu W, Tang H, et al. Self-assembly of stem cell membrane-camouflaged nanocomplex for microRNA-mediated repair of myocardial infarction injury. Biomaterials. 2020;257:120256. doi:10.1016/j.biomaterials.2020.120256
132. Nitzsche F, Muller C, Lukomska B, Jolkkonen J, Deten A, Boltze J. Concise review: MSC adhesion cascade-insights into homing and transendothelial migration. Stem Cells. 2017;35(6):1446–1460. doi:10.1002/stem.2614
133. Yang L, Lin Y, Zhang J, et al. Biomimetic metal-organic frameworks navigated biological bombs for efficient lung cancer therapy. J Colloid Interface Sci. 2022;625:532–543. doi:10.1016/j.jcis.2022.06.008
134. Mu X, Li J, Yan S, et al. siRNA delivery with stem cell membrane-coated magnetic nanoparticles for imaging-guided photothermal therapy and gene therapy. ACS Biomater Sci Eng. 2018;4(11):3895–3905. doi:10.1021/acsbiomaterials.8b00858
135. Osta WA, Chen Y, Mikhitarian K, et al. EpCAM is overexpressed in breast cancer and is a potential target for breast cancer gene therapy. Cancer Res. 2004;64(16):5818–5824. doi:10.1158/0008-5472.CAN-04-0754
136. Li R, He Y, Zhang S, Qin J, Wang J. Cell membrane-based nanoparticles: a new biomimetic platform for tumor diagnosis and treatment. Acta Pharm Sin B. 2018;8(1):14–22. doi:10.1016/j.apsb.2017.11.009
137. Glinsky VV, Glinsky GV, Glinskii OV, et al. Intravascular metastatic cancer cell homotypic aggregation at the sites of primary attachment to the endothelium. Cancer Res. 2003;63(13):3805–3811.
138. Huang X, Guo H, Wang L, Zhang Z, Zhang W. Biomimetic cell membrane-coated nanocarriers for targeted siRNA delivery in cancer therapy. Drug Discov Today. 2023;28(4):103514. doi:10.1016/j.drudis.2023.103514
139. Huang L, Zhou M, Abbas G, et al. A cancer cell membrane-derived biomimetic nanocarrier for synergistic photothermal/gene therapy by efficient delivery of CRISPR/Cas9 and gold nanorods. Adv Healthc Mater. 2022;11(16):e2201038. doi:10.1002/adhm.202201038
140. Zhang L, Zhang W, Peng H, et al. Bioactive cytomembrane@poly(citrate-peptide)-miRNA365 nanoplatform with immune escape and homologous targeting for colon cancer therapy. Mater Today Bio. 2022;15:100294. doi:10.1016/j.mtbio.2022.100294
141. Fang RH, Hu CM, Luk BT, et al. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett. 2014;14(4):2181–2188. doi:10.1021/nl500618u
142. Zhang L, Deng S, Zhang Y, et al. Homotypic targeting delivery of siRNA with artificial cancer cells. Adv Healthc Mater. 2020;9(9):e1900772. doi:10.1002/adhm.201900772
143. Ding L, Zhang X, Yu P, et al. Genetically engineered nanovesicles mobilize synergistic antitumor immunity by ADAR1 silence and PDL1 blockade. Mol Ther. 2023;31(8):2489–2506. doi:10.1016/j.ymthe.2023.04.011
144. Liu Y, Zhang D, An Y, et al. Non-invasive PTEN mRNA brain delivery effectively mitigates growth of orthotopic glioblastoma. Nano Tod. 2023;49:101790. doi:10.1016/j.nantod.2023.101790
145. Park JH, Mohapatra A, Zhou J, et al. Virus-mimicking cell membrane-coated nanoparticles for cytosolic delivery of mRNA. Angew Chem Int Ed. 2022;61(2):e202113671. doi:10.1002/anie.202113671
146. Gong C, Yu X, Zhang W, et al. Regulating the immunosuppressive tumor microenvironment to enhance breast cancer immunotherapy using pH-responsive hybrid membrane-coated nanoparticles. J Nanobiotechnol. 2021;19(1):58. doi:10.1186/s12951-021-00805-8
147. Tang H, Xue Y, Li B, et al. Membrane-camouflaged supramolecular nanoparticles for co-delivery of chemotherapeutic and molecular-targeted drugs with siRNA against patient-derived pancreatic carcinoma. Acta Pharm Sin B. 2022;12(8):3410–3426. doi:10.1016/j.apsb.2022.02.007
148. Shen T, Yang S, Qu X, et al. A bionic “Trojan horse”-like gene delivery system hybridized with tumor and macrophage cell membrane for cancer therapy. J Control Release. 2023;358:204–218. doi:10.1016/j.jconrel.2023.04.046
149. Zhou Y, Liang Q, Wu X, et al. siRNA delivery against myocardial ischemia reperfusion injury mediated by reversibly camouflaged biomimetic nanocomplexes. Adv Mater. 2023;35(23):e2210691. doi:10.1002/adma.202210691
150. Chen J, Wu Z, Ding W, et al. SREBP1 siRNA enhance the docetaxel effect based on a bone-cancer dual-targeting biomimetic nanosystem against bone metastatic castration-resistant prostate cancer. Theranostics. 2020;10(4):1619–1632. doi:10.7150/thno.40489
151. Zhang L, Zhao W, Huang J, et al. Development of a dendritic cell/tumor cell fusion cell membrane nano-vaccine for the treatment of ovarian cancer. Front Immunol. 2022;13:828263. doi:10.3389/fimmu.2022.828263
152. Li Y, Liu Y, Xu J, Chen D, Wu T, Cao Y. Macrophage-cancer hybrid membrane-camouflaged nanoplatforms for HIF-1α gene silencing-enhanced sonodynamic therapy of glioblastoma. ACS Appl Mater Interfaces. 2023;15(26):31150–31158. doi:10.1021/acsami.3c03001
153. Liu L, Chen Y, Liu C, et al. Effect of extracellular matrix coating on cancer cell membrane-encapsulated polyethyleneimine/DNA complexes for efficient and targeted DNA delivery in vitro. Mol Pharm. 2021;18(7):2803–2822. doi:10.1021/acs.molpharmaceut.1c00359
154. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:
155. Jeppesen DK, Fenix AM, Franklin JL, et al. Reassessment of exosome composition. Cell. 2019;177(2):428–445.e18. doi:10.1016/j.cell.2019.02.029
156. Yang B, Chen Y, Shi J. Exosome biochemistry and advanced nanotechnology for next-generation theranostic platforms. Adv Mater. 2019;31(2):e1802896. doi:10.1002/adma.201802896
157. Lenzini S, Bargi R, Chung G, Shin JW. Matrix mechanics and water permeation regulate extracellular vesicle transport. Nat Nanotechnol. 2020;15(3):217–223. doi:10.1038/s41565-020-0636-2
158. Roerig J, Mitrach F, Schmid M, et al. Synergistic siRNA Loading of Extracellular Vesicles Enables Functional Delivery into Cells. Small Methods. 2022;6(12):e2201001. doi:10.1002/smtd.202201001
159. Zhupanyn P, Ewe A, Büch T, et al. Extracellular vesicle (ECV)-modified polyethylenimine (PEI) complexes for enhanced siRNA delivery in vitro and in vivo. J Control Release. 2020;319:63–76. doi:10.1016/j.jconrel.2019.12.032
160. Zhang L, Lin Y, Li S, Guan X, Jiang X. In situ reprogramming of tumor-associated macrophages with internally and externally engineered exosomes. Angew Chem Int Ed. 2023;62(11):e202217089. doi:10.1002/anie.202217089
161. Tang T, Wei Y, Kang J, et al. Tumor-specific macrophage targeting through recognition of retinoid X receptor beta. J Control Release. 2019;301:42–53. doi:10.1016/j.jconrel.2019.03.009
162. Han R, Yu L, Zhao C, et al. Inhibition of SerpinB9 to enhance granzyme B-based tumor therapy by using a modified biomimetic nanoplatform with a cascade strategy. Biomaterials. 2022;288:121723. doi:10.1016/j.biomaterials.2022.121723
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


