Back to Journals » Infection and Drug Resistance » Volume 10
Cefazolin potency against methicillin-resistant Staphylococcus aureus: a microbiologic assessment in support of a novel drug delivery system for skin and skin structure infections
Authors Nicolau DP, Silberg BN
Received 11 February 2017
Accepted for publication 11 July 2017
Published 26 July 2017 Volume 2017:10 Pages 227—230
DOI https://doi.org/10.2147/IDR.S134497
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
David P Nicolau,1 Barry N Silberg2
1Center for Anti-Infective Research and Development, Hartford Hospital, Hartford, CT, USA; 2Department of Surgery, Sonoma West Medical Center, Sebastopol, CT, USA
Introduction: Despite aggressive medical and surgical management, the resolution of skin and skin structure infections is often difficult due to insufficient host response, reduced drug penetration, and a high prevalence of resistance organisms such as methicillin-resistant Staphylococcus aureus (MRSA). As a result of these factors, conventional management often consists of prolonged broad-spectrum systemic antimicrobials. An alternative therapy in development, ultrasonic drug dispersion (UDD), uses a subcutaneous injection followed by external transcutaneous ultrasound to deliver high tissue concentrations of cefazolin with limited systemic exposure. While it is postulated that these high concentrations may be suitable to treat more resistant organisms such as MRSA, the cefazolin minimum inhibitory concentration (MIC) distribution for this organism is currently unknown.
Materials and methods: We assessed the potency of cefazolin against a collection of 1,239 MRSA from 42 US hospitals using Clinical Laboratory Standard Institute-defined broth microdilution methodology.
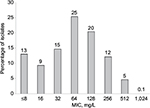
Results: The cefazolin MIC inhibiting 50% of the isolates was 64 mg/L; 81% had MICs ≤128 and nearly all (99.9%) had MICs ≤512 mg/L.
Conclusion: The overwhelming majority of MRSA had cefazolin MICs that were considerably lower than achievable tissue concentrations (≥1,000 mg/L) using this novel drug delivery system. While the currently defined cefazolin MRSA phenotypic profile precludes the use of parenteral administration, techniques that deliver local exposures in excess of these inhibitory concentrations may provide a novel treatment strategy for skin and skin structure infections.
Keywords: methicillin-resistant, Staphylococcus aureus, ultrasonic, infection, cefazolin
Erratum for this paper has been published
Introduction
Although the treatment paradigm for skin and skin structure infections (SSSIs) in diabetics and those with peripheral vascular disease frequently incorporates aggressive medical and surgical interventions, infection resolution in these patient populations is often difficult due to insufficient host response, reduced drug penetration, and a high prevalence of resistance organisms such as methicillin-resistant Staphylococcus aureus (MRSA).1 As a result of these factors, conventional medical management of these patients consists of the use of prolonged courses of broad-spectrum systemic antimicrobials.2 Despite the administration of systemic antimicrobials, this approach often results in poor infection outcomes and the need for amputation.1 Moreover, these currently utilized, prolonged antimicrobial regimens, unfortunately, also put patients at risk for adverse events such as superinfections (ie, infection with a new pathogen in the previously infected wound or at a previously uninfected site such as Clostridium difficile diarrhea) and/or the development of resistance in the originally infecting organism(s).
In an attempt to provide high local antimicrobial concentrations and avoid unnecessary systemic exposures, novel drug delivery techniques are being investigated. However, before the viability of these delivery systems can be fully understood, an assessment of the potency of the test agent must be undertaken against the target organism(s) of interest. Due to its longstanding use for prophylaxis and infection treatment, good systemic safety profile over a wide range of doses, and the ability to use intramuscular administration without local tissue damage, cefazolin possesses suitable characteristics for consideration of direct drug delivery to the infection site.3,4 While the intravenous administration of cefazolin is commonly given for both surgical prophylaxis and treatment of Gram-positive organisms such as methicillin-susceptible S. aureus (MSSA), due to the attainment of sufficiently high tissue concentrations which are in excess of typical MSSA minimum inhibitory concentrations (MICs) of 1–2 mg/L, achievable concentration using this route is considered too low for MRSA.5–7 Thus when considering the intravenous route of administration, organisms such as MRSA are considered intrinsically resistant to cefazolin. However, since this definition of resistance is based on achievable concentrations at the site of infection, if sufficiently high concentrations of an antimicrobial could be delivered in excess of the MIC using alternative drug delivery techniques, clinical efficacy may be recognized despite the classical definition of resistance based solely on the serum profile of the agent.
In an attempt to assess the utility of cefazolin for inclusion in a novel drug delivery system targeting skin infections, the activity of this compound against MRSA, one of the most frequently isolated and drug-resistant pathogens, would need to be determined in contemporary clinical isolates. Thus, the purpose of this study was to examine the in vitro microbiologic potency of cefazolin against large collection of MRSA derived from US hospitals.
Materials and methods
The MRSA included in the current investigation were collected between 2011 and 2013 from 42 US medical centers as part of a national surveillance program.7 All consecutive, non-duplicate organisms were identified by the local laboratory collecting the isolates, and reconfirmation of MRSA status was undertaken using BBL CHROMagar MRSA (BD, Franklin Lakes, NJ, USA) at the Center for Anti-Infective Research and Development (CAIRD), Hartford Hospital. Susceptibility testing was conducted at the CAIRD using reference broth microdilution techniques according to guidelines published by the Clinical and Laboratory Standards Institute.8,9 ATCC S. aureus 29213 was used for quality control on all MIC trays prior to and during the conduct of susceptibility testing. Colony counts were performed on each isolate to verify whether the correct inoculum was delivered to the MIC tray. As a result of the high achievable cefazolin concentrations with the ultrasonic drug dispersion (UDD) technique, the MIC range against cefazolin was extended up to a concentration of 1,024 mg/L.
Results
A total of 1,239 MRSA were tested against cefazolin and the MIC inhibiting 50% and 90% of the isolates was 64 and 256 mg/L, respectively. Eighty-one percent of these isolates had MICs ≤128 mg/L and 5% had an MIC=512 mg/L. Only a single isolate was noted to have an MIC of 1,024 mg/L. The MIC distribution for cefazolin against the MRSA population is displayed in Figure 1.
Discussion
Owing to the increasing compromise of the host and the prevalence of methicillin resistance among S. aureus, SSSIs have become one of the most challenging medical problems encountered in clinical arena.10 When considering this target pathogen, the increasing incidence of MRSA across the healthcare continuum has resulted in increased outpatient failures and higher rates of hospitalization for parental antimicrobials.10 In addition to the collective clinical implications, poor outcomes, and the need for frequent surgical intervention, the high prevalence of this disease entity represents an enormous cost of care liability to the healthcare system. As a result of these issues surrounding SSSIs, novel approaches to the management of these infectious processes are being sought. One methodology currently in clinical development is that of UDD. UDD is a procedure that incorporates a subcutaneous injection of the test agent in a sufficient volume of sterile solution that swells or tumesces the area of the infection and is then followed by external transcutaneous ultrasound which delivers ultrasound energy at 3 W/cm2 applied to the tissue for 3 minutes to diffuse the antimicrobial-containing solution throughout the target site.11
Due to the favorable systemic safety profile of cefazolin over a wide range of doses and its ability to be given by intramuscular injection without local tissue damage, cefazolin appears to be a good candidate for delivery via UDD. Preliminary studies revealed that cefazolin was well tolerated when given via UDD, while delivering high (≥1,000 mg/L) tissue concentrations of cefazolin with limited systemic exposure.11
This current study is unique in that the routine testing of cefazolin against MRSA is not conducted by clinical laboratories because this organism is considered to be intrinsically resistant to this antimicrobial as the concentrations routinely achieved at the infection site with conventionally administered intravenous doses are well below the expected MIC of this pathogen. Thus, contemporary comparative data on the potency of cefazolin against a large population of MRSA are not available. While this definition of intrinsic resistance for MRSA may be applicable to systemically administered cefazolin, the use of this resistance definition may not apply to local drug delivery techniques since the achievable concentrations in tissue may be well above the MIC of the target organism.2 Therefore, to fully evaluate the potential clinical viability of the UDD technique against this multidrug-resistant pathogen, a large-scale assessment of the potency profile of cefazolin against MRSA was required.
In the current study, we assessed the potency of cefazolin against a population of MRSA that had previously displayed typical phenotypic profiles to vancomycin, ceftaroline, daptomycin, linezolid, and tigecycline.7 When the 1,239 MRSA were tested against cefazolin, 99.9% of these organisms had MICs ≤512 mg/L. Moreover, most of the isolates had cefazolin MIC values that were considerably lower than 256 mg/L. As a result of these contemporary potency data, it appears that achievable tissue concentrations of cefazolin with the UDD technique are well in excess of the concentrations required to inhibit the growth of MRSA.
Conclusion
While systemic cefazolin is not considered a viable therapeutic option for MRSA-related SSSIs because of its relatively low tissue concentrations in the face of the high MICs as presented in this current study, local drug delivery techniques appear to represent a novel strategy to achieve sufficiently high cefazolin concentrations in excess of its MRSA MIC profile. Ultimately, clinical trial data will be required to support or refute the utility of cefazolin administered via the UDD technique for the management of MRSA skin infections; however, until these data are available, studies such as this which define potency relative to achievable drug concentrations at the site of infection provide important insights for the clinical development process of this novel therapeutic intervention.
Acknowledgments
The authors thank Christina Sutherland and the staff of the Center for Anti-Infective Research and Development, Hartford Hospital, for conducting the in vitro susceptibility testing. Abstract of this paper has been presented: Nicolau DP, Silberg BN. Assessing the Potency of Cefazolin against Methicillin-Resistance Staphylococcus aureus (MRSA): Microbiologic Data Supporting Ultrasonic Drug Dispersion for the Management of Skin & Skin Structure Infections (SSSIs) (Abstract No. C-1071). 55th Interscience Conference on Antimicrobial Agents and Chemotherapy/International Congress of Chemotherapy and Infection, San Diego, CA, September 2015.
Author contributions
David P Nicolau and Barry N Silberg conceived and designed the experiments, reviewed the raw data and quality control procedures, analyzed the data, and wrote the paper.
Disclosure
Drs Nicolau and Silberg are shareholders in Sonesence, the developer of the UDD technique. The authors report no other conflicts of interest in this work.
References
Nicolau DP, Stein GE. Therapeutic options for diabetic foot infections: a review with an emphasis on tissue penetration characteristics. J Am Podiatr Med Assoc. 2010;100(1):52–63. | ||
Ray A, Malin D, Nicolau DP, Wiskirchen DE. Antibiotic tissue penetration in diabetic foot infections: a review of the microdialysis literature and needs for future research. J Am Podiatr Med Assoc. 2015;105(6):520–531. | ||
Polk RE, Smith JE, Ducey K, Lower RR. Penetration of moxalactam and cefazolin into atrial appendage after simultaneous intramuscular or intravenous administration. Antimicrob Agents Chemother. 1982;22(2):201–203. | ||
Cefazolin for Injection, USP [package insert]. Schaumburg, IL: Sagent Pharmaceutics; 2012. | ||
Douglas A, Udy AA, Wallis SC, et al. Plasma and tissue pharmacokinetics of cefazolin in patients undergoing elective and semielective abdominal aortic aneurysm open repair surgery. Antimicrob Agents Chemother.2011;55(11):5238–5242. | ||
Brill MJ, Houwink AP, Schmidt S, et al. Reduced subcutaneous tissue distribution of cefazolin in morbidly obese versus non-obese patients determined using clinical microdialysis. J Antimicrob Chemother. 2014;69(3):715–723. | ||
Housman ST, Sutherland CA, Nicolau DP. Pharmacodynamic Profile of Commonly Utilised Parenteral Therapies against Methicillin-Susceptible and Methicillin-Resistant Staphylococcus aureus Collected from US Hospitals. Int J Antimicrob Agents. 2014;44(3):235–241. | ||
Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard-Ninth Edition, (M07-A9). Wayne, PA: CLSI; 2012. | ||
Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement (M100-S24). Wayne, PA: CLSI; 2014. | ||
Verastegui JE, Hamada Y, Nicolau DP. Transitions of care in the management of acute bacterial skin and skin structure infections: a Paradigm shift. Expert Rev Clin Pharmacol. 2016;9(8):1039–1045. | ||
Silberg BN. Direct antibiotic delivery into soft tissue infections using ultrasonic dispersion. Plast Reconst Surg. 2013; 132(4S-1):51–52. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.