Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Cell-Free Blood Cell Secretome (BCS) Counteracts Skin Aging: Multi-Center Prospective Regenerative Aesthetic Medicine Study Using Exokine®
Authors Kerscher M , Wagner-Schiffler S, Noah EM, Fischer T , Greiner-Krüger D, Sattler S, Kaptan T, Drabik A , Hamed G, Reinecke J, Wehling J
Received 25 January 2022
Accepted for publication 2 June 2022
Published 27 June 2022 Volume 2022:15 Pages 1157—1173
DOI https://doi.org/10.2147/CCID.S357810
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Martina Kerscher,1 Sylvia Wagner-Schiffler,2 Ernst Magnus Noah,3 Tanja Fischer,4 Daniela Greiner-Krüger,5 Sonja Sattler,6 Tanju Kaptan,7 Attyla Drabik,8 Glyn Hamed,9 Julio Reinecke,7 Jana Wehling10
1Division of Cosmetic Sciences, University of Hamburg, Hamburg, Germany; 2Dermatologische Praxis, Aachen, Germany; 3Noahklinik und Klinik für Plastische Chirurgie, Kassel, Germany; 4Haut und Lasercentrum, Potsdam, Germany; 5MediCorium, Zentrum für Dermatologie und Ästhetik, Oberursel, Germany; 6Rosenpark Klinik, Darmstadt, Germany; 7ORTHOGEN AG, Düsseldorf, Germany; 8Clinical Trial Support, Münster, Germany; 9Practice Dr. Wehling and Partner, Düsseldorf, Germany; 10Krankenhaus Neuwerk, Mönchengladbach, Germany
Correspondence: Glyn Hamed, Email [email protected]
Background: The “Inflammation Theory of Ageing” identifies pro-inflammatory cytokines and oxidative damage as one cause of cellular and mitochondrial deterioration and aging. Cell-free blood cell secretome (BCS) also known as autologous conditioned serum (ACS) has shown anti-inflammatory and regenerative mode of action in musculoskeletal disorders and radicular compression.
Aim: To confirm that BCS can improve signs of skin aging from a previous study in a multi-center setting.
Methods: Prospective, one-armed, multi-center interventional therapeutic study. Ninety-five women with skin firmness loss were treated with four intra-dermal injection sessions in both cheeks at 0, 2, 4 and 6 weeks. BCS was processed with Exokine® medical device according to manufacturer’s instructions. Primary endpoints were cutometric R0 and R3 at 12 and 24 weeks. GAIS, FACE-QTM, Patient Attractivity Self-Assessment and safety were evaluated.
Results: Mean skin firmness (R0) improved significantly from baseline 0.40 mm to 0.38 mm at week 12 and to 0.36 mm at week 24. Mean skin tiring (R3) improved significantly from baseline 0.45 mm to 0.42 mm at week 12 and to 0.40 at week 24. FACE-QTM “Satisfaction with Skin” significantly improved from baseline to weeks 12, 24 and 48. So did “Satisfaction with Facial Appearance” and “Psychological and Social Function”. “Satisfaction with Decision” and “Satisfaction with Outcome” were stable at week 24 and 48. At week 48 patients assessed their age 1.68 years younger vs Baseline. FACE-QTM aging appraisal improves from Baseline 52.94 to 65.23 at week 48. GAIS, by both physicians and patients, confirm improvement of skin.
Conclusion: For up to 48 weeks four intra-dermal injections with cell-free BCS increase facial skin firmness and resilience to tiring and patients’ satisfaction with their facial appearance and skin. Patients perceive their face as younger. BCS has the ability to sustainably rejuvenate facial skin safely.
Study Registration: Registration on German clinical trials register: DRKS00013014.
Keywords: cell-free blood cell secretome, BCS, autologous conditioned serum, ACS, skin quality, regeneration, autologous, rejuvenation
Introduction
According to the “Inflammation Theory of Ageing„ chronic inflammation in conjunction with oxidative stress and mitochondrial damage affect the regenerative capabilities and are key factors of premature ageing.1–4 Skin aging as a sub-topic of ageing in general is a complex phenomenon affecting multiple anatomical and cellular features, caused by various intrinsic and extrinsic influences.6,7 Intrinsic factors may include factors such as DNA damage, telomere shortening, hormone shifting as well as chronic inflammation and oxidative stress. Extrinsic factors, including ultraviolet (UV) radiation, smoking, pollution and nutrition, can further enhance inflammatory and oxidative stress, as underlying mechanisms for cellular aging.8 The similarities between skin wounding and skin aging are visible through the fact that initial inflammation seen in wounded skin is ROS-mediated, as are the changes seen in aging skin.9 Incidentally, BCS and Plasma have also been shown to neutralize ROS in cell culture10 and improve survival of skin cells irradiated with UV in vitro.
Biomolecular and biochemical changes largely contribute to the multi-factorial process of cellular (skin) aging.8 Such pathomechanisms affect the viability and composition of skin cells, the skin structure and deeper components. Typical age-related aspects on cellular level are decreased cellular activity and atrophy, loss or reduced synthesis of hyaluronic acid as well as collagen and loss of extracellular matrix integrity.8 The result is dehydration and dermal volume loss, clinically appearing in thin, dry and wrinkled skin. Age-related subcutaneous fat atrophy and bony changes paired with gravity lead to further changes in shape and appearance.11,12 Incidentally, a very recent paper showed improved fat graft survival/integration in a rat model when BCS was used as augmentation.56
Nonsurgical and minimally invasive skin anti-aging interventions, including intra-dermal (i.d.) injections are in high demand.13–15 The majority of the aesthetic field has been ruled by two main groups, namely Botulinum toxin A (BTX) and hyaluronic acids (HA) addressing two “main symptoms” of skin aging, wrinkles and volume loss. Wrinkles are one of the typical clinical features of skin aging, which can be associated with various different pathomechanisms including activity of mimic muscles, elastosis-related, gravity-related or a mixture of these. Depending on the wrinkle type different treatment modalities can become an option.
Aesthetic goals of BTX and HA are to (temporarily) improve the clinical and visual features of aged skin. BTX and HA have not been thought to exert strong effects on inflammatory processes nor regenerative deficiency. However, Humphrey et al16 presented a literature review on biological effects of BTX both in vitro and in vivo. It appears that BTX affects skin structure and biology which leads to a reorganization of collagen suggesting a higher skin quality.55 HA has also been seen to affect both cellular and structural aspects of skin, with studies on wound healing and aesthetics.17 Interestingly, combination of BCS with HA did not show any added effect.18
In the past decade, biological and regenerative approaches with the intent to restore cellular health have gained popularity across many medical fields, including aesthetics. The underlying mechanisms have become a main target for upcoming therapeutic modalities.12
Rejuvenating and Regenerative Aesthetic Treatments often rely on (Injectable) “biologicals”. For skin, this includes BCS (blood cell secretome) also known as ACS (autologous conditioned serum), platelet-rich plasmas (PRP) and “stem cells”. Such autologous techniques are often prepared and employed at site of care. A recently published clinical study by Kerscher et al shows how BCS significantly improves skin firmness and hydration after 12 and 24 weeks.5 In vitro qualification of BCS as a beneficial agent for cell division and quality was performed in a well-described skin model.19,20 This model not only showed cell activation but also skin development and keratinocyte differentiation gene expression patterns. Microarray- and qRT-PCR analysis identified upregulation of genes associated with regulation of cell cycle progression and skin development (FLG, FLG 2, S100A2); synthesis of unbranched hyaluronic acid (HAS3); cell growth and tissue repair (FGF17), chemotactic recruitment of leukocytes and angiogenesis (CXCL8) and mediation of epithelial wound healing (Serpine1).21
PRP is arguably the most popular easy-to-apply autologous procedure and has shown reasonable benefit. The effect of PRP is attributed to growth factors liberated by platelets activated in situ. However, PRP composition is highly variable in quality.22 Some groups have taken up the challenge to more clearly define optimized laboratory and treatment protocols.23–25
The term “stem cells” subsumes many types of body cells that have retained some or all potential to differentiate into multiple directions. Usual stem cell preparations often are not well defined – as with PRP – and may contain only low numbers of such cells. It has now become clear that stem cells do not actually replace damaged cells but rather educate the surrounding tissue through secreted factors, their secretome.26,27 Current research and development tends to look at standardized secretome preparations to address the unwelcome heterogeneity of stem cell preparations. Incidentally, secretomes of PRP are looked at; also to overcome the mentioned limitations of PRP preparations.
BCS is blood serum fortified with the secretome of blood cells released under conditions which nature has designed to: 1. stop bleeding, 2. inhibit infection, 3. initiate healing, 4. restore function. BCS represents the autologous and genuine harmonic whole blood response to a blood spill situation, which also includes the secretome of blood borne “stem cells”. It is a secretome released by the individual’s current blood composition under standardized sterile conditions. Numerous publications have described the contents of BCS.28 The list includes, but is not limited to, growth factors, cytokines, lipid mediators and exosomes. Sterile filtration before clinical application contributes to a low number of adverse events. BCS is in clinical use for 20 years, is easy to obtain and addresses inflammatory pathomechanisms.
BCS has proven its effectiveness in controlled studies in degenerative orthopedic indications.29–34 The underlying dysfunctional phenomena found in osteoarthritis, tendinitis, cartilage damages, and other inflammatory and degenerative components of orthopedic diseases bear relevance as pathophysiological mechanisms for aging skin. Issues like chronified inflammation (often sub-clinical), oxygen radicals, oxidative stress, mitochondrial dysfunction, immune dysfunction, senescence of cells and loss of extracellular matrix integrity are underlying pathomechanisms that play a universal role in, but are not limited to, musculoskeletal and skin ageing.8
Overall, stem cells, PRP and BCS have in common the provision of secreted factors potentially beneficial for tissue integrity including skin health. The potential of topical and injectable growth factors and cytokines for skin rejuvenation is a growing topic of discussion and clinical practice.35
Objectives
The aim of this study was the statistical verification of the therapeutic effects on skin quality and subjective appearence of Exokine® medical device processed cell free BCS under a long term (48 weeks) observation in a representative sample size at six clinical sites.
Methods
Study Design
This therapeutic, investigator-initiated study is designed as a prospective, one-armed, multi-center, interventional study with a calculated sample size of 95 patients.
We decided not to perform a two-armed study because, so far, no evidence-based standard of treatment exists for regeneration of reduced facial skin quality. A placebo-controlled design using only intra-dermal injections without active substance in a placebo arm was not implemented for (a) practical and for (b) ethical reasons. In the participating centers routine patients were hard to recruit with the prospect to possibly receive placebo treatment. Any adverse events arising from placebo treatment are viewed more critical since skin ageing is not seen as a (serious) disease, but rather as a “natural” phenomenon. The aim of the study was not to compare BCS with a comparator, rather the confirmation of previous results5,18 in a multi-center setting.
Participants
Female patients aged 30–65 years who presented to the study centers with confirmed skin firmness loss: Cutometry for value R0 ≥0.31 mm was included in this analysis. The study was conducted from August 2018 to February 2020. Patients accepted not to alter their usual skincare routine during the study, nor to start Botulinum toxin treatments, Hyaluronic Acid treatments, skin booster or laser treatments. Inclusion and Exclusion criteria are shown in Table 1.
 |
Table 1 Inclusion and Exclusion Criteria. The Study Was Conducted as a Multi-Center Study at Six Different Sites in Germany: Hamburg, Darmstadt, Potsdam, Kassel, Aachen and Oberursel |
BCS Preparation
60 mL blood was taken from each patient with 6 Exokine® medical devices. Blood was stored in these devices for extended coagulation at 37℃ for 6 hours, then centrifuged for 10 minutes at 3000 g. BCS was obtained and aliquoted via a 0.22 μm syringe tip filter (Millex GP, Merck Millipore, Tullagreen, Carrigtwohill, Cork, Ireland) to 1 mL syringes and stored at −18 ℃ for the treatment sessions (storage possible for 12 months).
Interventions
Patients underwent a series of 4 treatment sessions at 0, 2, 4, and 6 weeks. At each session, 4 mL of cell-free BCS was injected manually with a serial puncture technique. Intra-dermal injections were placed approximately 1 cm apart in a max. skin depth of 0.5–2 mm on both cheeks. Syringes of 1 mL volume with 30 G, 4 mm mesotherapy needles (RI.MOS SRL, Mirandola, Italy) were used to administer the intra-dermal injection per cheek.
The technique and handling of BCS processing and facial skin injections is standardized and specific training was a precondition for the involved physicians and health-care staff to participate in the study.
Outcomes
Skin status evaluation was performed at screening. Screening values were used as baseline.
Skin firmness, skin tiring and FACE-QTM Questionnaires “Satisfaction with Skin and Face” and “Satisfaction with psychological and social functions” were measured at baseline, 12, 24 and 48 weeks.36 GAIS score was measured at 6 weeks additionally. FACE-QTM Questionnaires “Aging Appraisal” and “Satisfaction with decision and outcomes” were measured at 24 and 48 weeks. See Figure 1 for summary.
 |
Figure 1 A timeline with all interventions and the timepoints at which questionnaires were requested. |
Cutometry is an objective tool to determine skin quality. Skin firmness is correlated to collagen content. Cutometric firmness (R0) is consequently correlated to (age related) collagen loss of the skin.37,38 Skin tiring (R3) displays how resistant the skin is to repetitive stress.
The resistance of the skin to the negative pressure (firmness) and its ability to return into its original position (elasticity) are displayed as curves (penetration depth in mm/time) in real time during the measurement. From these curves, a variety of measurement parameters can be calculated related to elastic and visco-elastic properties of skin structure and skin aging.
R0 (Uf) describes pliability/firmness in mm (amplitude at the end of the first suction phase) (Figure 2). R3/R9/R10 describe tiring effects in mm (Fatigue) visible for repeated measurement circles (Figure 2). The technique has been demonstrated to produce data that reliably documents the change between baseline and follow-up timepoints.
 |
Figure 3 Skin firmness (R0) and Skin tiring (R3) recorded at weeks 12, 24 and 48 show significant improvement vs baseline. R0 and R3 are highly significant at week 24 with ***p < 0.001 compared to baseline. Week 12 shows significance with *p < 0.05 for R0 and significance with **p < 0.01 for R3. Week 48 shows significance with **p < 0.01 for R0 and ***p < 0.001 for R3. Table S1 provides the full data set. |
 |
Figure 4 FACE-QTM Data evaluated by questionnaire “Satisfaction with skin” was assessed at Baseline, week 12, 24 and 48. Figure 4 shows change from baseline. Week 12 shows 20.2, week 24 shows 14.6 and week 48 shows 14.4 score points improvement vs baseline. Statistical significance at all timepoints: *** p < 0.001. Table S2 in the supplementary Document provides the full data set. |
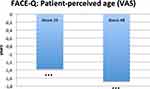 |
Figure 5 Patient-perceived age at week 24 and 48. Displayed is the difference to perceived age in years at Baseline. ***p < 0.0001. |
 |
Figure 6 GAIS score assessed by physician at week 6, 12, 24 and 48. Week 6, 12, and 24 show high significance with ***p < 0.001. One physician scored one patient as “worse”. |
 |
Figure 7 GAIS score assessed by patient at week 6, 12, 24 and 48. All time points show high significance with ***p < 0.001. No patient scored herself as “worse”. |
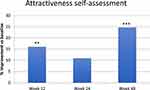 |
Figure 8 Self assessed attractiveness improved significantly when compared to baseline with **p < 0.01 at week 12 and ***p < 0.001 at week 48. Table S7 in the supplementary document shows all numbers analyzed. |
The measurement of skin quality (R0: inverse skin firmness and R3: tiring representing the passive behaviour of the skin to force) was performed by using cutometry with (Cutometer® dual MPA 580; Courage+Khazaka GmbH, Cologne, Germany) 13 repetitions at 450 mbar of negative pressure. Both cheeks were examined twice independently, the two measurements of each side were averaged, then an overall average of both cheeks was calculated. A list of published studies using Cutometry is available online.38
The Global Aesthetic Improvement Scale (GAIS) is a well-established tool for self- and physician-assessment. Aesthetic evaluation was performed using the GAIS, a qualitative, 5-point, relative-improvement scale, which is commonly used as a clinically meaningful instrument. GAIS was modified to allow evaluation of the cheeks alone. This was done for each cheek, and then an average of both was calculated.
The FACE-QTM is a new patient-reported outcome instrument (PRO) composed of numerous independently functioning scales and checklists designed to measure outcomes important to facial aesthetic patients. They are designed to evaluate the satisfaction of patients with their skin, their face, the decision about the procedure, the satisfaction with the outcome, the psychological and social function in relation to their facial appearance. The FACE-QTM Satisfaction with face and skin contains, eg, questions about the symmetry, the proportions, the freshness, the attractiveness and hydration of the facial skin. The questions regarding psychological and social function ask how much patients agree or disagree – with their facial appearance in mind. Conversion of score points to percentages was done for each FACE-QTM score according to user’s manual.36,41,42
GAIS and FACE-QTM questionnaires were chosen as subjective endpoints to further determine, if cutometry data are correlated with perceived skin quality changes. Each questionnaire will be briefly outlined in the according result section.
Endpoints
This study has four primary endpoints: Change in skin firmness (R0) from baseline to week 12 and from baseline to week 24 as well as skin tiring (R3) from baseline to week 12 and from baseline to week 24.
Secondary endpoints of the study are long-term changes in skin firmness (R0) and skin tiring (R3) at 48 weeks as well as changes in FACE-QTM Questionnaires and changes in GAIS score at any timepoint and safety of BCS treatment.
Sample Size
The sample size calculation was based on the four primary outcomes.
For each primary outcome, a two-sided test problem was established: H0: μ=0 versus H1: μ ≠ 0 where μ denotes the mean of the intraindividual difference of values.
Therapeutic effectiveness was considered clinically relevant with a mean in the primary endpoints of at least Δ/σ=0.35 for each hypothesis. The tests were carried out for the two-sided global significance level α = 0.05. Because of multiple comparisons (4 tests) Bonferroni correction was used.
A minimum sample size of 95 evaluable patients is necessary to demonstrate a significant therapeutic effect with the paired t-test in the primary statistical analysis with a power of 80%.
We have not defined a drop out rate because we considered every drop out as a therapy failure.
Statistical Methods
Statistical analysis was performed with descriptive methods. Moreover, inferential analyses were carried out using appropriate significance tests and confidence intervals. Effect Size (ES) Calculation is based on J. Cohen’s Statistical Power Analysis for the behavioral Sciences43 and evaluated according to Sawilowsky 0.2 small; 0.5 medium; 0.8 strong; 1.2 very large and 2.0 huge.44 Moreover, inferential analyses were carried out using appropriate significance tests and confidence intervals. Missing values have not been replaced.
For each of the four primary outcomes, a two-sided test problem was established and solved accordingly to the statistical hypothesis given above. Instead of using paired t-test we choose the exact Wilcoxon test with a global significance level of α = 0.05 (Bonferroni correction for local level α = 0.0125), because of non-parametric data structure. The interpretation of the results occurred on a confirmatory basis.
The secondary endpoints have been evaluated with a local significance level of α = 0.05.
Safety criteria were evaluated exploratively.
Statistical analysis of primary and secondary endpoints was performed with the full analysis set (FAS) according to the intention-to-treat (ITT) principle. The ITT patient population includes all patients enrolled regardless of possible protocol deviations/violations (eg, premature termination of the study or discontinuation of study medication).
The statistical analysis was performed with SPSS version 27.
Results
Recruitment
Ninety-five patients from 6 different sites in Germany were included. Table 2 shows the distribution of patients by study sites. For general patient characteristics, see Tables 3 and 4.
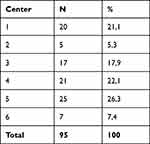 |
Table 2 Study Sites in Germany with the Respective Number and Percentage Distribution of Included Patients |
 |
Table 3 Information About the Study Population. Patients Tanning Behavior and Summary of Sun Exposure Through Tanning Salon Visits, Hours of Sun During Vacations or Outdoor Activities in Germany |
 |
Table 4 Patient Characteristics Regarding Weight, Skin Condition, Skin Sensitivity and Phototype According to Fitzpatrick. There Were No Patients with Phototype V or VI |
Outcomes
Primary Endpoints
Skin Firmness and Tiring
Skin firmness (R0) and Skin tiring (R3) were evaluated by cutometry. Both cheeks were examined twice independently, the two measurements of each side were averaged, then an overall average of both cheeks was calculated.
Supplementary Table S1 provides the cutometry data. Mean inverse skin firmness (R0) is 0.40 mm ±0.0697 mm at baseline and decreases to 0.38 mm ±0.0849 mm at week 12 (ES = 0.32, p < 0.0148) and to 0.36 mm ±0.0886 mm at week 24 (ES = 0.58, p < 0.0001). Week 48 shows 0.37 mm ±0.0891 mm (ES = 0.35, p < 0.0024). Predefined primary endpoints are met. Skin firmness improves significantly from Baseline to weeks 12 and 24. Skin firmness also improves from Baseline to week 48.
Mean skin tiring (R3) is 0.45 mm ± 0.0744 mm at baseline and decreases to 0.42 mm ±0.0939 mm at week 12 (ES = 0.34, p < 0.0064) and to 0.40 mm ±0.0953 mm at week 24 (ES = 0.62, p < 0.0001). Week 48 shows 0.41 mm ± 0.0993 mm (ES = 0.43, p < 0.0005). Predefined primary endpoints are met. Skin tiring improves significantly from Baseline to week 12 and 24. Skin tiring also improves from Baseline to week 48. Overview see Figure 3.
Secondary Endpoints
Face-Qtm
Baseline of FACE-QTM Satisfaction with facial appearance is 42.56 (40.50 to 44.62) ±10.13 increasing to 56.31 (54.07 to 58.54) ±10.98 (ES = 0.94, p < 0.0001) at week 12. Week 24 shows 52.17 (49.89 to 54.44) ±11.17 (ES = 0.7, p < 0.0001) and week 48 51.99 (49.38 to 54.6) ±12.81 (ES = 0.65, p < 0.0001).
FACE-QTM Satisfaction with skin was also assessed at weeks 12, 24 and 48. Baseline values increase from 39.64 ±14.37 to 59.82 ±13.0 (ES = 1.07, p < 0.0001) at week 12. Weeks 24 and 48 show almost identical improvements with 54.20 ±12.48 (ES = 0.85, p < 0.0001) and 53.99 ±14.15 (ES = 0.85, p < 0.0001), respectively. Overview see Figure 4.
The results show that patient’s satisfaction with skin as well as facial appearance is highest at week 12 and remains significantly improved until 48 weeks. Data of FACE-QTM “Satisfaction with facial appearance” and “Satisfaction with skin” are included in Supplementary Table S2.
FACE-QTM Data on Psychological and Social Function were obtained at Baseline, Week 12, 24 and 48. The results are in the below text and summarized in Supplementary Table S3.
Baseline for FACE-QTM Data on Psychological Function shows 59.75 ±16.79. The improvement seen at week 12 is to 68.96 ±14.45 (ES = 0.58, p < 0.0001). Weeks 24 and 48 show 66.74 ±17.44 (ES = 0.41, p < 0.0001) and 66.43 ±15.54 (ES = 0.38, p < 0.0003), respectively.
FACE-QTM Data for Social Function is 57.54 ±15.88 at Baseline and shows high improvement at week 12 with 66.03 ±16.48 (ES = 0.58, p < 0.0001). Weeks 24 and 48 show 63.81 ±17.42 (ES = 0.47, p < 0.0001) and 62.9 ±17.76 (ES = 0.34, p < 0.0013), respectively.
FACE-QTM “Satisfaction with Decision” and “Satisfaction with Outcome” Data was assessed on week 24 and week 48 into the treatment. The results show almost no difference; both FACE-QTM outcomes do not change after 48 weeks’ time. In the Supplementary Table S4 shows all data.
Age Appraisal and Patient Perceived Age
The Aging appraisal score and the Patient perceived age Visual Analogue Scale are two parts of the FACE-QTM aiming to assess how old patients think they look and how they feel about the age in respect to their face. The questionnaires were obtained at baseline, week 24 and week 48. The results demonstrate that patients feel 1.68 years younger in average in comparison to baseline after 48 weeks, and aging appraisal improved from 52.94 ±16.43 to 65.23 ±18.03 (ES = 0.73, p < 0.0001) at week 48. Complete data set is shown in Supplementary Table S5. Overview see Figure 5.
GAIS
The GAIS is a questionnaire that is answered by physician and patient. They answer whether or not they see improvement on each cheek. At week 6, 90.5% of the physicians reported improvement (p < 0.0001). Although it decreases to 58.9% at week 48, the majority of physicians record improvement. Patients had a superior self-assessment regarding their improvement. 76.8% saw an improvement at 48 weeks (p < 0.0001). Supplementary Table S6 provides all the information collected by GAIS. Overview see Figures 6 and 7.
Patient Attractiveness Self-Assessment
The Patient Attractiveness Self-Assessment contains questions that aim to analyse patients’ perceptions of their own attractivity. They were asked to choose from the following categories: very unattractive, quite unattractive, rather unattractive, moderate attractive, rather attractive, quite attractive, very attractive. Answers were subcategorized into Improvement and non-improvement. Improvement was defined when patients viewed their attractiveness at least “rather attractive”. All patients that viewed themselves at least “rather attractive” were summed. Data are provided in Supplementary Table S7. Overview see Figures 8.
Safety Results
Adverse events (AEs) were documented over the whole study period and classified according to seriousness, intensity, and relationship to treatment. Intensity was classified as: mild (not affecting day-to-day activities), moderate (affected day-to-day activities), or severe (day-to-day activities or work were no longer possible). Relationship to treatment was adjudicated as to whether they are device related, procedure related, or unrelated.
Adverse Events
All AEs were documented during the course of the study. The most frequent AEs were hematoma at the site of injection. Sixteen of the 17 observed hematomas were considered mild. Urticaria (4 incidents), as a side effect of serum injection, is an ordinary AE should the patient have a disposition. With 95 Patients and 4 injection sessions per patient, a total number of 380 injection sessions took place. Thirty-six times an adverse event was reported, 9.5% AEs in total. No infections or dermal necrosis were observed at any time. See Table 5.
 |
Table 5 Adverse Events |
Summary of Results
This study demonstrates beneficial effects and safety up to 48 weeks of intra-dermal BCS injections on skin quality as well as patient wellbeing with face and skin by objective and subjective outcome measures.
Skin firmness (R0) and skin tiring (R3) were chosen as primary endpoints as objective measures analysed by cutometry. Both parameters show significant improvement at week 24 and 48. The effects of BCS on skin quality appear to be strongest at week 24 with and less strong by week 48, nonetheless remaining significant.
Secondary endpoints, consisting of subjective questionnaires, demonstrate significant improvement in skin satisfaction, attractiveness and perceived age.
Impressive is (i) the patient’s satisfaction with their skin with a strong ES of 1.07 at week 12 remaining significantly improved at week 48 and (ii) the patients-perceived age of 1.68 years younger at week 48 compared to baseline.
Self-assessed attractiveness significantly improved at 12 and 48 weeks.
Both GAIS assessments by the doctor and the patient show improvement from baseline up to week 24, whilst at week 48 the patient assessed GAIS maintained significant improvement. FACE-QTM outcomes with respect to satisfaction with facial appearance, satisfaction with skin, psychological and social function, aging appraisal and patient-perceived age concur with these results.
In 380 treatments (95 patients and 4 injection sessions per patient), 36 AEs of mild to moderate intensity have been observed with the majority being mild hematoma at the injection site. No serious AEs such as infections or dermal necrosis were observed.
Discussion
The future of anti-ageing in aesthetic dermatology lies in regenerative approaches, healthy aging and inflammation-resolving boosters with autologous therapies. With its causative modes of action, BCS has the capability to become such a novel, pan-facial, holistic and unique treatment arm in the field of skin aging.
Ageing, while sharing numerous characteristics with diseases, is not generally considered as a pathology. The scientific opinions regarding this topic are in flux. Nonetheless, there is an unmet medical need for patients who look for sustained (skin) health with little side effects in the sense of “beauty as an expression of health” (see “perfectissima et absolutissima sanitas”:46); a need BTX and HA may possibly help to satisfy. Treatment modalities like BCS, PRP and stem cell therapy follow biological and autologous principles and aim to narrow this gap, whilst creating a unique and novel treatment pillar in the aesthetic field. BCS incidentally has been shown to support human adipose stem cell immunomodulary behaviour in vitro.54
Not only in Aesthetics, but also in other medical fields, different measuring tools may produce less than coherent results. GAIS, FACE-QTM et al have a strong psychological component which may be influenced by fluctuations of mood, illumination or even decreased eyesight, both in self-assessment and physician-assessment. Both too good and too bad evaluations are possible. One concept, “Response shift”46,47 tries to define the possible changes in patient and observer perception over time and applies sophisticated methods. This is one reason why, not only in aesthetics, more objective measurements are sought in addition. Cutometry, as one such tool, has been used for many years to effectively document changes in skin properties. It reproducibly documents changes following skin treatment.
The following paragraph was compiled to put our own results and treatment modalities (injectables into the facial skin) into perspective and compare our findings to those of others using cutometry.
Luebberding et al and Krueger et al studied cutometry for skin mechanics as a function of age.39,40 They found an imperfect age correlation to cutometric performance of skin implying that the characteristics are more strongly dependent on the individual person and the skin location. To our knowledge, no publication has produced a clear and linear correlation of R0, R3 with patient age with a significant patient number. Cutometry is however a good technique to document mechanical skin changes including firmness and resilience to tiring resulting from (aesthetic) interventions.38 We found few publications comparable to this one, with regard to skin location, treatment modality and cutometric follow-up ranging from 4 weeks to 48 weeks (see Table 6).
Table 6 shows comparisons between studies performed with injectables, in skin quality of the facial cheek. These studies were selected because they also used cutometry as objective measuring tool for skin quality (R0, R3, R5). The present study differs fundamentally from the other 5 for the following reasons in hierarchical order: i) multi-center ii) follow-up time iii) number of patients. For all points mentioned, the data scatter of the presented study is inevitably larger than in single center studies with a low number of patients and a short follow-up time. Therefore, the achieved efficacies at 24 and 48 weeks in this study are impressive.
 |
Table 6 Publications with Similarly Conducted Studies with Regard to Injection Location, Injection Frequency and Method as Well as Cutometry Measurements |
BCS has a long history of scientific research and clinical application in human orthopedic pain medicine as well as veterinary medicine.29–34 BCS has biomolecular impact on various pathways including the innate immune system, mitochondrial health and reduced radical production. It resolves inflammation and promotes regeneration in order to restore homeostasis and healthy conditions in diseased or aged tissues. These are the overarching goals of regenerative therapies, aesthetic or other. Cells are the fundamental building blocks of any tissue. BCS improves cellular health helping the tissue to regain its appropriate function and structure. Thus, by form and function, BCS differs from modalities such as hyaluronan, fillers and BTX.
Shirokova et al conducted a study investigating the clinical and biochemical effects of intra-articular BCS injections in patients with symptomatic knee osteoarthritis. Biopsy analysis showed that BCS injections lead to significant improvement in viscosity of synovial fluid after 6 months, potentially through induction of HA synthesis. A significant reduction of footprints of reactive oxygen/nitrogen species (conjugated dienes and nitric oxide) was observed after 3 months, indicating a resolution of oxidative stress through BCS. Shirokova’s data imply a BCS-triggered induction of rejuvenation-associated changes of joint metabolism.33 Her observations, (i) reduction of oxidative stress and resolution of inflammation resulting in pain reduction and increased Interleukin 1 Receptor Antagonist (IL-1Ra) concentration, (ii) lower activity of proteolytic enzymes with subsequent less degradation of collagen, hyaluronic acid and other components of the extracellular matrix, may help to explain improvements in skin.
BCS has shown in several tendon injury studies that it accelerates healing. Both structure and COL1 (Collagen Type I) gene expression improved.49–51 In an in vitro skin model COL1 and HAS2 (HA-Synthase 2) mRNAs were upregulated. The same model demonstrated elevated cell proliferation rates.21
Growth factors like TGFb and PDGF, both strongly present in BCS, are known to further induce hyaluronic acid synthesis and collagen formation.52,53
Skin firmness is directly correlated to collagen content.37 Consequently, an improvement in skin firmness, as observed in this and other studies, suggests an association between BCS and collagen content/synthesis in the skin, mirroring published data from an orthopedic context.
Intra-dermal BCS injections have unique properties – with the regenerative characteristic comprising various biomolecular and biochemical features.
The inherent predefined role and generation of the blood secretome may not suffice the pharmaceutical criteria of standardization, as it is a self-assembling composition. However, Exokine® device derived BCS is not dependent on overly concentrated/dosed factors as in single active substance formulations but rather represents a colorful orchestra of mediators.
At this point in time, the authors believe that treatment with Exokine® device derived BCS may provide excellent input for an improved skin homeostasis.
Limitations
The study has a small sample size and is neither controlled nor randomized. The study was performed with the participation of a regular clientele of the centers to generate data more related to real-world conditions.
The needling may also have contributed to the observed results since micro-needling has shown to reduce scars and induce collagen synthesis. However, the number of punctures in this study is smaller than in classical micro needling procedures such as dermal rollers.
The primary objective of the customer as study participant was to have improved facial looks which stands in the way of participating in a controlled study, given the risk that receiving placebo may not fulfill the desired effect.
Conclusion
Intra-dermal BCS injections resulted in objective and subjective improvement in quality of aged skin. Patients perceive their face as younger and the satisfaction with one’s face and skin remains significantly higher until 48 weeks of follow-up.
Some biomolecular characteristics of BCS lend themselves to explanation of the observed clinical effects. BCS can restore skin quality and health in a safe and potentially rejuvenating manner.
Data Sharing Statement
No de-identified patient information will be shared.
All data to be shared are available in the manuscript and the Supplementary Materials provided with the publication for as long as the publication is available.
Ethics and Consent Statements
This study was conducted in accordance with the Declaration of Helsinki and International Conference on Harmonization Guidelines for Good Clinical Practice. Before the start of the study, approval of the Ethik-Kommission der Ärztekammer Hamburg (Ethics committee of the Hamburg Medical Association. Ref. Number: PV5739, 2018-05-28) was obtained, and all patients gave written, informed consent to participate.
Funding
This study was funded by Orthogen AG.
Disclosure
Prof. Dr. Martina Kerscher reports personal fees and study support by providing study materials from Orthogen, during the conduct of the study. Dr Tanja Fischer reports grants from Orthogen, during the conduct of the study. Dr Tanju Kaptan was an Employee of Orthogen AG. Dr Glyn Hamed reports personal fees from Orthogen AG, during the writing of the manuscript and outside the submitted work. Dr Julio Reinecke is an employee of Orthogen, outside the submitted work; In addition, Dr Julio Reinecke shares a patent WO2017080669A1 pending to Orthogen AG. The other authors report no conflicts of interest in this work.
References
1. Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15(9):505–522. PMID: 30065258; PMCID: PMC6146930. doi:10.1038/s41569-018-0064-2
2. Fülöp T, Larbi A, Witkowski JM. Human inflammaging. Gerontology. 2019;65(5):495–504. PMID: 31055573. doi:10.1159/000497375
3. Lee YI, Choi S, Roh WS, Lee JH, Kim TG. Cellular senescence and inflammaging in the skin microenvironment. Int J Mol Sci. 2021;22(8):3849. PMID: 33917737; PMCID: PMC8068194. doi:10.3390/ijms22083849
4. Sreedhar A, Aguilera-Aguirre L, Singh KK. Mitochondria in skin health, aging, and disease. Cell Death Dis. 2020;11(6):444. PMID: 32518230; PMCID: PMC7283348. doi:10.1038/s41419-020-2649-z
5. Kerscher M, Hertz-Kleptow D, Drabik A, Kaptan T, Reinecke J. Cell-Free Blood Cell Secretome (BCS) counteracts skin aging and restores firmness and elasticity. J Drugs Dermatol. 2021;20(6):682–688. PMID: 34076388. doi:10.36849/JDD.2021.5018
6. Farage MA, Miller KW, Elsner P, Maibach HI. Intrinsic and extrinsic factors in skin ageing: a review. Int J Cosmet Sci. 2008;30(2):87–95. PMID: 18377617. doi:10.1111/j.1468-2494.2007.00415.x
7. Katsambas AD, Katoulis AC. Topical retinoids in the treatment of aging of the skin. Adv Exp Med Biol. 1999;455:477–482. PMID: 10599385. doi:10.1007/978-1-4615-4857-7_70
8. Frank M, Caceres B. Inflammaging: a concept analysis. J Nurse Pract. 2015;11(2):258–261. doi:10.1016/j.nurpra.2014.08.005
9. Cameli N, Mariano M, Cordone I, Abril E, Masi S, Foddai ML. Autologous pure platelet-rich plasma dermal injections for facial skin rejuvenation: clinical, instrumental, and flow cytometry assessment. Dermatol Surg. 2017;43(6):826–835. PMID: 28375975. doi:10.1097/DSS.0000000000001083
10. Brossi PM, Baccarin RYA, Massoco CO. Do blood components affect the production of reactive oxygen species (ROS) by equine synovial cells in vitro? Pesquisa Veterinária Brasileira. 2012;32(12):1355–1360. doi:10.1590/S0100-736X2012001200023
11. Caso G, McNurlan MA, Mileva I, Zemlyak A, Mynarcik DC, Gelato MC. Peripheral fat loss and decline in adipogenesis in older humans. Metabolism. 2013;62(3):337–340. PMID: 22999012; PMCID: PMC3531563. doi:10.1016/j.metabol.2012.08.007
12. Wollina U, Wetzker R, Abdel-Naser MB, Kruglikov IL. Role of adipose tissue in facial aging. Clin Interv Aging. 2017;12:2069–2076. PMID: 29255352; PMCID: PMC5723114. doi:10.2147/CIA.S151599
13. Buntrock H, Reuther T, Prager W, Kerscher M. Efficacy, safety, and patient satisfaction of a monophasic cohesive polydensified matrix versus a biphasic nonanimal stabilized hyaluronic acid filler after single injection in nasolabial folds. Dermatol Surg. 2013;39(7):1097–1105. PMID: 23506356. doi:10.1111/dsu.12177
14. Kerscher M, Bayrhammer J, Reuther T. Rejuvenating influence of a stabilized hyaluronic acid-based gel of nonanimal origin on facial skin aging. Dermatol Surg. 2008;34(5):720–726. PMID: 18384619. doi:10.1111/j.1524-4725.2008.34176.x
15. Reuther T, Bayrhammer J, Kerscher M. Effects of a three-session skin rejuvenation treatment using stabilized hyaluronic acid-based gel of non-animal origin on skin elasticity: a pilot study. Arch Dermatol Res. 2010;302(1):37–45. PMID: 19730872. doi:10.1007/s00403-009-0988-9
16. Humphrey S, Jacky B, Gallagher CJ. Preventive, cumulative effects of botulinum toxin type a in facial aesthetics. Dermatol Surg. 2017;43(Suppl 3):S244–S251. PMID: 33065950. doi:10.1097/DSS.0000000000001404
17. Fallacara A, Baldini E, Manfredini S, Vertuani S. Hyaluronic acid in the third millennium. Polymers. 2018;10(7):701. PMID: 30960626. doi:10.3390/polym10070701
18. Kerscher M, Wagner-Schiffler S, Nachtweide D, Drabik A, Kaptan T. Cell-free Autologous Conditioned Serum (ACS) significantly increases skin elasticity and the combination with hyaluronic acid (HA) shows no additive effects – results of the OrthoSkin 2 Clinical Study. J Am Acad Dermatol. 2021;79(3):AB66.
19. Marquardt Y, Amann PM, Heise R, et al. Characterization of a novel standardized human three-dimensional skin wound healing model using non-sequential fractional ultrapulsed CO2 laser treatments. Lasers Surg Med. 2015;47(3):257–265. doi:10.1002/lsm.22341;
20. Schmitt L, Marquardt Y, Amann P, et al. Comprehensive molecular characterization of microneedling therapy in a human three-dimensional skin model. PLoS One. 2018;13(9):1–11. doi:10.1371/journal.pone.0204318
21. Marquardt Y, Heise R, Huth S, Baron JM In vitro studies investigating the effects of Autologous Conditioned Serum (ACS) injection on human full thickness 3D skin models.
22. Maisel-Campbell AL, Ismail A, Reynolds KA, et al. A systematic review of the safety and effectiveness of platelet-rich plasma (PRP) for skin aging. Arch Dermatol Res. 2020;312(5):301–315; PMID: 31628542. doi: 10.1007/s00403-019-01999-6
23. Graziani F, Ivanovski S, Cei S, Ducci F, Tonetti M, Gabriele M. The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin Oral Implants Res. 2006;17(2):212–219. PMID: 16584418. doi:10.1111/j.1600-0501.2005.01203.x
24. Bansal H, Leon J, Pont JL, et al. Platelet-rich plasma (PRP) in osteoarthritis (OA) knee: correct dose critical for long term clinical efficacy. Sci Rep. 2021;11(1):3971. PMID: 33597586; PMCID: PMC7889864. doi:10.1038/s41598-021-83025-2
25. Chahla J, Cinque ME, Piuzzi NS, et al. A call for standardization in platelet-rich plasma preparation protocols and composition reporting: a systematic review of the clinical orthopaedic literature. J Bone Joint Surg Am. 2017;99(20):1769–1779. PMID: 29040132. doi:10.2106/JBJS.16.01374
26. Gunawardena TNA, Rahman MT, Abdullah BJJ, Abu Kasim NH. Conditioned media derived from mesenchymal stem cell cultures: the next generation for regenerative medicine. J Tissue Eng Regen Med. 2019;13(4):569–586. PMID: 30644175. doi:10.1002/term.2806
27. Praveen Kumar L, Kandoi S, Misra R, Vijayalakshmi S, Rajagopal K, Verma RS. The mesenchymal stem cell secretome: a new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. 2019;46:1–9. PMID: 30954374. doi:10.1016/j.cytogfr.2019.04.002
28. Wehling P, Moser C, Frisbie D, et al. Autologous conditioned serum in the treatment of orthopedic diseases: the orthokine therapy. BioDrugs. 2007;21(5):323–332. PMID: 17896838. doi:10.2165/00063030-200721050-00004
29. Becker C, Heidersdorf S, Drewlo S, de Rodriguez SZ, Krämer J, Willburger RE. Efficacy of epidural perineural injections with autologous conditioned serum for lumbar radicular compression: an investigator-initiated, prospective, double-blind, reference-controlled study. Spine. 2007;32(17):1803–1808. doi:10.1097/BRS.0b013e3181076514
30. Baltzer AW, Moser C, Jansen SA, Krauspe R. Autologous conditioned serum (Orthokine) is an effective treatment for knee osteoarthritis. Osteoarthritis Cartilage. 2009;17(2):152–160. PMID: 18674932. doi:10.1016/j.joca.2008.06.014
31. Auw Yang KG, Raijmakers NJ, van Arkel ER, et al. Autologous interleukin-1 receptor antagonist improves function and symptoms in osteoarthritis when compared to placebo in a prospective randomized controlled trial. Osteoarthritis Cartilage. 2008;16(4):498–505. PMID: 17825587. doi:10.1016/j.joca.2007.07.008
32. Wright-Carpenter T, Klein P, Schäferhoff P, Appell HJ, Mir LM, Wehling P. Treatment of muscle injuries by local administration of autologous conditioned serum: a pilot study on sportsmen with muscle strains. Int J Sports Med. 2004;25(8):588–593. PMID: 15532001. doi:10.1055/s-2004-821304
33. Shirokova L, Noskov S, Gorokhova V, Reinecke J, Shirokova K. Intra-articular injections of a whole blood clot secretome, autologous conditioned serum, have superior clinical and biochemical efficacy over platelet-rich plasma and induce rejuvenation-associated changes of joint metabolism: a prospective, controlled open-label clinical study in chronic knee osteoarthritis. Rejuvenation Res. 2020;23(5):401–410. PMID: 31847701. doi:10.1089/rej.2019.2263
34. Pishgahi A, Abolhasan R, Shakouri SK, et al. Effect of dextrose prolotherapy, platelet rich plasma and autologous conditioned serum on knee osteoarthritis: a randomized clinical trial. Iran J Allergy Asthma Immunol. 2020;19(3):243–252. PMID: 32615658. doi:10.18502/ijaai.v19i3.3452
35. Fabi S, Sundaram H. The potential of topical and injectable growth factors and cytokines for skin rejuvenation. Facial Plast Surg. 2014;30(2):157–171. PMID: 24810127. doi:10.1055/s-0034-1372423
36. Klassen AF, Cano SJ, Schwitzer JA, Scott AM, Pusic AL. Scales for health-related quality of life, early life impact, satisfaction with outcomes, and decision to have treatment: development and validation. Plast Reconstr Surg. 2015;135(2):375–386. PMID: 25626785. doi:10.1097/PRS.0000000000000895
37. Reilly DM, Lozano J. Skin collagen through the lifestages: importance for skin health and beauty. Plast Aesthet Res. 2021;8:2. doi:10.20517/2347-9264.2020.153
38. Literature list cutometer® [homepage on the Internet] cologne, Germany: courage + khazaka electronic GmbH; 2022. Available from: https://www.courage-khazaka.de/images/Downloads/Studien/Studies_Cutometer.pdf.
39. Luebberding S, Krueger N, Kerscher M. Mechanical properties of human skin in vivo: a comparative evaluation in 300 men and women. Skin Res Technol. 2014;20(2):127–135. PMID: 23889488. doi:10.1111/srt.12094
40. Krueger N, Luebberding S, Oltmer M, Streker M, Kerscher M. Age-related changes in skin mechanical properties: a quantitative evaluation of 120 female subjects. Skin Res Technol. 2011;17(2):141–148. PMID: 21281361. doi:10.1111/j.1600-0846.2010.00486.x
41. Pusic AL, Klassen AF, Scott AM, Cano SJ. Development and psychometric evaluation of the FACE-Q satisfaction with appearance scale: a new patient-reported outcome instrument for facial aesthetics patients. Clin Plast Surg. 2013;40(2):249–260. PMID: 23506765. doi:10.1016/j.cps.2012.12.001
42. Klassen AF, Cano SJ, Schwitzer JA, et al. Development and psychometric validation of the FACE-Q skin, lips, and facial rhytids appearance scales and adverse effects checklists for cosmetic procedures. JAMA Dermatol. 2016;152(4):443–451. PMID: 26934294; PMCID: PMC4833666. doi:10.1001/jamadermatol.2016.0018
43. Cohen J. Statistical Power Analysis for the Behavioral Sciences.
44. Sawilowsky SS. New Effect Size Rules of Thumb. J Mod Appl Stat Methods. 2009;8(2):597–599. doi:10.22237/jmasm/1257035100
45. Gadebusch Bondio M Medizinische Ästhetik: kosmetik und plastische Chirurgie zwischen Antike und früher Neuzeit. Humanistische Bibliothek Reihe I: Abhandlungen, Band: 56. s.l München: Fink; 2005.
46. Howard JS, Mattacola CG, Howell DM, Lattermann C. Response shift theory: an application for health-related quality of life in rehabilitation research and practice. J Allied Health. 2011;40(1):31–38. PMID: 21399850.
47. Hammas K, Sébille V, Brisson P, Hardouin J-B, Blanchin M. How to investigate the effects of groups on changes in longitudinal patient-reported outcomes and response shift using rasch models. Front Psychol. 2020;11:613482. PMID: 33424726; PMCID: PMC7786435. doi:10.3389/fpsyg.2020.613482
48. Hersant B, SidAhmed-Mezi M, Niddam J, et al. Efficacy of autologous platelet-rich plasma combined with hyaluronic acid on skin facial rejuvenation: a prospective study. J Am Acad Dermatol. 2017;77(3):584–586. PMID: 28807118. doi:10.1016/j.jaad.2017.05.022
49. Geburek F, Lietzau M, Beineke A, Rohn K, Stadler PM. Effect of a single injection of autologous conditioned serum (ACS) on tendon healing in equine naturally occurring tendinopathies. Stem Cell Res Ther. 2015;6(1):126. PMID: 26113022; PMCID: PMC4513386. doi:10.1186/s13287-015-0115-0
50. von Wehren L, Pokorny K, Blanke F, Sailer J, Majewski M. Injection with autologous conditioned serum has better clinical results than eccentric training for chronic Achilles tendinopathy. Knee Surg Sports Traumatol Arthrosc. 2019;27(9):2744–2753. PMID: 30900032. doi:10.1007/s00167-019-05465-8
51. Majewski M, Ochsner PE, Liu F, Flückiger R, Evans CH. Accelerated healing of the rat Achilles tendon in response to autologous conditioned serum. Am J Sports Med. 2009;37(11):2117–2125. PMID: 19875360. doi:10.1177/0363546509348047
52. Tiedemann K, Malmström A, Westergren-Thorsson G. Cytokine regulation of proteoglycan production in fibroblasts: separate and synergistic effects. Matrix Biol. 1997;15(7):469–478. PMID: 9106158. doi:10.1016/s0945-053x(97)90020-2
53. Suzuki M, Asplund T, Yamashita H, Heldin CH, Heldin P. Stimulation of hyaluronan biosynthesis by platelet-derived growth factor-BB and transforming growth factor-beta 1 involves activation of protein kinase C. Biochem J. 1995;307:817–821. PMID: 7741713; PMCID: PMC1136722. doi:10.1042/bj3070817
54. Blázquez R, Sánchez-Margallo FM, Reinecke J, et al. Conditioned serum enhances the chondrogenic and immunomodulatory behavior of mesenchymal stem cells. Front Pharmacol. 2019;10:699. PMID: 31316380; PMCID: PMC6609570. doi:10.3389/fphar.2019.00699
55. Choudhury S, Baker MR, Chatterjee S, Kumar H. Botulinum toxin: an update on pharmacology and newer products in development. Toxins. 2021;13(1):58. PMID: 33466571; PMCID: PMC7828686. doi:10.3390/toxins13010058
56. Baykara G, Sungur N, Ozer K, et al. Autologous Conditioned Serum Increases Fat Graft Viability More than Platelet-Rich Plasma in a Controlled Rat Model. Plast Reconstr Surg. 2022;149(5):1123–1136. doi:10.1097/PRS.0000000000009029
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

