Back to Journals » International Journal of Nanomedicine » Volume 17
Cefquinome Sulfate Oily Nanosuspension Designed for Improving its Bioavailability in the Treatment of Veterinary Infections
Authors Mao Y, Chen Y , Liu C, He X, Zheng Y, Chen X, Wang Y, Chen W, Wu Y, Shen Y, Yang H, Ma S
Received 24 November 2021
Accepted for publication 10 May 2022
Published 2 June 2022 Volume 2022:17 Pages 2535—2553
DOI https://doi.org/10.2147/IJN.S348822
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Mian Wang
Yujuan Mao,1 Yumeng Chen,2 Chang Liu,2 Xingyue He,2 Yi Zheng,1 Xiaolan Chen,1 Ying Wang,1 Wei Chen,1 Yanling Wu,1 Yan Shen,2 Haifeng Yang,1 Songbo Ma3
1Jiangsu Animal Husbandry and Veterinary College, Taizhou, Jiangsu, 225300, People’s Republic of China; 2State Key Laboratory of Natural Medicines, Center for Research Development and Evaluation of Pharmaceutical Excipients and Generic Drugs, Department of Pharmaceutics, School of Pharmacy, China Pharmaceutical University, Nanjing, People’s Republic of China; 3Department of Oral and Maxillofacial Surgery, Taizhou People’s Hospital, Taizhou, Jiangsu, 225300, People’s Republic of China
Correspondence: Haifeng Yang, Email [email protected]; Songbo Ma, Email [email protected]
Introduction: Cefquinome sulfate (CS) is the first fourth-generation antibiotic for animals, which has a wide antibacterial spectrum, strong antibacterial activity and low drug resistance. However, it is accompanied by problems of poor therapeutic efficacy. In this context, the use of nanosuspensions have been found to be an attractive strategy. The main objective of this work is to develop a new oily nanosuspension to improve bioavailability and stability of CS formulations.
Methods: After screening the formulations, cefquinome sulfate oily nanosuspension (CS-NSP) was prepared by mortar grinding, using propylene glycol dicaprolate/dicaprate (Labrafac™ PG) as oil medium and caprylocaproyl polyoxyl-8 glycerides (Labrasol®) as stabilizer. The properties of CS-NSP were investigated by testing its physicochemical characteristics, stability, in vitro release, hemolysis, and muscle irritation. The in vivo pharmacokinetics of CS-NSP was studied using rats.
Results: Results show that CS-NSP presents suitable stability, physicochemical properties and safety. Moreover, a rapid release and high bioavailability of CS-NSP have also been verified in the study. Pharmacokinetic experiments in vivo showed that the bioavailability of CS-NSP was about 1.6 times that of commercial cefquinome sulfate injection (CS-INJ, Chuangdao®) (p< 0.01). These advantages of CS-NSP were carried out by small particle size and low viscosity, being associated with the use of Labrafac PG and stabilizer Labrasol.
Conclusion: The results proved that the new preparation is safe and effective and is expected to become a promising veterinary nanodelivery system.
Keywords: cefquinome sulfate, oily nanosuspension, Labrafac PG, labrasol, pharmacokinetics
Introduction
Cefquinome sulfate is a fourth-generation amino-thiazolyl cephalosporin,1 which has been developed solely for veterinary use.2 It shows strong antibacterial activity on fighting with a broad spectrum.3,4 It is widely used in the clinical treatment of respiratory and breast infections in pigs and cattle.5,6 Meanwhile, it exhibits strong bactericidal effect, low toxic and side effects, and without teratogenic, carcinogenic and mutagenic features in clinical use.7,8
A commercial product of cefquinome sulfate available on the market is cefquinome sulfate injection (Chuangdao®, 10 mL: 0.25 g, Jiangxi), which is an oily suspension marketed in China. Chuangdao only needs to be injected intramuscularly once a day, thanks to the use of soybean oil and ethyl oleate as oil medium, but it is also accompanied by poor stability, low bioavailability and muscular stimulation.9 Many approaches have been explored for improving these problems including using novel formulations such as microspheres,10 micorparticles,11 and liposomes.9 However, the high production costs and complex preparation processes limit the industrial production and application of these drug delivery systems. These shortcomings require the development of a new delivery system of cefquinome sulfate to improve its therapeutic efficacy and reduce its production costs.
According to Noyes-Whitney and Ostwald-Freundlich equations,12 the dissolution and bioavailability of drugs can be greatly improved by making nanosuspensions of drugs.13–15 In this case, the use of nanosuspensions has been found to be an attractive strategy to improve bioavailability and reduce the difficulty of industrial production of CS. However, drugs made into nanosuspensions are generally insoluble in water, but CS is soluble in water. In order to solve this problem, a new type of oily nanosuspension was explored in this study.
The properties of nanosuspensions prepared by mortar grinding are related to factors such as particle size and the use of oil phase and stabilizers. Labrafac PG is transparent oily liquid with much lower viscosity (9–12mPa.s, 20°C) than soybean oil and similar to the viscosity of ethyl oleate. Labrafac PG can be used not only as oil phase of liquid preparation,16 but also as a liquid oily core in lipid nanocapsules.17 And it was found to be an ideal oily medium for oily nanosuspensions due to its low viscosity, better stability and low muscle irritation at the injection site. Labrasol® has excellent solubilization and penetration promotion ability with hydrophile-lipophile balance (HLB) of 14,18 usually used as surfactant in microemulsion.16 In the screening of stabilizers in this study, it was found that labrasol as a stabilizer could effectively reduce the particle size and increase the stability of oily nanosuspension. Meanwhile, oleoyl polyoxyl-6 glycerides (Labrafil® M 1944 CS) can be used in combination with labrasol for self-microemulsifying drug-delivery system (SMEDDS).19,20 In order to further increase the stability of the oily nanosuspension system, Labrafil M 1944 CS was added and proved to be effective.
In this study, we designed a novel oily nanosuspension with Labrafac PG as oily medium, Labrasol and Labrafil M 1944 CS as stabilizers, to improve the strategy of cefquinome sulfate in the treatment of animal bacterial infection. This new oily nanosuspension not only improves the stability and bioavailability of cefquinome sulfate, but also reduces muscle irritation and the cost of production, a great therapeutic effectiveness has been promoted consequently.
Materials and Methods
Materials
Cefquinome sulfate (purity: 81.7%) was purchased from Wuhan Dongkangyuan Technology Co., Ltd (Wuhan, China). Formic acid (chromatographically pure) was purchased from Aladdin Reagent Co., Ltd (Shanghai, China). Acetonitrile (chromatographically pure) was obtained from Anhui Tiandi High Purity Solvent Co., Ltd (Anhui, China). Commercial cefquinome sulfate injection (Chuangdao, 10mL: 0.25g) was obtained from Jiangxi Chuangdao Animal Health Products Co., Ltd (Jiangxi, China). Labrafac PG (analytical pure), Labrasol (analytical pure) and Labrafil M 1944 CS (analytical pure) was purchased from GATTEFOSSE Trading Co., Ltd (Shanghai, China). Glycerol monostearate (purity: 99%) was purchased from Jiangsu Ruiduo Bioengineering Co., Ltd (Jiangsu, China). Tween80 (analytical pure) was purchased from Sinopharm Chemical Reagent Co., Ltd (China). Tween60 (purity: 99%) was purchased from Shanghai Guchen Biotechnology Co., Ltd (Shanghai, China). PVPK30 (analytical pure) was obtained from Anhui Shanhe Pharmaceutical Accessories Co., Ltd (Anhui, China). N-hexane (analytical pure) was obtained from Shanghai Titan Technology Co., Ltd (Shanghai, China).
Female Wistar rats weighing 250–300 g were purchased from Qinglongshan Farms (SCXK2019-0010, Nanjing, China). All rats were healthy and free of clinically observable ocular surface disease. All animal experiments complied with the requirements of the National Institute of Health Guide for Care and procedures were approved by the China Pharmaceutical University Animal Experiment Center.
Preparation of CS-NSP
Stabilizers, including 306.0 mg of Labrasol, 30.6 mg of glyceryl monostearate and 102.0 mg labrafil M 1944 CS are accurately weighed by ML104 electronic balance (Mettler Toledo Instrument Co., Ltd, Shanghai, China), and then they are added into 5 mL Labrafac PG to form oil phase dispersion by swirling for 3 minwith QL-902 vortex mixer (Haimen Qilinbeier Instrument Manufacturing Co., Ltd, Haimen, China). Then a mixture of 153 mg of cefquinome sulfate and a small amount of oil phase dispersion are ground in a mortar for a period of time until the drug is evenly dispersed. After adding the remaining oil phase, the dispersion should continue to be ground for three hours to be completely uniform.
Selection of Preparation Method
Medium grinding method: according to the above preparation process, the drug with the same formula amount is completely mixed with the oil dispersion to obtain 5 mL cefquinome sulfate crude suspension, and then it can be added into a grinding bottle filled with zirconia grinding beads and magnetic stirrer, with the volume ratio of dispersion to grinding beads being 3:2. Place this grinding bottle on a magnetic stirrer and stir it at 1200 rpm for 3 hours to obtain the preparation.
Mortar grinding method: the mortar grinding method is the same preparation process introduced in the above period of preparation of CS-NSP.
After dilution with Labrafac PG, the particle size and PDI of cefquinome sulfate nanosuspensions with the same formula prepared by these two methods are measured at 0 h and 48 h, and the particle size and particle size changes are observed to evaluate the advantages and disadvantages of these two preparation methods.
Screening of Stabilizers
When the formula concentration is 25 mg/mL, formula volume is 5 mL and the mass ratio of drug to stabilizer is 2:1, Tween60, Tween80, PVPK30, Labrasol, glyceryl monostearate and Labrafil M 1944 CS are used as stabilizers to prepare formula cefquinome sulfate nanosuspensions by mortar grinding method. The particle size of each nanosuspension, PDI are measured to evaluate effect of each stabilizer.
Screening of Stabilizer Ratio
Labrasol, glyceryl monostearate and Labrafil M 1944 CS, three stabilizers with better stabilizing effect, were preliminarily screened, and the proportion of these three stabilizers was screened respectively, and the selected ratio of drugs to Labrasol were 2:1, 1:1, 1:2, the selected ratio of drugs to glyceryl monostearate were 2:1, 5:1, 7:1, the selected ratio of drugs to Labrafil M 1944 CS were 2:1, 3:2, 1:1. According to the defined formula concentration (25 mg/mL) and formula volume (5 mL), the mortar grinding method was used to determine the optimum dosage of the three stabilizers.
Screening of Grinding Time
Three stabilizers, including Labrasol, glyceryl monostearate and Labrafil M 1944 CS were weighed according to the determined mass ratio of drug to stabilizers (drug: Labrasol: glyceryl monostearate: Labrafil M 1944 CS=15:30:3:10), then they were added into Labrafac PG and swirled for 3 min to mix evenly. The formula quantity of cefquinome sulfate was weighed and added into the mortar. Then mortar grinding method was used to add oil phase and grind the mixture of drug and oil phase for 0.5, 1, 1.5, 2, 2.5 and 3 h, respectively to prepare CS-NSP The particle size and DPI of the preparations after grinding for 0.5, 1, 1.5, 2, 2.5 and 3 h, respectively were measured and the effects on grinding time were evaluated.
Screening of Dilution Ratio for Size Determination
According to the same preparation process in the section “Preparation of CS-NSP”, cefquinome sulfate nanosuspensions were prepared. The particle size and PDI of nanosuspensions diluted 5, 10, 20, 25, 30, and 40 times by Labrafac PG were measured respectively, and the optimal dilution ratio was evaluated.
Formula Process Validation
Three batches of cefquinome sulfate nanosuspension were prepared in parallel by mortar grinding method according to the best formula process selected by formula, and its particle size and PDI were determined.
Quantification of Cefquinome Sulfate
The concentration of cefquinome sulfate was calculated by HPLC (Shimadzu, Kyoto, Japan) equipped with a diode array detector (SPD-20AT). HPLC analysis was performed on a reverse C18 column (4.6×250 mm, 5 µm, Topsil C18, Welch Materials, Shanghai, China). The mobile phase consisted of a mixture of 0.1% formic acid aqueous solution: acetonitrile (90:10, v/v). The detection wavelength was 270 nm, the flow rate was 1 mL/min, the injection volume was 20 μL, and the column temperature was 30°C. The accuracy, recovery, linearity, and limit of quantitation of HPLC method were validated.21
Viscosity Measurement
Ten milliliters of CS-NSP was taken and the viscosity of it was measured by viscometer at 12 RPM (CED-E-VM-085DV2T rotor viscometer, Bolefei Company, USA), which was compared with the CS-INJ. All the experiments were conducted in triplicate.
Content Determination of CS-NSP
Then, 0.2 mL of CS-NSP was put into a centrifuge tube, where 2.5 mL of N-hexane saturated with cefquinome reference substance was added. Then the mixture was shaken to disperse and centrifuged for 10 min (8500 rpm) (KDC-140HR, Anhui Zhongke Zhongjia Scientific Instrument Co., Ltd, Anhui, China). The supernatant was discarded and precipitate was operated twice by the same method, and the final precipitate was evaporated at room temperature. A small amount of mobile phase then was mixed with achieved precipitate and ultrasonicated (KQ5200DB, Kunshan Ultrasonic Instrument Co., Ltd, Kunshan, China) for a period of time until the precipitate was completely dissolved. Then its content were detected by HPLC (please see quantification procedures in section “Quantification of cefquinome”).
Characterization of CS-NSP
Appearance and Transmission Electron Microscopy (TEM)
The appearance of self-made CS-NSP was observed by comparing with CS-INJ. We put 0.4 mL of CS-NSP into a centrifuge tube, then 2.5 mL of N-hexane saturated with cefquinome reference substance was also added into the tube. The mixture of CS-NSP and N-hexane was then shaken to disperse completely and centrifuged for 10 min (8500 rpm). The supernatant was discarded and the precipitate operated twice by the same method. After taking the precipitate, it was placed on the special copper net for transmission electron microscope and dried naturally at room temperature. The particle morphology was observed by transmission electron microscope at an accelerating voltage of 80 kV. (EVO transmission electron microscope, Dongguan Sanben Precision Instrument Co., Ltd, Dongguan, China.9
Particle Size and Particle Size Distribution
The CS-NSP was prepared according to the optimized formula, and the particle size and particle size distribution of the nanosuspension were determined by a ZetaPlus laser particle size analyzer (Brookhaven Instruments Corporation, Holtsville, NY, USA) after taking the right amount and diluting it 20 times with Labrafac PG. All the experiments were conducted in triplicate.
Fourier Transform Infrared (FTIR) Spectroscopy
Fourier transform infrared spectroscopy was done to determine the cefquinome sulfate’s functional group to compare physicochemical characterization. For this purpose, samples were reduced to powder which was precipitated by n-hexane and analyzed as KBr pellets by using a FTIR spectrometer (Nicolet IS 10, USA) and characterized in wavelengths with a range of 4000 to 400 cm−1.22
Differential Scanning Calorimetry (DSC)
DSC was performed using a differential scanning calorimeter (DSC-250, USA). Ten milligrams CS-NSP was crimped separately in an aluminum pan and heated from 0 to 200°C at a heating rate of 10°C/min.23 During the scanning nitrogen gas at a flow rate of 20 mL/min was continuously provided. An empty aluminum pan was used as reference. The same weight of CS-INJ and physical mixture were tested as control. The melting points (Tm) were determined using TA-Universal Analysis 2000 software (version 4.7A).
X-ray Diffraction (XRD)
XRD patterns were recorded on an X diffractometer (Phillip PW 1130/00 diffractometer, The Netherlands),24 employing Cu radiation source operating at 40 mA.23 CS-NSP, CS-INJ, and API were scanned at 2θ values from 3° to 40° at a scanning rate of 4° minute−1.
In vitro Release Study
In vitro dissolution studies were performed for CS-NSP and CS-INJ by using dissolution tester RC806 (Tianjing Tian datianfa, China) following the guidelines of “People's Republic of Veterinary Pharmacopoeia”.25
The CS-NSP and CS-INJ containing 25 mg cefquinome sulfate were placed in the dissolution medium47 which contains phosphate buffer (pH 7.4, 500 mL) with 0.05% Tween 80 at 37±0.5°C under a paddle rotating at 75 rpm. Each time, samples (1 mL) were withdrawn at 3, 5, 10, 20, 30, 45, and 60 min and equal amount of fresh dissolution medium was added. Withdrawn samples were filtered by 0.22 μm organic filtration and cefquinome sulfate in the sample was detected by HPLC. The study was performed in triplicate and cumulative release percentage (%) was calculated by the following equation: cumulative release percentage (%) = 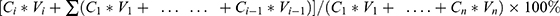 . (Ci is the concentration at time point of i, Vi is the volume of the dissolution medium at time point of I, Cn is the concentration at the last time point, Vn is the volume of the dissolution medium at the last time point).
. (Ci is the concentration at time point of i, Vi is the volume of the dissolution medium at time point of I, Cn is the concentration at the last time point, Vn is the volume of the dissolution medium at the last time point).
Syringeability and Stability
Syringeability
The syringeability was assessed as the ability of a suspension to pass easily through a hypodermic needle.26 It is a measurement to determine syringeability by determining the time required to withdraw the suspension under the specified pressure for 5-gauge syringe-needle system. The plunger end of the 1 mL size syringe was forced to the top to keep a stable negative pressure environment which was used for the specified pressure. After inserting the needle into the suspension, the time when the suspension reached to the needle scale of 1 mL was measured as the function of syringeability. The flow velocity calculated according to the formula: flow velocity (mm/s) = L/T (L: the length from the needle to the scale of 1 mL, T: the withdraw time) solutions were tested as control. All the experiments were conducted in triplicate.
The parameters studied included ease of withdrawal, freedom from clogging, foaming, tendency, evenness of flow, and aspiration quality.
Sedimentation Volume Ratio Determination
Place the cefquinome sulfate oily nanosuspension and the commercial cefquinome sulfate suspension in a 5 mL centrifuge at room temperature for 48 h to determine the original height (H0) and the ultimate height (H) at the fixed time of the formulations according to the scale. The sedimentation volume ratio is calculated according to the formula F=H/H0.27
Physical Stability
Based on the optimal formulation, three batches of CS-NSP were used for stability evaluation. These CS-NSP were packed in clean and dry centrifuge tube, and stored under the accelerated condition for 10 days as prescribed by ICH guidelines.28 Group 1, Group 2, and Group 3 were separately stored under accelerated conditions at illuminated light 4500 lx, relative humidity 92% and temperature 60°C, which were respectively used to perform illuminated light stability, humidity stability and thermal stability. Samples were withdrawn and evaluated for particle size, color, drug content and in vitro cumulative release percentage profile.29 Evaluations were performed at 0, 5, and 10 days and all the experiments were conducted in triplicate.
In vitro Hemolysis Assays
We collected 3 mL blood from the eye socket of one mouse and placed it into a preprepared centrifuge tube coated with 2% heparin sodium to prepare anticoagulated blood. The blood was stirred by a glass to remove fibrin, diluted in physiological saline solution at a 1:5 volume ratio, and centrifuged at 1500 rpm for 20 min.30 The supernatant was removed and the remaining sample should be diluted in physiological saline solution at the same volume ratio to centrifuge. Repeat four times to collect the red blood cells to be diluted with physiological saline solution to prepare a 2% red blood cells suspension.
Briefly, 1.0 mL of 2% red blood cell suspension were added to tubes labeled 1–10, with 1.0 mL physiological saline solution in test tube 9, as a negative control, and 1.0 mL ultra-pure water in test tube 10, as the positive control group. Then, 0.05 mL marketed injection and nanosuspension which had been diluted to a concentration gradient of 0.5, 1.0, 1.5, 2.0 mg/mL were respectively added into tube labeled 1–4 and labeled 5–8 with the concentration gradient to do comparative analysis, with 0.95 mL physiological saline each tube. Finally, the mixtures gently vortexed, and incubated for 2 h at 37°C. After 2 h, all tubes were centrifuged at 2000 rpm for 10 min, and measure the absorbance of supernatant at 570 nm in triplicate.31 The hemolysis ratio is calculated according to the formula hemolysis ratio (%)=(AS-A0)/(A1-A0)×100% (AS: absorbance of test group, A0: absorbance of negative control group, A1: absorbance of positive control group).32
In vivo Muscle Irritation Test
The injection site irritation of the cefquinome sulfate nanosuspension in mice was observed by the cross-comparison method. Different samples were divided into five groups which separately contain 0.2 mL CS-NSP, 0.1 mL CS-NSP, 0.2 mL CS-INJ, and 0.2mL Labrafac PG, all of them were individually injected in the right side of quadriceps on the dose of 25 mg/mL in mice and the same volume of the saline solution at the left side. After injection, the muscle changes at the injection site were visually observed for 48 h and the animals were sacrificed. The muscle tissue near the injection site was dissected to observe their appearance and the tissue was fixed with 4% paraformaldehyde.33,34 The muscle on injection site was also collected for histopathological examination using the hematoxylin & eosin staining method.
In vivo Pharmacokinetics Study
Randomly selected healthy rats were randomly divided into two groups with five rats in each group. One group was received CS-INJ and the other was received CS-NSP. The absorption of CS-NSP in rats was evaluated by comparing the pharmacokinetic parameters of the two groups. Both commercial and self-made preparations were injected intramuscularly at the dose of 40 mg/kg.35 Blood samples were collected at 0.17, 0.33, 0.5, 0.75, 1, 1.5, 2.5, 3.5, 4.5, 6, and 8.5 hs after administration and placed in heparinized Eppendorf tubes.10 Blood samples were centrifuged instantly at 5000 rpm for 10 min.36 After centrifugation, the upper plasma was stored in a low temperature environment to complete the collection of blood samples. Precipitation of plasma proteins were performed through addition of acetonitrile (100 μL) to the plasma samples (100 μL), which were vortexed for 2 min followed by centrifugation at 10000 rpm for 10 min. Supernatant was transferred to ultra-pure water of the same volume, and mixture was vortexed for 1 min. Samples were assayed by high performance liquid chromatography at 270 nm (please see quantification procedures in section “Quantification of cefquinome”).37
Pharmacokinetic parameters (t1/2, half-life; Tmax, the peak time; Cmax, the peak concentration; AUC, area under the plasma concentration–time curve; AUMC, area under the moment curve; MRT, mean residence time; V, volume of distribution; CL, clearance) were calculated using a noncompartmental model by PK Solver 2.0 software (Nanjing, China). Relative bioavailability (F) was calculated by AUCCS-NSP * XCS-INJ/(AUCCS-INJ * XCS-NSP) *100%, where AUCCS-NSP and AUCCS-INJ are the AUC from CS-NSP and CS-INJ group, X is administration dose.
Statistical Analysis
SPSS version 22.0 (SPSS Inc.) was used for statistical analysis. t-test was used to analyze the significant difference (p≤0.05, p≤0.01). All data were expressed in mean ±SD.
Results and Discussion
Preparation of CS-NSP
Selection of Preparation Method
The particle size and PDI results of cefquinome sulfate nanosuspensions prepared by medium grinding and mortar grinding are shown in Table 1. It can be seen that the particle size and PDI of nanosuspension prepared by medium grinding are significantly larger than those obtained by mortar grinding. After standing at room temperature for 48 h, the particle size of nanosuspension obtained by mortar grinding is relatively stable, while the particle size of nanosuspension obtained by medium grinding is obviously increased and the stability is poor.
 |
Table 1 Effects of Different Preparation Methods on Particle Size and PDI of CS-NSP, n=3, mean ±SD |
The high-pressure homogenization process will consume a lot of energy, which may induce particles to gather together and accelerate Ostwald ripening,38 thus affecting the stability of nanosuspensions. Therefore, the high-pressure homogenization method is not used in this preparation.
Media grinding is a commonly used method for preparing nanosuspensions, but according to the data in Table 1, this method is not suitable for preparing this cefquinome sulfate nanosuspensions, which not only has large particle size and wide particle size distribution, but also has great drug loss. The reason for this may be that the viscosity of oily nanosuspension is higher and the oil has a great adsorption force on the grinding beads, which makes it difficult for them to be dispersed at a certain speed. Therefore, the collision friction between drugs and grinding beads is not enough to make the drug particles reach nanometer level, and also leads to uneven grinding and wide particle size distribution. In order to avoid the excessive drug loss and reduce drug particle size and distribution to make the suspension more stable, mortar grinding is considered for oily nanosuspension preparation. It is simple to operate with low cost and small drug loss. After determination, the mortar grinding method is selected to prepare cefquinome sulfate oily nanosuspension.
Screening of Stabilizers
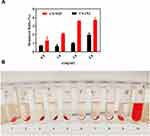
The particle size and PDI results of six kinds of stabilizers are shown in Figure 1A. It can be seen that different stabilizers have a great impact on the particle size and PDI of nano suspension, and there are significant differences among the six treatment groups (p<0.05). Among the six stabilizers, cefquinome sulfate nanosuspensions prepared from glyceryl monostearate, Labrafil M 1944 CS and Labrasol have preferable particle size and PDI scale (the particle size range is 900–1200 nm and the PDI range is 0.8–1.6) and the particle size of the preparation prepared by Labrasol is 940 nm, which is relatively better among the three stabilizers.
Screening of Stabilizer Ratio
Labrasol is an oil-based nonionic water dispersed surfactant. Its viscosity is similar to that of oil-phase Labrafac PG used in this paper, and they are very miscible. On the one hand, it can provide steric hindrance to prevent the aggregation between drug particles; On the other hand, it will play a role in solubilization and improve the dispersion stability of drugs in oil phase. The stabilization effect is related to the concentration. It can be seen from Figure 1B that when the ratio of drug to Labrasol was 1:2, the drug particle size is the smallest, which showed significant difference compared the other two groups (p<0.05). Because the amount of Labrasol was insufficient in the initial stage, the excess drug molecules combined to form precipitation leading to the large particle size. With the increase of Labrasol, the preparation tended to be stable Considering that Labrasol is an effective absorption enhancer, the ratio of drug to Labrasol 1:2 was chosen in the further study.
Glycerol monostearate is a nonionic surfactant, which maintains the stability of nanosuspension by spatial action. In nanosuspension, there is a complex process of adsorption and desorption of stabilizer on the surface of drug particles, which is closely related to the concentration of stabilizer. As can be seen from Figure 1C, when the ratio of drug to glycerol monostearate is 7:1, glycerol monostearate cannot provide enough steric hindrance to organize the aggregation of nanoparticles. Therefore, the particle size of the prepared nanosuspension is large, when the ratio of drug to glyceryl monostearate decreased to 5:1, the drug particle size was smaller, and the drug particle size and PDI did not change significantly with increasing the dosage of the auxiliary materials. Based on the principle of reducing the use of excipients, the ratio of drugs to glycerol monostearate was 5:1.
Labrafil® M 1944 CS is a nonionic surfactant and solubilizer, which can reduce the surface energy of drug particles in nanosuspension and maintain the stability of nanosuspension. It can be seen from Figure 1D that when the ratio of drug to Labrafil M 1944 CS was 2:1, it is difficult to significantly increase the viscosity of the system and provide enough steric hindrance to stabilize the nanosuspension system. When the ratio decreased to 3:2, the particle size and PDI of the drug were the smallest compared with the other two groups. Therefore, the mass ratio of drug to Labrafil M 1944 CS was 3:2.
Screening of Grinding Time
It can be seen from Figure 1E that grinding time has a great influence on the particle size and PDI of nanosuspension. With the extension of time, both drug particle size and PDI decrease, and grinding time has the most obvious influence on PDI. With the increase of grinding time, grinding will be more adequate and uniform, so nanosuspension will be more uniform and PDI will decrease obviously. When the grinding time reaches 3 h, the drug particle size and PDI have reached an ideal state. Therefore, the appropriate grinding time is 3 h.
Screening of Dilution Ratio for Size Determination
It can be seen from Figure 1F that when the dilution ratio reaches about 20–25 times, the kilo counts per second of particle size meter is between 200 and 500, which meets the requirements of instrument detection and the measured particle size results are normal, When the dilution ratio is further increased, both particle size and PDI increase, which may be due to the excessive addition of Labrafac PG, which destroys the internal stability of nanosuspension system and aggregation may occur between particles. Therefore, the appropriate dilution ratio is 20 times.
Formula Process Validation
The process validation results as shown in Table 2. To sum up, the formula composition of CS-NSP prepared by mortar grinding method was preliminarily determined as follows: drug: Labrasol: glyceryl monostearate: Labrafil M 1944 CS=15:30:3:10 (the mass ratio of drug to Labrasol is 1:2, the mass ratio of drug to glyceryl monostearate is 5:1, the mass ratio of drug to Labrafil M 1944 CS is 3:2, the grinding time is determined to be 3 h, and the dilution ratio is 20 times. The average particle size of CS-NSP we prepared was 772.70±82.03, and average PDI was 0.828±0.45.
 |
Table 2 Process Validation Results, n=3, mean ±SD |
Viscosity Measurement
The viscosity measurement results of CS-NSP are shown in Table 3. It can be seen from Table 4 that the viscosity of CS-NSP is much lower than that of CS-INJ. There was significant difference in viscosity between CS-NSP and CS-INJ (p<0.05). For injection, the advantages of low viscosity are: good needle permeability, good compliance of livestock and convenient to industrial production.
 |
Table 3 Comparison of Viscosity and Content Determination Between CS-NSP and CS-INJ with the Same Concentration in the Market, n=3, mean ±SD |
 |
Table 4 Pharmacokinetic Parameters in Rats After Intramuscular Administration of CS-INJ and CS-NSP at a Dosage of 40 mg CS/kg Body Weight, n=5, mean ±SD |
Content Determination
After drug extraction treatment, the contents of CS-INJ and self-made CS-NSP with the same marked concentration were determined, and the results are shown in Table 3.
From the content determination results in Table 3, it can be seen that the contents of the commercial oil suspension and the self-made CS-NSP meet the requirements of 2020 edition of Chinese Veterinary Pharmacopoeia (95–105%).
Characterization of CS-NSP
Appearance and Transmission Electron Microscopy (TEM)
Use transmission electron microscopy to observe the morphology of particles and take photos, as shown in Figure 2A. The cefquinome sulfate nanosuspension prepared by mortar grinding has a uniform milky yellow suspension with good appearance, as shown in Figure 2B. The measured particle size is smaller than that measured by the particle size tester, because the laser particle size is statistical data, which may be the performance of single particles and aggregates, while the electron microscope particle size is generally a statistical single particle. In the suspension, the dispersion performance of drug particles in the dispersion medium is not similar to that of monodispersed slurry, so the electron microscope particle size will be smaller.
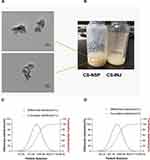 |
Figure 2 (A) Transmission electron microscopy of CS-NSP. (B) Appearance of CS-NSP and CS-INJ. (C) Particle size distribution of CS-NSP and (D) CS-INJ. |
Particle Size and Particle Size Distribution
The particle size distribution of CS-NSP and CS-INJ is shown in Figure 2C and D. It can be seen that the particle size range of CS-NSP is about 200–2000 nm, the average particle size is 725.15±79.19 nm, the polydispersity coefficient is 0.782±0.42, and the particle size distribution is symmetrical and evenly dispersed.
Fourier Transform Infrared (FTIR) Spectroscopy
The FTIR test results indicated in Figure 3A show that the typical bands commonly found in pure cefquinome sulfate were the band at 3402.1 cm−1, which indicated the presence of N-H bonds in primary amine group,39 the band at 1034.7 cm−1, which showed C-O-N bonds, the band at 1796.2 cm−1, which assigned C=O bonds in cyclic amide, and the band at 1611.0 cm−1, 1482.7 cm−1, which mean C=C and C=N bonds. These peaks were also observed in the powder of CS-NSP with slightly difference in intensities, but it showed that there was no chemical reaction between drug and excipients and without new chemical bond.
Differential Scanning Calorimetry (DSC)
The DSC curves of samples are shown in Figure 3B. Due to previous experimental investigations, the CS-NSP showed sharp endothermic peaks at 173.59 and the bulk drug displayed a melting point of 179.11, indicating their crystalline nature. Physical mixture of accessories showed flat line with no melting endotherm means that excipients of Labrasol and Labrafil M 1944 CS have no solvent peak at 0–200°C.40,41
The oil-surfactant-cosurfactant system has provided sufficient stabilization to the drug so that the cefquinome sulfate still exists in crystalline form. Moreover, the temperature difference of 5.52°C in melting point with bulk drug might due to the slight change in crystal form caused by grinding during the preparation, which can make the deformation of the crystal form boundary and increase amorphous components.42
X-ray Diffraction (XRD)
The XRD patterns of pure cefquinome sulfate, the powder of CS-NSP and CS-INJ presented in Figure 3C. The characteristic peaks appeared in the XRD pattern of the pure cefquinome sulfate at a diffraction angle of 8.200°, 12.667°, 16.219°, 20.259°, 24.657° (2θ), suggesting that the drug is present as a crystalline state. The XRD pattern of powder of CS-NSP which was precipitated by n-hexane was also observed that some peaks shown such as 12.518°, 24.520°, 26.549°, and the intensity of peaks reduced. These observations indicate that the drug in CS-NSP was crystalline state.
In vitro Release Study
The cumulative release percentage profiles of the CS-NSP and CS-INJ in dissolution medium for 60 min are shown in Figure 4. The CS-NSP showed an increase in vitro release than CS-INJ, whose cumulative release percentage was 97.84% at 5 min higher than CS-INJ of 27.74%.
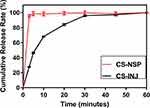 |
Figure 4 The comparison of cumulative release percentage profiles in CS-NSP and CS-INJ. |
The enhanced cumulative release percentage was mainly attributed to the small particle size and increased surface area of drug, which facilitated drug release.43 Meanwhile, grinding during preparation increase in lattice defects to make the drug in disordered crystalline state that exhibited higher solubilization.23 In the end, Labrasol in CS-NSP improved the hydrophilic ability of the oil system, forcing water molecules to move to the oil solution and weakened the oil barrier.44
Syringeability and Stability
Syringeability
Results showed that the flow velocity reached to 2.91 mm/s in the CS-NSP and increased almost 3.6 times in the CS-INJ which reached to 0.78 mm/s means the excellent syringeability. The CS-NSP displayed excellent flow properties, such as faster flow velocity, freedom from clogging or foaming. Within the range studied, the particle size and viscosities were three parameters. The viscosity of CS-INJ (54.90 cp at 25°C) was five times higher than the CS-NSP (13.13cp at 25°C). Due to increasing viscosity can lead to higher head loss and longitudinal drag force caused by the pressure to push particles toward needle scale of 1 mL, the flow of the suspension through the syringe more become more difficult. Meanwhile, injecting a high number of small particles would impose lower risk of needle blockage and the particle size of CS-INJ were four times higher compared to CS-NSP. Therefore, the excellent syringeability shortens the injection time and reduces the pain of injection contributing to the adherence.
Sedimentation Volume Ratio Determination
After standing for 24 h, the sedimentation volume ratio was significantly higher of the CS-NSP in comparison to the CS-INJ, just as shown on Figure 5A. The sedimentation volume ratio was 0.51 and 0.19 at 24 h for CS-NSP and CS-INJ, respectively, and there was 2.68-fold higher for CS-NSP compared to the CS- INJ.
According to Stokes' law, the sedimentation volume ratio depends on particle size and viscosity of suspensions.45 During preparation, appropriate stabilizer and 3 h stirring time reduced the drug particle size so that the small particles were suspended for a long time. Although the internal particles settled after a short time, the interparticle attraction and bonding were loose, which also contributed to high sedimentation volume ratio.46
Physical Stability
From Figure 5B–H, it was observed that there were significant changes on evaluation parameters after the study. From Figure 5B and E, the color of Group 1 was obviously changed from white to yellow. The formulation showed drug precipitation and lost 13.6% of its bulk drug after storage for 10 days. To reduce the oxidization under illuminated light, antioxidants and a dark environment are the key factors for preparation. From Figure 5C and E, the color of Group 2 was uniform of white, but the drug in Group 2 absorbed moisture which contributed the impurity of 5, 6, 7, 8-tetrahydroquinoline increased significantly and the bulk drug content loss of 3.4%. Meanwhile, small particles agglomerated to large particles contributed the particle size from 1682 nm to 3698 nm. From the Figure 5D and E, the color of Group 3 changed drastically from white to red means the discoloration of drug accelerated and resulted in degradation which contributed to the loss of 5.23% for the bulk drug, so the preparation should be stored at room temperature. In contrast, the cumulative release percentage profiles of these groups after storage for 10 days were similar to samples when these were just prepared (Figure 5F, G and H). In conclusion, the CS-NSP should be stored in shade and kept dry.
In vitro Hemolysis Assays
The hemolysis rate of the CS-NSP shown on Figure 6A at different concentration at 570 nm were lower than 5% means that it meets the national requirements for hemolysis rate of injections. The negative control solution was stratified, and there was no red blood cells residue at the bottom of the tube. The upper solutions of tube labeled 1–8 contained two layers which is the oil layer and the colorless and clear supernatant that could be resuspended by shaking the tube just as Figure 6B, indicating good hemocompatibility and no red blood cell agglutination.
In vivo Muscle Irritation Test
After intramuscular injection, the mice did not show any clinical sign of pain and without lumps or swelling on the muscle tissue. Combined with the staining results shown in Figure 7, it can be seen that the elongated skeletal muscle fibers with a blue stained cell nucleus, and the small interstitial tissue without inflammatory cell infiltration. The pathological figures of the injection site showed no significant differences compared to the marketed injection means that the nanosuspension has no irritation which might be due to the low viscosity and small irritation effect of Labrafac PG, the small drug particles, and the good emulsifying ability of Labrasol.
 |
Figure 7 H&E staining image at the injection site of (1) normal saline group, (2) 0.2 mL Labrafac PG group, (3) 0.2 mL CS-NSP group, (4) 0.1 mL CS-NSP group, and (5) 0.2 mL CS-INJ, respectively. |
In vivo Pharmacokinetics
The plasma drug concentration – time curves for CS-INJ and CS-NSP after intramuscular injection to rats are presented in Figure 8. The plasma concentration of CS in CS-INJ group and CS-NSP group decreased rapidly about 1 h after administration, and approached the quantitative limit at 8.5 h. After noncompartment model fitting, the pharmacokinetic parameters of the two groups are shown in Table 4. The Cmax and AUC0-∞ values of CS-NSP were about twice that of CS-INJ (p<0.01). The CL value of CS-NSP was about three-fifths that of CS-INJ (p<0.05). The relative bioavailability values of CS-NSP were 158.18%. It is proved that the CS-NSP group has faster absorption and higher bioavailability when injected with the same dose of cefquinome sulfate, indicating that the oily nanosuspension alleviated the limitation of cefquinome sulfate absorption in vivo. CL of CS-NSP group decreased, indicating that the elimination rate of CS-NSP was slower than CS-INJ in rats, and that more drugs could exert their efficacy in vivo.
Both CS-INJ and CS-NSP belong to oil suspension, so the rate-limiting process of absorption of cefquinome sulfate in vivo is the dissolution of cefquinome sulfate in tissue liquid, which is related to drug solubility, particle size, liquid viscosity and the use of additives. In the in vitro release experiment of this study, it was found that the dissolution of CS-NSP was very rapid, indicating that cefquinome sulfate may also be rapidly dissolved into body fluid after injection into the body. The average particle size of the CS-NSP was reduced to half of that of the commercial preparation, and the viscosity of the oil dispersion medium (Labrafac PG) was lower, which increased the diffusion and dissolution rate of the drug and increased the absorption of the drug. The new stabilizer Labrasol not only increases the stability of the suspension, but also promotes the absorption of the drug. Other additives (glycerol monostearate, Labrafil M 1944 CS) can dissolve cell membrane lipids, increase membrane permeability, decrease surface tension and increase drug wettability, thus increasing the dissolution and absorption of cefquinome sulfate. These factors also affect the distribution and elimination of cefquinome sulfate in rats in varying degrees, highlighting the advantages of cefquinome sulfate oily nanosuspension.
Conclusion
The formulation, physicochemical properties and pharmacokinetics of cefquinome sulfate oily nanosuspension were reported in this paper. CS-NSP was prepared by mortar grinding method. The best formula was drug: Labrasol: glyceryl monostearate: Labrafil M 1944 CS=15:30:3:10, and add an appropriate amount of Labrafac PG as oily dispersion medium to make CS-NSP reach the required concentration. The characterization in vitro showed that the particle size and viscosity of oily nanosuspension decreased. The dissolution test in vitro showed that the dissolution of oily nanosuspension was faster than that of commercial injection. In vivo pharmacokinetic studies showed that the self-made cefquinome sulfate oily nanosuspension had advantages in absorption, distribution and elimination in rats. Conclusively, the new preparation is safe and effective and is expected to become a new type of veterinary preparation with great application prospects.
Acknowledgments
This work was supported by Animal Medicine Science and Technology Innovation Team of Jiangsu Agri-animal Husbandry Vocational College (Grant no. NSF2021TC02); Qinglan Project of Colleges and Universities in Jiangsu Province (Sujiaoshi2019[3]); 333 high-level talent training project of Jiangsu Province; Jiangsu Students’ innovation and entrepreneurship training program (Grant no. 202112806013Y); Innovation and Entrepreneurship Training Program for Undergraduate (202210316146, 202210316147). We also thank Jing Zhang and Qingjie Zhang from China Pharmaceutical University for their formulation preparation assistance.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Limbert M, Isert D, Klesel N, et al. Antibacterial activities in vitro and in vivo and pharmacokinetics of cefquinome (HR 111V), a new broad-spectrum cephalosporin. Antimicrob Agents Chemother. 1991;35(1):14–19. doi:10.1128/AAC.35.1.14
2. Parlevliet JM, Lynn JW, Paccamonti DL. The use of cefquinome in equine semen extender. J Equine Vet Sci. 2011;31(3):139–142. doi:10.1016/j.jevs.2010.12.015
3. Zhou YF, Zhao DH, Yu Y, et al. Pharmacokinetics, bioavailability and PK/PD relationship of cefquinome for Escherichia coli in Beagle dogs. J Vet Pharmacol Ther. 2015;38(6):543–548. doi:10.1111/jvp.12225
4. Thomas E, Thomas V, Wilhelm C. Antibacterial activity of cefquinome against equine bacterial pathogens. Vet Microbiol. 2006;115(1–3):140–147. doi:10.1016/j.vetmic.2005.12.019
5. Lee DH, Birhanu BT, Lee EB, et al. Pharmacokinetic and pharmacodynamic integration for optimal dosage of cefquinome against Streptococcus equi subsp. Equi in Foals Vet Res. 2020;51(1):131. doi:10.1186/s13567-020-00853-2
6. Funaki H, Uzuka Y, Tanabe S, et al. Therapeutic efficacy of cefquinome against acute respiratory disease in holstein steers. J Jpn Vet Med Assoc. 2001;54:451–454. doi:10.12935/jvma1951.54.451
7. Khusro A, Aarti C, Buendía-Rodriguez G, Arasu MV, Al-Dhabi NA, Barbabosa-Pliego A. Adverse effect of antibiotics administration on horse health: an overview. J Equine Vet Sci. 2021;97:103339. doi:10.1016/j.jevs.2020.103339
8. Ahmad I, Hao H, Huang L, et al. Integration of PK/PD for dose optimization of Cefquinome against Staphylococcus aureus causing septicemia in cattle. Front Microbiol. 2015;6:588. doi:10.3389/fmicb.2015.00588
9. Fu Q, Fu HL, Huan L, et al. Preparation of cefquinome sulfate proliposome and its pharmacokinetics in rabbit. Iran J Pharm Res. 2013;12(4):611–621.
10. Zhang S, Dai W, Lu Z, et al. Preparation and evaluation of cefquinome-loaded gelatin microspheres and the pharmacokinetics in pigs. J Vet Pharmacol Ther. 2018;41(1):117–124. doi:10.1111/jvp.12429
11. Du X, Zu S, Chen F, et al. Preparation and characterization of cefquinome sulfate microparticles for transdermal delivery by negative-pressure cavitation antisolvent precipitation. Powder Technol. 2016;294:429–436. doi:10.1016/j.powtec.2016.03.008
12. Kesisoglou F, Panmai S, Wu Y. Nanosizing–oral formulation development and biopharmaceutical evaluation. Adv Drug Deliv Rev. 2007;59(7):631–644. doi:10.1016/j.addr.2007.05.003
13. Sinha B, Müller RH, Möschwitzer JP. Bottom-up approaches for preparing drug nanocrystals: formulations and factors affecting particle size. Int J Pharm. 2013;453(1):126–141. doi:10.1016/j.ijpharm.2013.01.019
14. Lu Y, Li Y, Wu W. Injected nanocrystals for targeted drug delivery. Acta Pharm Sin B. 2016;6(2):106–113. doi:10.1016/j.apsb.2015.11.005
15. Ghosh I, Bose S, Vippagunta R, Harmon F. Nanosuspension for improving the bioavailability of a poorly soluble drug and screening of stabilizing agents to inhibit crystal growth. Int J Pharm. 2011;409(1–2):260–268. doi:10.1016/j.ijpharm.2011.02.051
16. Mallappa MK, Kesarla R, Banakar S. Calcium Alginate-Neusilin US2 nanocomposite microbeads for oral sustained drug delivery of poor water soluble drug aceclofenac sodium. J Drug Deliv. 2015;2015:826981. doi:10.1155/2015/826981
17. Yadava SK, Basu SM, Chauhan M, et al. Low temperature, easy scaling up method for development of smart nanostructure hybrid lipid capsules for drug delivery application. Colloids Surf B Biointerfaces. 2020;190:110927. doi:10.1016/j.colsurfb.2020.110927
18. McCartney F, Jannin V, Chevrier S, et al. Labrasol® is an efficacious intestinal permeation enhancer across rat intestine: ex vivo and in vivo rat studies. J Controlled Release. 2019;310:115–126. doi:10.1016/j.jconrel.2019.08.008
19. Balakrishnan P, Lee B-J, Oh DH, et al. Enhanced oral bioavailability of Coenzyme Q10 by self-emulsifying drug delivery systems. Int J Pharm. 2009;374(1):66–72. doi:10.1016/j.ijpharm.2009.03.008
20. Oh DH, Kang JH, Kim DW, et al. Comparison of solid self-microemulsifying drug delivery system (solid SMEDDS) prepared with hydrophilic and hydrophobic solid carrier. Int J Pharm. 2011;420(2):412–418. doi:10.1016/j.ijpharm.2011.09.007
21. Li X, Wu W, Su D, Wang Z, Jiang H, Shen J. Pharmacokinetics and bioavailability of cefquinome in healthy piglets. J Vet Pharmacol Ther. 2008;31(6):523–527. doi:10.1111/j.1365-2885.2008.00989.x
22. Das A, Nayak AK, Mohanty B, Panda S. Solubility and dissolution enhancement of etoricoxib by solid dispersion technique using sugar carriers. ISRN Pharm. 2011;2011:819765. doi:10.5402/2011/819765
23. Guo T, Zhang Y, Zhao J, Zhu C, Feng N. Nanostructured lipid carriers for percutaneous administration of alkaloids isolated from Aconitum sinomontanum. J Nanobiotechnology. 2015;13:47. doi:10.1186/s12951-015-0107-3
24. Bunaciu AA, Udriştioiu EG, Aboul-Enein HY. X-ray diffraction: instrumentation and applications. Crit Rev Anal Chem. 2015;45(4):289–299. doi:10.1080/10408347.2014.949616
25. Yadav PS, Yadav E, Verma A, Amin S. Development, characterization, and pharmacodynamic evaluation of hydrochlorothiazide loaded self-nanoemulsifying drug delivery systems. Sci World J. 2014;2014:274823. doi:10.1155/2014/274823
26. Cilurzo F, Selmin F, Minghetti P, et al. Injectability evaluation: an open issue. AAPS Pharm Sci Tech. 2011;12(2):604–609. doi:10.1208/s12249-011-9625-y
27. Palur K, Archakam SC, Lingasani N, Diviti R, Komarla Kumarachari R, Velusamy S. RP-HPLC method for the estimation of ceftiofur hydrochloride in bulk form. J Pharm Res. 2013;7(3):246–251.
28. National Pharmacopoeia Commission. Chinese Pharmacopoeia [M]. Beijing: China Pharmaceutical Science and Technology Press; 2020:1088.
29. Ahsan T, Chen J, Zhao X, Irfan M, Wu Y. Extraction and identification of bioactive compounds (eicosane and dibutyl phthalate) produced by Streptomyces strain KX852460 for the biological control of Rhizoctonia solani AG-3 strain KX852461 to control target spot disease in tobacco leaf. AMB Express. 2017;7(1):54. doi:10.1186/s13568-017-0351-z
30. Cao J, Huang J, Gui S, Chu X. Preparation, synergism, and biocompatibility of in situ liquid crystals loaded with sinomenine and 5-Fluorouracil for treatment of liver cancer. Int J Nanomedicine. 2021;16:3725–3739. doi:10.2147/IJN.S207607
31. Dou JP, Wu Q, Fu CH, et al. Amplified intracellular Ca (2+) for synergistic anti-tumor therapy of microwave ablation and chemotherapy. J Nanobiotechnology. 2019;17(1):118. doi:10.1186/s12951-019-0549-0
32. Tan X, Gao P, Li Y, et al. Poly-dopamine, poly-levodopa, and poly-norepinephrine coatings: comparison of physico-chemical and biological properties with focus on the application for blood-contacting devices. Bioact Mater. 2021;6(1):285–296. doi:10.1016/j.bioactmat.2020.06.024
33. Tao Y, Yang F, Meng K, et al. Exploitation of enrofloxacin-loaded docosanoic acid solid lipid nanoparticle suspension as oral and intramuscular sustained release formulations for pig. Drug Deliv. 2019;26(1):273–280. doi:10.1080/10717544.2019.1580798
34. Mazutti da Silva SM, Rezende Costa CR, Martins Gelfuso G, et al. Wound healing effect of essential oil extracted from Eugenia dysenterica DC (Myrtaceae) leaves. Molecules. 2018;24(1):2. doi:10.3390/molecules24010002
35. Shan Q, Liang C, Wang J, Li J, Zeng Z. In vivo activity of cefquinome against Escherichia coli in the thighs of neutropenic mice. Antimicrob Agents Chemother. 2014;58(10):5943–5946. doi:10.1128/AAC.03446-14
36. Rafiei P, Haddadi A. Docetaxel-loaded PLGA and PLGA-PEG nanoparticles for intravenous application: pharmacokinetics and biodistribution profile. Int J Nanomedicine. 2017;12:935–947. doi:10.2147/IJN.S121881
37. Elazab ST, Schrunk DE, Griffith RW, et al. Pharmacokinetics of cefquinome in healthy and Pasteurella multocida-infected rabbits. J Vet Pharmacol Ther. 2018;41(3):374–377. doi:10.1111/jvp.12489
38. Zhan H, Yang X, Wang C, Liang C, Wu M. Multiple growth stages and their kinetic models of anatase nanoparticles under hydrothermal conditions. J Phys Chem C. 2010;114(34):14461–14466. doi:10.1021/jp1062308
39. Pasaribu KM, Gea S, Ilyas S, et al. Fabrication and in-vivo study of micro-colloidal Zanthoxylum acanthopodium-loaded bacterial cellulose as a burn wound dressing. Polymers. 2020;12(7):1436. doi:10.3390/polym12071436
40. Tran T, Xi X, Rades T, Müllertz A. Formulation and characterization of self-nanoemulsifying drug delivery systems containing monoacyl phosphatidylcholine. Int J Pharm. 2016;502(1–2):151–160. doi:10.1016/j.ijpharm.2016.02.026
41. Milović M, Simović S, Lošić D, Dashevskiy A, Ibrić S. Solid self-emulsifying phospholipid suspension (SSEPS) with diatom as a drug carrier. Eur J Pharm Sci. 2014;63:226–232. doi:10.1016/j.ejps.2014.07.010
42. Archana K, Aman A, Jabeen A. Effect of superfine grinding on structural, morphological and antioxidant properties of ginger (Zingiberofficinale) nano crystalline food powder. Mater Today. 2021;43:3397–3403.
43. Xie S, Zhang X, Luo W, et al. Formulation, characterization and pharmacokinetics of long-acting ceftiofur hydrochloride suspension. Curr Drug Deliv. 2021;18(2):224–233. doi:10.2174/1567201817666200903165119
44. Wang T, Shen L, Zhang Y, Li H, Wang Y, Quan D. “Oil-soluble” reversed lipid nanoparticles for oral insulin delivery. J Nanobiotechnology. 2020;18(1):1–11. doi:10.1186/s12951-020-00657-8
45. Fatimi A, Tassin J-F, Axelos MA, Weiss P. The stability mechanisms of an injectable calcium phosphate ceramic suspension. J Mater Sci. 2010;21(6):1799–1809. doi:10.1007/s10856-010-4047-z
46. Ogaji JI, Omachi JA, Iranloye TA. Effect of Adansonia digitata gum on some physicochemical properties of paracetamol pediatric suspension formulations. Int J Res Pharm Sci. 2012;2(2):129–141.
47. Wang B, Xinbiao Y, Jianying C. The method for improving the dissolution rate of cefquinome sulfate and its dissolution test method: CN111714501A[P]; 2020.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.