Back to Journals » OncoTargets and Therapy » Volume 12
CDK4/6 Inhibitor Palbociclib Amplifies the Radiosensitivity to Nasopharyngeal Carcinoma Cells via Mediating Apoptosis and Suppressing DNA Damage Repair
Authors Xie X, Zheng W , Chen T, Lin W, Liao Z, Liu J, Ding Y
Received 10 October 2019
Accepted for publication 26 November 2019
Published 16 December 2019 Volume 2019:12 Pages 11107—11117
DOI https://doi.org/10.2147/OTT.S234221
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Gaetano Romano
Xianhe Xie,1,* Weili Zheng,1,2,* Ting Chen,1,2 Wanzun Lin,1,2 Ziyuan Liao,1 Junjin Liu,1 Yin Ding1
1Department of Medical Oncology, The First Affiliated Hospital of Fujian Medical University, Fuzhou 350005, Fujian, People’s Republic of China; 2Department of Central Laboratory, The First Affiliated Hospital of Fujian Medical University, Fuzhou 350005, Fujian, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xianhe Xie
Department of Medical Oncology, The First Affiliated Hospital of Fujian Medical University, 20th Chazhong Road, Fuzhou 350005, Fujian, People’s Republic of China
Tel +86 183 0591 5132
Fax +86 591 8798 1028
Email [email protected]
Background: Radiotherapy is the primary approach for nasopharyngeal carcinoma (NPC). Although high survival rates can be obtained with radiation for early stage lesions, distant metastasis and local recurrence frequently occur. In this study, we pioneeringly investigated the antitumor activity and underlying mechanism of cyclin-dependent kinase 4/6 inhibitor (palbociclib) combined with radiation on NPC cells.
Methods: Evaluation of radiation enhancement with palbociclib was based on results from CCK8 and clonogenicity assays for cell proliferation, flow cytometry for apoptosis, ROS level and cell cycle and immunocytochemistry for DNA double-strand break repair.
Results: Palbociclib inhibited cellular growth of RB-proficient CNE-1 and CNE-2 via reducing RB phosphorylation and arresting cell cycle. Combination regimens of palbociclib plus radiation were significantly superior to palbociclib or radiation only through inhibiting cellular growth and inducing apoptosis. Moreover, the antitumor activity of both concurrent palbociclib plus radiation and radiation followed by palbociclib consistently preceded that of palbociclib followed by radiation. Meanwhile, the two preferable combination regimens possessed higher proportion of G2/M phase cells, evidently inhibited DNA double-strand break repair and eventually triggered tumor cell apoptosis.
Conclusion: Our study demonstrated that palbociclib could provoke a strong antitumor activity as a potential adjuvant to radiation therapy for NPC harboring RB expression.
Keywords: nasopharyngeal carcinoma, palbociclib, radiotherapy, apoptosis, DNA damage repair
Introduction
Nasopharyngeal carcinoma (NPC), deriving from the nasopharynx epithelium, is associated with a unique pattern of geographical distribution. NPC occurs dominantly in the eastern and southeastern parts of Asia, and northern and eastern Africa.1 Although the 5-year control rate of local NPC by radiotherapy can reach 80% to 90%, a proportion of individuals still develop into distant metastasis and local recurrence, which is mainly attributed to radiation resistance.2 Hence, it is crucial to find novel drugs or therapeutic modalities to enhance radiosensitivity and improve prognosis of NPC.
Palbociclib, a potently oral and highly specific inhibitor targeting cyclin-dependent kinases 4 and 6 (CDK4/6) that commits the transition between G1 and S phase of cell cycle, induces inactivation of the retinoblastoma (RB) tumor-suppressor protein and G1-phase cell-cycle arrest.3 Palbociclib is the first CDK4/6 inhibitor approved by the FDA as first-line treatment of advanced breast cancer.4 Notably, palbociclib proves to be promising in managing RB-proficient germ-cell tumors, liposarcoma, melanoma in some clinical trials. Moreover, preclinical evidence reveals its effectiveness in colorectal cancer, non-small-cell lung cancer, pancreatic cancer and ovarian cancer in combination or alone strategies.5 Palbociclib also facilitates radiosensitivity in glioblastoma, atypical teratoid rhabdoid tumor and non-small-cell lung cancer.6,7 However, whether palbociclib may serve as a favorable antitumor agent when combining with radiation in NPC remains to be established.
In this study, we verified the hypothesis that palbociclib enhanced the radiosensitivity of CNE-1 and CNE-2 cells in vitro. To determine an optimal administration sequence, various regimens of palbociclib plus radiation treatment (RT) were analyzed. The results demonstrated that palbociclib augmented the antitumor activity of radiation by delaying DNA double-strand break repair and inducing cell-cycle arrest, consequently promoted cellular apoptosis. Moreover, this study indicated that treatment of concurrent palbociclib plus radiation and radiation followed by palbociclib preferably enhanced radiosensitivity.
Materials and Methods
Reagents and Antibodies
Palbociclib (Selleck Chemicals, Shanghai, China); Antibodies specific for pRB (Ser780, #9307, 1:3000), RB (#9309, 1:3000), CDK4 (#12790, 1:3000), CDK6 (#3136, 1:3000) and p16 (#80772, 1:3000) were obtained from Cell Signaling Technologies. Antibodies against γ-histone-H2AX (ab124781, 1:300), 53BP1 (ab175933, 1:300) and β-actin (ab8227, 1:3000) were ordered from Abcam (Abcam, Cambridge, UK). ECL luminescence reagent (abs920) was obtained from Absin (Absin Bioscience Inc., Shanghai, China).
Cell Culture
CNE-1 and CNE-2 cells were gifted from Central Laboratory of The First Affiliated Hospital of Fujian Medical University, approved by Review Board of The First affiliated hospital of Fujian Medical University, and cultured in RPMI-1640 (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (HyClone, Logan, UT, USA) at 37 °C in a humidified incubator containing 5% CO2.
Cell Viability Assay
Cell viability was determined by CCK8 assay. Cells were seeded into 96-well plates and incubated in the presence of various concentrations of palbociclib for 72 hrs, 10 μL of CCK-8 dye solution was added to each well and then incubated at 37°C for 1 hr. The optical density (OD) was read at a wavelength of 450 nm. Positive cells with the CCK-8 solution were deemed viable. The half survival concentration of palbociclib was determined. All measurements were repeated in triplicate.
Cell-Cycle Analysis
For assessment of the effect on cell cycle, cells were pretreated with palbociclib for 18 hrs, digested with 0.25% pancreatin into single-cell suspensions and detected with the cell cycle detection kit (Beyotime Biotechnology) following the manufacturer’s instructions. The cell cycle was executed by BD Accuri C6 and analyzed with the FlowJo software. All analyses were repeated in triplicate.
Apoptosis Assay
Apoptosis was assessed using the Annexin V-FITC Apoptosis Detection Kit (Beyotime Biotechnology). After administration of palbociclib and radiation, cells were collected and dyed with Annexin V-FITC and PI solution at room temperature for 15 mins in the dark, respectively, followed by flow cytometric analysis.
Clonogenic Survival Assay
Cells were seeded into 6-well plates and incubated for 16 hrs to adhere. They were treated with radiation doses of 2, 4, 6, and 8 Gy and with palbociclib at the time of irradiation, 18 hrs before irradiation, or 6 hrs after irradiation. Approximately after 2-weeks incubation, colonies of >50 cells were counted under a light microscope following stained with 0.5% crystal violet. Plating efficiencies (PE) and surviving fraction (SF) were calculated as following: PE (%) = number of colonies/original cell-seeding density×100%, SF = number of colonies/ (number of cells seeded × PE). The multi-target click model was used to describe the SF, SF = 1- (1-e-D/D0) N. The radiobiological parameters: D, radiation dose; e, the bottom of the natural logarithm; D0, the mean death dose; N, extrapolate number; Dq, quasi-threshold dose, and SF2, the SF following exposure to 2 Gy radiation were determined analyzing the survival curve. The sensitization enhancing ratio (SER) was calculated as a ratio of D0.
ROS Measurement
Mitochondrial ROS was analyzed by incubating cells with MitoSOX Red mitochondrial superoxide indicator (ThermoFisher Scientific, Waltham, MA) for 10 mins, kept on ice in the dark and immediately detected by flow cytometric analysis. ROS levels were quantified as mean fluorescence intensity (MFI).
Immunofluorescence Assay
Cells were seeded on coverslips in a 6-well plate, then treated with palbociclib and exposed to a radiation dose of 6 Gy. They were fixed with 4% paraformaldehyde for 20 mins at 1 and 24 hrs post irradiation, permeabilized with 0.25% Triton X-100 for 15 mins, and blocked in 5% FBS for 40 mins at room temperature. Cells were immunostained with mouse-anti-γ-histone-H2AX antibody (1:300) and rabbit-anti-53BP1 antibody (1:300) overnight at 4°C, followed by incubation with goat-anti-mouse/Alexa568 antibody (1:200) and rabbit-anti-rabbit/Alexa488 antibody (1:200) for 1 hr at room temperature. The coverslips were stained with 4′,6-diamidino-2- phenylindole (1:10,000) to visualize nuclei. Images were captured with an Olympus fluorescence microscope (Olympus Optical Co., Tokyo, Honshu, Japan).
Western Blotting
Western blotting analysis was performed using the whole cell lysates prepared in RIPA buffer (150 mM NaCl, 10 mM Tris, pH 7.3, 0.1% sodium dodecyl sulfate (SDS), 1%Triton X-100, 1% deoxycholate and 5µM ethylenediaminetetraacetic acid) containing protease inhibitors. Lysates were then centrifuged at 12,000 r/min, 4°C for 5 mins. The protein concentration was measured with a BCA Protein Assay Kit (Beyotime Biotechnology) and 20µg of protein per sample was separated with 10% SDS-PAGE, electro-transferred to a nitrocellulose membrane, blocked with 5% bovine serum albumin (BSA, Sigma-Aldrich, USA) in TBST buffer, and incubated with primary antibodies at 4°C overnight. Then, the blots were incubated with a horseradish peroxidase-conjugated secondary antibody for 1 hr at room temperature, visualized with a FlourChemE system (Protein Simple) following the manufacturer’s instructions.
Statistical Analysis
Student’s t test and one-way analysis of variance were used. P < 0.05 was considered statistically significant.
Results
RB Pathway Status and Cell-Cycle Change in CNE-1 and CNE-2 Cells Response to Palbociclib and Radiation
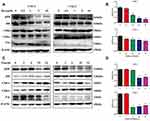
In order to analyze the capability of palbociclib intervening RB pathway in CNE-1 and CNE-2 cells, Western blot was applied to evaluate the level of protein expression of key pathway components. Protein such as pRB, RB, CDK4, CDK6 was detectable and variable in both CNE-1 and CNE-2 cell lines (Figure 1A and C). However, p16 expression was extremely low in CNE-2 in comparison with p16-positive CNE-1. This study also displayed that palbociclib attenuated RB phosphorylation in a concentration-dependent fashion in both CNE-1 and CNE-2 after being incubated with various concentration of palbociclib for 18 hrs (Figure 1A and B), but palbociclib did not prominently affect the expression of total RB protein in CNE-2 as well as RB-modifying enzymes CDK4 and CDK6 in both CNE-1 and CNE-2. This study also suggested that disruption of RB phosphorylation with palbociclib achieved the maximum in CNE-1 (18-hrs exposure) and in CNE-2 (6-hrs exposure), whereas RB phosphorylation gradually increased beyond 18 hrs (Figure 1C and D).
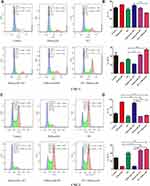
Meanwhile, to better understand the antitumor effect of palbociclib and RT, we compared the cell-cycle distribution effects of various regimens. For CNE-1 cells (Figure 2A and B), synchronous radiation and radiation followed by palbociclib significantly amplified the relatively radiosensitive G2/M cell proportion compared with RT-only, palbociclib-only, and palbociclib followed by RT groups (21.45% and 27.3% versus 16.2%, 12.75% and 11.15%). For CNE-2 cells (Figure 2C and D), concurrent radiation and radiation followed by palbociclib analogously augmented G2/M cell proportion and remarkably decreased the percentage of radioresistant G1-phase relative to palbociclib-only and palbociclib followed by RT groups (G2/M: 47.65% and 50.5% versus 7.18% and 7.62%, G1: 16.55% and 19.4 versus 54.2% and 50.85%). Compared with monotherapy effects, concurrent regimen and palbociclib following RT increased proportion of G2/M cells and reduced radioresistant G1 cells. Thereby, these two combination regimens obtained a high proportion of apoptotic cells attributed to more outstanding radiosensitivity.
Apoptosis Variation in CNE-1 and CNE-2 Cells Response to Palbociclib and Radiation
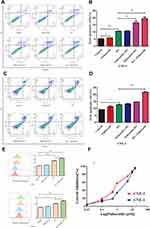
Next, we surveyed the effect of palbociclib plus radiation on apoptosis in CNE-1 and CNE-2 Cells. The cells were treated with palbociclib either before, concurrent, or after radiation for 48 hrs, and apoptosis was analyzed with flow cytometry using Annexin V-FITC/PI double staining. For CNE-1 (Figure 3A and B), both palbociclib and radiation noticeably mediated more apoptotic cells relative to control group (5.97% and 10.685% versus 5.335%). Among combination treatments, cells receiving palbociclib concurrently and after radiation emerged remarkably higher proportion of apoptotic cells in relation to RT-only group (16.6% and 19.6% versus 10.685%). Instead, RT following palbociclib did not significantly increase the proportion of apoptotic cells in contrast with the RT-only group (10.705% versus 10.685%). For CNE-2 (Figure 3C and D), the proportion of apoptotic cells was significantly increased with RT-only treatment compared with the control group (12.635% versus 9.375%) while that was not significantly changed with palbociclib-only treatment (11.42% versus 9.375%). Contrastly, palbociclib after RT markedly prompted apoptosis relative to the RT-only group (21.655% versus 12.635%).
To explore the mechanism of palbociclib increased apoptosis in irradiated cells, we next evaluated mitochondrial ROS level which was noted for apoptosis mediator when cells were exposed to radiation and chemotherapy. Our data (Figure 3E) revealed that radiation evidently augmented mitochondrial ROS level while palbociclib failed in both CNE-1 and CNE-2. Furthermore, there was an evident elevation in CNE-1 and CNE-2 treated with palbociclib following radiation compared with RT-only treatment.
Consequently, the results indicated that apoptosis induced by palbociclib in irradiated cells was partly attributed to elevation of ROS.
Growth Inhibition in CNE-1 and CNE-2 Cells Response to Palbociclib and Radiation
To evaluate the inhibitory effect of palbociclib on cell viability, CNE-1 and CNE-2 cells were added with various concentrations of palbociclib for 72 hrs, and the cell proliferation ability was measured by CCK8 assay. Palbociclib noticeably attenuated the cell viability in both CNE-1 and CNE-2 cells (Figure 3F), with IC50 values at 2.05 μM and 0.86 μM, respectively. Next, we investigated the growth effect and radiosensitivity of the treated CNE-1 and CNE-2 cells with palbociclib plus RT through colony formation assays. All combined regimens more potently restrained the number of surviving clonogenic CNE-1 and CNE-2 cells than that of RT-only group (Figure 4). D0, Dq, N, SF2 were also generally lower in all combined regimens than that in the RT-only group (Table 1), which represented higher sensitivity to RT of irradiated cells. Furthermore, the maximal reduction of SF values was observed in the palbociclib following the radiation group. Instead, the minimal decrease occurred in palbociclib before the radiation group, and concurrent palbociclib and radiation group were intermediate. N was increased in the palbociclib following radiation group compared with RT-only and other combined-treatment group suggesting that the capacity of the cells for repair was elevated (Table 1). Therefore, exposure of cells to palbociclib before radiation demonstrated the least SER (CNE-1=1.05, CNE-2=1.02, respectively), whereas palbociclib following radiation showed the favorite SER (CNE-1=1.475, CNE-2=1.20).
 |
Table 1 The Radiobiological Parameters of CNE-1 and CNE-2 Cells Exposed to Radiation with or Without Palbociclib |
Palbociclib Dampened Radiation-Induced DNA Repair in CNE-1 and CNE-2 Cells
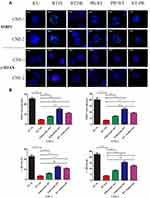
To elaborate the effect of palbociclib on DNA double-strand break (DSB) repair in CNE-1 and CNE-2 cells undergoing radiation, fluorescence immunocytochemistry of phosphorylated γ-H2AX and 53BP1 (DSB marker) were performed to demonstrate and quantify DNA damage in CNE-1 and CNE-2 cell lines which were prepared with palbociclib and radiation. Our data revealed that 6 Gy irradiation markedly elevated γ-H2AX and 53BP1 immunocytochemical positivity (Mean values for γ-H2AX and 53BP1 foci/nucleus: CNE-1: 22.94, CNE-2: 22.4 for γ-H2AX; CNE-1:25.48, CNE-2: 20.26 for 53BP1) in one hour after radiation (Figure 5A and B). Evident declines of γ-H2AX and 53BP1 foci occurred 24 hrs after radiation (CNE-1:3.46 and CNE-2:3.94 for γ-H2AX; CNE-1:4.58 and CNE-2:3.28 for 53BP1). Palbociclib plus radiation significantly enhanced the levels of γ-H2AX and 53BP1 relative to cells exposed to radiation alone at 24 hrs. The higher levels were measured in concurrent palbociclib and RT group and palbociclib following the RT group in both CNE-1 and CNE-2 cells (Figure 5B). (Mean values for γ-H2AX and 53BP1 foci/nucleus: CNE-1:PA->RT=6.08, RT+PA=12.62, RT->PA=10.82 for γ-H2AX; PA->RT=7.96, RT+PA=15.12, RT->PA=11.02 for 53BP1; CNE2: PA->RT=6.6, RT+PA=12.52, RT->PA=9.22 for γ-H2AX, PA->RT=8.46, RT+PA=15.54, RT-PA=12.48 for 53BP1).
Discussion
The p16-cyclinD1-CDK (4/6)-Rb pathway is of great importance in mediating cell cycle G1/S transition. When RB is phosphorylated, cyclin-dependent kinase Cdk4 and Cdk2 complexes would release E2F and allow the expression of S-phase-associated proteins.8 According to whole exome sequencing (WES) studies, numerous solid tumors including NPC possess CCND1 amplification and overexpression while CDKN2A inactivation,9 which heightens activity of CDK4/6 and loss of function of Cdk inhibitors like p16, p27, p53, and ultimately promotes the phosphorylation and activation of RB protein.10 Heretofore, it was documented that NPC-PDXs (patient-derived xenograft) in mouse model and clinical metastatic NPC tumors had CCND1 amplification and cyclin-dependent kinase inhibitor CDKN2A deletion,11 which implies our study targeting activated CDK4/6 in NPC cells is feasible.
Positive RB protein expression is imperative for palbociclib to exert anti-tumor activity. This is also observed in our research that RB-proficient CNE-1 and CNE-2 are respondent to palbociclib (Figure 1). Previous studies in vitro suggested that cells lacking p16 expression and with nonamplified CDK4 are more sensitive to palbociclib in glioblastoma.11 In our study, proliferation of p16-deficient CNE-2 cell line was restrained at lower concentration of palbociclib compared with p16-proficient CNE-1. Thereby, we assumed that p16 might serve as a potential determinant of tumor response to palbociclib in NPC. However, our study showed palbociclib response was not associated with the expression of additional factors including CDK4, CDK6, whether they may contribute to response for palbociclib remains obscure.
Cell-cycle alteration is a crucial affecting factor of radiation sensitivity. Palbociclib is an orally bioavailable, potently reversible inhibitor of CDK4/6. It is reported to arrest cell cycle at G1-phase by blocking RB phosphorylation, ultimately restrain cell proliferation.12 In our study, it was exhibited that the cell cycle was arrested at G1-phase in CNE-1 and CNE-2 cells treated with only palbociclib and palbociclib followed by irradiation. It is deemed that G1/S checkpoint is deficient in a large proportion of cancer cells and G2/M checkpoint arrest tends to be a critical determinant for the radiation sensitivity in cancer cells.13 Our data demonstrated that the proportion of G2/M phase cells evidently accumulated in palbociclib during irradiation group and after irradiation group compared with the palbociclib followed by the irradiation group, which might partially interpret desirable antitumor effect of palbociclib in the two superior groups.
It has been revealed that radiation induces DNA damage, triggering interrelated responses encompassing cell-cycle arrest and cell death (apoptosis or senescence) directly or following DNA replication during the S phase in cancer cells.14 However, when cells are cell-cycle arrested, several DNA repair pathways would be activated to cure DNA breaks, which ultimately lead to lower radiation sensitivity. To augment intrinsic tumor radiosensitivity, it is rational to combine CDK inhibitors that contribute to cellular stress response to ionizing radiation (IR).15 Our study revealed that palbociclib significantly extended the period of unrepaired DNA breaks caused by IR in three regimens of palbociclib and RT. Moreover, inhibition of IR-induced double-strand break repair by palbociclib is more favorite in concurrent palbociclib and RT group as well as palbociclib following RT group than palbociclib followed by RT group.
Apoptosis is a primary type of cell death when tumor cells are exposed to radiation. The ratio of apoptosis rest with cell type and radiation dose. It is considered that RB-proficient cells exposed to the CDK4/6 inhibitor exhibited apoptotic response while CDK4/6 inhibitor has little effect on apoptosis in RB-deficient tumors cells.16 It is reported that exposure to radiation elevated the level of ROS and generated ceramide which eventually induced apoptosis.17 In the present study, palbociclib increased the apoptotic rate of irradiated CNE-1 and CNE-2 cells both with RB expression relative to RT only. Furthermore, administration of palbociclib following RT generated more apoptotic cells than RT following palbociclib. Our data attributed palbociclib promoting apoptosis in irradiated cells to ROS escalation. In our study, combination of palbociclib following RT significantly augmented ROS level compared with radiation alone. Hence, it is indicated that palbociclib may be a promising adjuvant agent for NPC radiotherapy through mediating cell apoptosis to enhance radiation sensitivity.
Conventionally, radiotherapy and chemotherapy are primary approaches for NPC, but distant metastasis and local recurrence of NPC remain an intractable issue.18,19
Preclinical studies manifested that CDK4/6 inhibitor could enhance radiosensitivity in NSCLC20 and glioblastoma.21 In agreement with these findings, we first investigated whether CDK4/6 inhibitor palbociclib might be beneficial to irradiated NPC cells through designing various modalities of palbociclib and radiation. Ultimately, our research demonstrated that combination of palbociclib and RT, especial for RT followed by palbociclib and concurrent palbociclib and RT regimens, heightened antitumor activity in NPC cells.
Acknowledgments
This work was supported by the Natural Science Foundation of Fujian Province (2015J01457 and 2019J01457). We would like to extend my sincere gratitude to our senior, Chen Lin, who Gifted CNE-1 and CNE-2 cells.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chua MLK, Wee JTS, Hui EP, Chan ATC. Nasopharyngeal carcinoma. Lancet (London, England). 2016;387(10022):1012–1024. doi:10.1016/S0140-6736(15)00055-0
2. He H, Lin K, Su Y, et al. Overexpression of beta-catenin decreases the radiosensitivity of human nasopharyngeal carcinoma CNE-2 cells. Cell Physiol Biochem. 2018;50(5):1929–1944. doi:10.1159/000494873
3. Klein ME, Kovatcheva M, Davis LE, Tap WD, Koff A. CDK4/6 inhibitors: the mechanism of action may not be as simple as once thought. Cancer Cell. 2018;34(1):9–20. doi:10.1016/j.ccell.2018.03.023
4. Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375(20):1925–1936. doi:10.1056/NEJMoa1607303
5. O’Leary B, Finn RS, Turner NC. Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol. 2016;13(7):417–430. doi:10.1038/nrclinonc.2016.26
6. Hashizume R, Zhang A, Mueller S, et al. Inhibition of DNA damage repair by the CDK4/6 inhibitor palbociclib delays irradiated intracranial atypical teratoid rhabdoid tumor and glioblastoma xenograft regrowth. Neuro-Oncology. 2016;18(11):1519–1528. doi:10.1093/neuonc/now106
7. Tao Z, Le Blanc JM, Wang C, et al. Coadministration of trametinib and palbociclib radiosensitizes KRAS-mutant non-small cell lung cancers in vitro and in vivo. Clin Cancer Res. 2016;22(1):122–133. doi:10.1158/1078-0432.CCR-15-0589
8. Wong CH, Ma BBY, Hui CWC, Lo KW, Hui EP, Chan ATC. Preclinical evaluation of ribociclib and its synergistic effect in combination with alpelisib in non-keratinizing nasopharyngeal carcinoma. Sci Rep. 2018;8(1):8010. doi:10.1038/s41598-018-26201-1
9. Hsu CL, Lui KW, Chi LM, et al. Integrated genomic analyses in PDX model reveal a cyclin-dependent kinase inhibitor Palbociclib as a novel candidate drug for nasopharyngeal carcinoma. J Exp Clin Cancer Res. 2018;37(1):233. doi:10.1186/s13046-018-0873-5
10. Chow YP, Tan LP, Chai SJ, et al. Exome sequencing identifies potentially druggable mutations in nasopharyngeal carcinoma. Sci Rep. 2017;7:42980. doi:10.1038/srep42980
11. Cen L, Carlson BL, Schroeder MA, et al. p16-Cdk4-Rb axis controls sensitivity to a cyclin-dependent kinase inhibitor PD0332991 in glioblastoma xenograft cells. Neuro-Oncology. 2012;14(7):870–881. doi:10.1093/neuonc/nos114
12. Sherr CJ, Beach D, Shapiro GI. Targeting CDK4 and CDK6: from discovery to therapy. Cancer Discov. 2016;6(4):353–367. doi:10.1158/2159-8290.CD-15-0894
13. Wilson GD. Radiation and the cell cycle, revisited. Cancer Metastasis Rev. 2004;23(3–4):209–225. doi:10.1023/B:CANC.0000031762.91306.b4
14. Helleday T, Petermann E, Lundin C, Hodgson B, Sharma RA. DNA repair pathways as targets for cancer therapy. Nat Rev Cancer. 2008;8(3):193–204. doi:10.1038/nrc2342
15. Ma BB, Bristow RG, Kim J, Siu LL. Combined-modality treatment of solid tumors using radiotherapy and molecular targeted agents. J Clin Oncol. 2003;21(14):2760–2776. doi:10.1200/JCO.2003.10.044
16. Thangavel C, Boopathi E, Liu Y, et al. Therapeutic challenge with a CDK 4/6 inhibitor induces an RB-dependent SMAC-mediated apoptotic response in non-small cell lung cancer. Clin Cancer Res. 2018;24(6):1402–1414. doi:10.1158/1078-0432.CCR-17-2074
17. Mortezaee K, Najafi M, Farhood B, et al. Modulation of apoptosis by melatonin for improving cancer treatment efficiency: an updated review. Life Sci. 2019;228:228–241. doi:10.1016/j.lfs.2019.05.009
18. Wee J, Tan EH, Tai BC, et al. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J Clin Oncol. 2005;23(27):6730–6738. doi:10.1200/JCO.2005.16.790
19. Elser C, Siu LL, Winquist E, et al. Phase II trial of sorafenib in patients with recurrent or metastatic squamous cell carcinoma of the head and neck or nasopharyngeal carcinoma. J Clin Oncol. 2007;25(24):3766–3773. doi:10.1200/JCO.2006.10.2871
20. Naz S, Sowers A, Choudhuri R, et al. Abemaciclib, a selective CDK4/6 inhibitor, enhances the radiosensitivity of non-small cell lung cancer in vitro and in vivo. Clin Cancer Res. 2018;24(16):3994–4005. doi:10.1158/1078-0432.CCR-17-3575
21. Barton KL, Misuraca K, Cordero F, et al. PD-0332991, a CDK4/6 inhibitor, significantly prolongs survival in a genetically engineered mouse model of brainstem glioma. PLoS One. 2013;8(10):e77639. doi:10.1371/journal.pone.0077639
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.