Back to Journals » Cancer Management and Research » Volume 12
CD56 Expression Is Associated with Biological Behavior of Pancreatic Neuroendocrine Neoplasms
Authors Chen X , Guo C , Cui W , Sun K, Wang Z, Chen X
Received 16 February 2020
Accepted for publication 20 May 2020
Published 17 June 2020 Volume 2020:12 Pages 4625—4631
DOI https://doi.org/10.2147/CMAR.S250071
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Xin Chen,1 Chuangen Guo,2 Wenjing Cui,1 Ke Sun,3 Zhongqiu Wang,1 Xiao Chen1
1Department of Radiology, The Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing 2100029, People’s Republic of China; 2Department of Radiology, The First Affiliated Hospital, College of Medicine Zhejiang University, Hangzhou 310003, People’s Republic of China; 3Department of Pathology, The First Affiliated Hospital, College of Medicine Zhejiang University, Hangzhou 310003, People’s Republic of China
Correspondence: Zhongqiu Wang; Xiao Chen Tel +086-025-86619843
; +086-025-86617141
Email [email protected]; [email protected]
Purpose: CD56 is a neural cell adhesion molecule that plays a role in the cohesiveness of neuroendocrine cells. The aim of this study was to explore the biological values of CD56 expression in pancreatic neuroendocrine neoplasms (PNENs) and its role in predicting PNENs grades.
Patients and Methods: A total of 138 patients with histological-proven PNENs was included (66 G1, 46 G2 and 26 G3). The clinicopathological characteristics, including mitosis count, ki67 index, chromogranin A (CgA), synaptophysin (Syn) and CD56 expression, were evaluated. We assessed the diagnostic performance of markers in predicting PNEN G3 and the association between CD56 expression and risk of G3 or organs invasion.
Results: Lack of CD56 immunoreaction (CD56-) was more common in PNEN G3 than G1/G2 (31% vs 0– 2%, p < 0.01). The sizes of CD56- tumors were larger than CD56 positive tumors in PNEN G3 (p < 0.01). The odds ratio (OR) of CD56- expression was 13.6 [95% confidence interval (CI): 2.1– 88.1] in predicting PNEN G3. The OR of CD56- expression was 6.5 (95% CI: 1.1– 38.6) and 31.9 (95% CI: 1.09– 938.3) in predicting organs invasion and neuroendocrine carcinoma in PNEN G3, respectively. Tumor size (area under the curve [AUC] = 0.77 and size+CD56- expression [AUC = 0.84]) had acceptable performance in predicating PNEN G3.
Conclusion: Lack of CD56 immunoreaction may be a predictor and biological behavior marker for PNEN G3.
Keywords: pancreatic neuroendocrine neoplasm, grade, pancreatic neuroendocrine carcinoma, CD56
Introduction
Pancreatic neuroendocrine neoplasms (PNENs) are rare tumors, which only account for 1–2% of all pancreatic neoplasms.1 The incidence of PNENs has been increased during the past decades. The incidence rose from 1.1 to 5.2 per 100,000 inhabitants per year over the years 1973–2004.2 In 2017, PNENs were classified into three groups, pancreatic neuroendocrine tumor (PNET) grade 1 (G1), NET G2 and PNEN G3 by the World Health Organization (WHO) based on the mitotic count and Ki-67 proliferation index.3
The treatment strategies are substantially different between PNET G1/G2 and PNEN G3. For PNETs G1/G2, targeted therapy and somatostatin analogs (octreotide) are also valuable besides surgical resection.4 For PNEN G3, cytotoxic chemotherapy is usually adopted besides surgical resection. Therefore, it is critical to identify the tumor grades, especially for the differentiation between PNEN G3 and PNET G1/G2. Mitotic count and Ki-67 proliferation index are recommended to use in WHO classification. Several studies also showed that imaging approached, such as computed tomography (CT) and magnetic resonance imaging (MRI) were valuable in differentiation between PNEN G3 and PNET G1/G2.5–7 One study showed that tumor size was also a good indicator.6 Are there any other valuable biomarkers for identifying PNEN G3 from PNET G1/G2?
Several neuroendocrine markers have been used for pathological diagnosis of PNETs, including chromogranin A (CgA), neuron-specific enolase (NSE), and synaptophysin (Syn). CD56 is a neural cell adhesion molecule (NCAM) that plays a role in the cohesiveness of cells in nervous systems and neuroendocrine cells.8 Case studies show that CD56 expresses in PNETs. Kanehira and Khoury9 also reported that CD56 expressed in 89% PNETs (n = 18, PNEN G3 = 5). Konukiewitz et al10 showed that CD56 was expressed in 9 of 12 poorly differentiated pancreatic neoplasm with a Ki67-index above 20%. However, only small number of PNETs was included in those studies. The role of CD56 expression in PNETs has not been completely clarified. In this study, we aimed to show the CD56 expression in PNETs. Moreover, we evaluated the role of CD56 expression in predicting risk of PNEN G3 and organs invasion.
Patients and Methods
Study Patients
This study was approved by institutional review board of the First Affiliated Hospital, College of Medicine Zhejiang University. As a retrospective study, formal consent was waived. From February 2012 to May 2018, 145 patients with histological-proven PNETs were included in this study. Three patients were excluded because they only underwent biopsy. Four patients were excluded due to the absence of CD56 staining. Finally, 138 patients were included for further analysis. They were divided into NET G1, NET G2 and PNEN G3 according to the 2017 WHO classification for neuroendocrine tumors. We also recorded the demographic information, such as age and gender. Information in tumor location and sizes were also collected. Every precaution was taken to respect the confidentiality of patient data and to comply with the Declaration of Helsinki.
Histological Analysis
The tumor tissues were fixed in 10% formalin for 24 hours. Then they were embedded in paraffin blocks and sectioned for hematoxylin-eosin (H&E) staining (4-μm). Immunochemical staining was performed to detect the Ki67, CgA, Syn and CD56 expression. Briefly, each slide was heat-treated using retrieval solution, blocked the endogenous peroxidase activity, and incubated with the primary antibodies for 30–60 min and second antibodies for 30 min. Then the streptavidin-biotin complex method with 3,3′-diaminobenzidine (DAB) was used as chromogen. Pathological specimens were observed by light microscopy. The percentage of positive cells was calculated. CD56 positive usually showed faint intensity, and staining for other neuroendocrine markers, including chromogranin A, was diffusely positive.11 The percentage of positive cells between 1% and 5% was considered as negative expression and 5–25% as focal positive.12,13 In our study, percentage greater >10% was considered as positive (Figure 1).
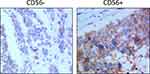 |
Figure 1 The illustration of the negative and positive CD56 expression. |
PNETs Grade
Tumor grades of PNETs were evaluated by manually counting the number of mitoses per 10 high-power fields (HPF) and detecting the Ki-67 index (percentage of positive cells in areas of highest nuclear labeling). PNETs were divided into NET G1, NET G2, and PNEC according to the 2017 WHO classification for neuroendocrine tumors. NET G1: mitotic count < 2/10 HPF and/or Ki-67≤2%; NET G2: mitotic count 2–20/10 HPF and/or Ki-67 index is 3–20%; PNEN G3: mitotic count > 20/10 HPF and/or Ki-67 index > 20%. Tumor invasions to adjacent organs, including stomach, duodenum, gallbladder and bile duct, and lymph node metastasis were also recorded.
We further divided G3 PNEN into well-differentiated G3 PNETs and pancreatic neuroendocrine carcinoma (PNEC) based on recent reports.12,13 Briefly, the tumor that had one of the following morphological features was considered as PNEC: solid structure; extensive necrosis; severe cytological atypia; large nuclei with salt and pepper chromatin; inconspicuous nucleoli in most cells (small cell type); conspicuous nucleoli in most cells (large cell type).
Statistical Analysis
Data analysis was performed by using SPSS 16.0 (SPSS Inc, Chicago, IL) and MedCalc software (Mariakerke, Belgium). Quantitative data were shown as means ± SD and were analyzed one-way analysis of variance (ANOVA) or Mann–Whitney U-test, such as age and tumor sizes. Categorical data were shown as the number of cases (percentage) and were analyzed by using Chi-square test. The receiver operating characteristic (ROC) curve was used to assess the diagnostic performance in predicting PNEC. Logistic regression analysis was used to show the association between CD56 expression and risk of PNEC, and organs invasion or ki67 index > 50% in PNEN G3. Age, gender or tumor size were considered as confounders. P < 0.05 was considered a significant statistical difference.
Results
The clinical characteristics of patients are shown in Table 1. The mean age of the patients was 54.2 years old. There were 69 men and 69 women. 71 lesions were located in head-neck and 67 were in body-tail. The number of NET G1, G2 and PNEN G3 were 66, 46 and 26, respectively. 122 tumors were nonfunctional PNETs. The average size was 3.4 cm.
 |
Table 1 Clinical Characteristics of Patients |
We also showed the gender distribution, age and tumors sizes (Table 2). Significant differences were observed in gender distribution and tumor sizes. 70% of patients with PNEN G3 were male, and 36% of patients with G1/G2 were male (p < 0.05). The sizes of PNEN G3 were larger than G1 or G2 (p < 0.05).
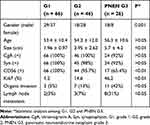 |
Table 2 Immuohistochemical Evaluation in Pancreatic Neuroendocrine Neoplasms (PNENs) |
Subsequently, we showed the CgA, Syn, and CD56 expression in PNETs (Table 2). No significant differences were observed in CgA and Syn expression among PNET G1, G2 and PNEN G3. For G1 and G2 PNETs, CgA and Syn were expressed in 98–100% tumors. For PNEN G3, those positive expressions were observed in 92% tumors. Significant difference was found in CD56 expression. Lack of immunoreaction to CD56 was more common in PNEN G3 than G1 or G2 (34.6% vs 0–2%) (p < 0.05). More organs invasions and lymph node metastasis were observed in PNEN G3 than G1/G2 PNETs (p < 0.05).
We also analyzed the sizes of CD56+ and CD56- tumors (Figure 2). For all PNETs, the sizes of CD56- tumors were larger than those of CD56+ tumors (7.8 vs 2.9 cm). Similar results were observed in sizes of CD56+ and CD56- tumors for G3 PNEN (9.3 vs 3.8 cm). CD56- PNEN G3 showed higher ki67 index than CD56+ PNEN G3, but no significant differences were observed (p = 0.059). PNEN G3 with high ki67 index (>50%) was more common in CD56- tumors than CD56+ tumors (6/9 vs 4/13, p = 0.046).
 |
Figure 2 The sizes of CD56+ and CD56- tumors in all pancreatic neuroendocrine neoplasms (PNENs) and PNEN grade 3 (PNEN G3). |
We also showed the CgA, Syn, and CD56 expression between well-differentiated G3 and PNEC (Table 3). Lack of CD56 expression was more common in PNEC than well-differentiated G3 (p = 0.05). No significant differences were observed in CgA and Syn expression.
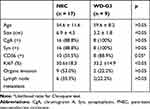 |
Table 3 Immuohistochemical Evaluation in Well-Differentiated (WD) Grade 3 Pancreatic Neuroendocrine Neoplasms (WD-G3 PNENs) and PNEC |
The association between tumor sizes, lack of immunoreaction to CD56 and risk of PNEN G3 was assessed by using logistic regression model. The odds ratio (OR) of size >4.0 cm was 5.7 [95% confidence interval (CI): 2.1–15.4) compared with those size ≤4.0 cm in predicting PNEN G3 (Table 4) after adjusted with the confounders. The OR of lack of immunoreaction to CD56 was 19.1 (95% CI: 4.7–77.5) compared with those with CD56+ tumor in predicting PNEN G3 (Table 4). After adjusted with the confounders, the OR was 13.6 (95% CI: 2.1–88.1). We also showed the risk of organs invasion between CD56- and CD56+ tumors in PNEN G3. The OR of CD56- tumors was 9.5 (95% CI: 1.1–82.8) compared with those CD56+ tumor (Table 4). After adjusted with the confounders, the OR was 6.5 (95% CI: 1.1–38.6).
 |
Table 4 The Association Between Sizes, CD56 Negative Expression and PNENs Grade, Organs Invasion, Ki67 Index and NEC in PNEN G3 |
In addition, we showed the risk of ki67 index >50% between CD56- and CD56+ tumors in PNEN G3. The OR of CD56- tumors was 14.7 (95% CI: 1.6–138.9) compared with those CD56+ tumor (Table 4) after adjusted with the confounders. The risk of PNEC was higher in those tumors had lack of CD56 expression than that had CD56 expression (OR=31.9, 95% CI: 1.09–938.3).
The diagnostic performances of tumor size alone and size + CD56 in predicting PNEN G3 from PNETs are shown in Figure 3. The area under curve was 0.77 (95% CI: 0.71–0.85) for tumor size (67.1% sensitivity and 80.8% specificity) and 0.84 (95% CI: 0.74–0.89) for size+CD56 expression (70.4% sensitivity and 85.1% specificity).
Discussion
CD56 is a type of neural cell adhesion molecule. CD56 expresses in PNETs.9,10 However, the biological role of CD56 in PNETs has not been totally clarified besides a neuroendocrine marker. In the current study, we showed the status of CD56 expression in 138 PNETs. Our data indicate that PNET G1/G2 was positive for CD56. Lack of CD56 expression is more common in PNEN G3. In addition, lack of CD56 expression is associated with tumor sizes and organs invasion in PNEN G3.
The treatment strategies and prognosis of PNETs are associated with tumor grades.4,14 PNEN G3 showed poorer prognosis than PNET G1/G2. Mitotic count and ki67 index are used for PNET grading. Several studies reported that imaging features were associated with the PNET grades,6,7,15 such as contrast-enhanced ratio and apparent diffusion coefficients (ADCs). Kim et al6 also showed that tumor size was an acceptable indicator for PNEN G3. Our data is consistent with this previous study.
Neuroendocrine markers, such as CgA, Syn and CD56 have been widely used in PNET pathological diagnosis. In addition, several studies have shown that serum CgA level is related to the prognosis16–19 and disease progression.20 However, the association between neuroendocrine markers and PNET grades has not been totally understood. CD56 is the cluster of differentiation assignment for the neural cell adhesion antigen.21 It is widely expressed in cerebellum, cerebral cortex and spinal cord.21 The immunochemical staining for CD56 has been used in the diagnosis of neuroendocrine tumors.8 CD56 expression is not all positive in PNETs.9 Fuksiewicz et al17 demonstrated that 25% of PNEN G3 was negative for CD56. Similar result was reported in renal neuroendocrine carcinoma.22 In our study, we found that CD56 was positive in 98–100% PNET G1/G2, which was consistent with the previous study.23 However, 31% of PNEC showed lack of CD56 expression. CD56- tumor was more common in PNEN G3 than PNET G1/G2. For those PNETs with lack of CD56 expression, the risk of PNEN G3 is higher than that of PNET G1/G2. Lack of CD56 expression may be an indicator of PNEN G3. A recent study showed that the prognosis of PNETs with non-triple positive staining was worse than those with triple positive staining (CgA, Syn and CD56)24 which indicated that negative expression may be a predictor of bad outcomes. In addition, non-triple-positive staining was associated with large tumors size, high Ki-67 index and high mitotic rate,24 which was similar to our data. However, the specific role of CD56 was not observed. Interestingly, lack of CD56 expression was also reported in pancreatic carcinoma25 and MIA PaCa-2 cells.26 A study indicated that there was a close relationship between PNEN G3 and pancreatic ductal adenocarcinomas (PDAC).27 Lack of CD56 expression in PNEN G3 may also support the previous data.
Li et al18 also indicated that CD56 was associated with overall survival rate of PNETs. CD56 can induce biological effects besides as a neuroendocrine differentiation marker. In the present study, we observed that PNEN G3 with lack of CD56 expression showed larger sizes, easier invasion to adjacent organs and higher ki67 index than that CD56+ PNEN G3. ki67 index >50% was more common in CD56- PNEN G3. CD56 plays an important role in the cell-cell or cell-matrix adhesion, detachment, and cell aggregation.28 For those tumors that cannot express CD56, the ability of cells adhesion may be weak and tumor cells are easy to detach from the mass. Therefore, tumor grows fast and adjacent invasion is more common in CD56- tumor than CD56+ one. In the 2017 WHO classification for neuroendocrine tumors, G3 PNEN was classified into well-differentiated PNET and PNEC. Our data showed that the risk of PNEC was higher in those tumors that had lack of CD56 expression. We speculated that CD56 expression may be a potential biomarker for the differentiation between well-differentiated PNET G3 and PNEC. Further studies are required.
There are several limitations in our study. First, the sample size for PNEC was relatively small because PNEN G3 is a rare pancreatic lesion. Multicenter study would be necessary to obtain more clear research results. Second, we did not obtain the survival data and response to chemotherapy in our cases. Therefore, the association between CD56 expression and prognosis or chemotherapy response was not clarified in our study.
In conclusion, our data show that lack of CD56 expression is more common in PNEC than PNET G1/G2. Lack of CD56 expression may be a potential indicator of PNEN G3. Furthermore, our data also indicate that CD56 expression is associated with tumors size, organs invasion and ki67 index in PNEN G3. Lack of CD56 expression may be also a potential marker of biological behavior for PNEN G3.
Acknowledgments
This study was supported by the Primary Research & Development Plan of Jiangsu Province (BE2017772) and Zhejiang Medical Science and Technology Project (2017KY331).
Disclosure
The authors declare no conflicts of interest.
References
1. Wang Y, Miller FH, Chen ZE, et al. Diffusion-weighted MR imaging of solid and cystic lesions of the pancreas. RadioGraphics. 2011;31(3):E47–E64. doi:10.1148/rg.313105174
2. Kuo JH, Lee JA, Chabot JA. Nonfunctional pancreatic neuroendocrine tumors. Surg Clin North Am. 2014;94(3):689–708. doi:10.1016/j.suc.2014.02.010
3. Singhi AD, Klimstra DS. Well-differentiated pancreatic neuroendocrine tumours (PanNETs) and poorly differentiated pancreatic neuroendocrine carcinomas (PanNECs): concepts, issues and a practical diagnostic approach to high-grade (G3) cases. Histopathology. 2018;72(1):168–177. doi:10.1111/his.13408
4. Rindi G, Wiedenmann B. Neuroendocrine neoplasms of the gut and pancreas: new insights. Nat Rev Endocrinol. 2012;8(1):54–64. doi:10.1038/nrendo.2011.120
5. Guo C, Chen X, Xiao W, Wang Q, Sun K, Wang Z. Pancreatic neuroendocrine neoplasms at magnetic resonance imaging: comparison between grade 3 and grade 1/2 tumors. Onco Targets Ther. 2017;10:1465–1474. doi:10.2147/OTT.S127803
6. Kim DW, Kim HJ, Kim KW, et al. Neuroendocrine neoplasms of the pancreas at dynamic enhanced CT: comparison between grade 3 neuroendocrine carcinoma and grade 1/2 neuroendocrine tumour. Eur Radiol. 2015;25(5):1375–1383. doi:10.1007/s00330-014-3532-z
7. Lotfalizadeh E, Ronot M, Wagner M, et al. Prediction of pancreatic neuroendocrine tumour grade with MR imaging features: added value of diffusion-weighted imaging. Eur Radiol. 2017;27(4):1748–1759. doi:10.1007/s00330-016-4539-4
8. Wick MR. Immunohistology of neuroendocrine and neuroectodermal tumors. Semin Diagn Pathol. 2000;17(3):194–203.
9. Kanehira K, Khoury T. Neuroendocrine markers expression in pancreatic serous cystadenoma. Appl Immunohistochem Mol Morphol. 2011;19(2):141–146. doi:10.1097/PAI.0b013e3181f5023d
10. Konukiewitz B, Schlitter AM, Jesinghaus M, et al. Somatostatin receptor expression related to TP53 and RB1 alterations in pancreatic and extrapancreatic neuroendocrine neoplasms with a Ki67-index above 20%. Mod Pathol. 2017;30(4):587–598. doi:10.1038/modpathol.2016.217
11. Notohara K, Hamazaki S, Tsukayama C, et al. Solid-pseudopapillary tumor of the pancreas: immunohistochemical localization of neuroendocrine markers and CD10. Am J Surg Pathol. 2000;24(10):1361–1371. doi:10.1097/00000478-200010000-00005
12. Rindi G, Klersy C, Albarello L, et al. Competitive testing of the WHO 2010 versus the WHO 2017 grading of pancreatic neuroendocrine neoplasms: data from a large international cohort study. Neuroendocrinology. 2018;107(4):375–386. doi:10.1159/000494355
13. Kim H, An S, Lee K, et al. Pancreatic high-grade neuroendocrine neoplasms in the Korean population: a multicenter study. Cancer Res Treat. 2020;52(1):263–276. doi:10.4143/crt.2019.192
14. Pape UF, Jann H, Muller-Nordhorn J, et al. Prognostic relevance of a novel TNM classification system for upper gastroenteropancreatic neuroendocrine tumors. Cancer. 2008;113(2):256–265. doi:10.1002/cncr.23549
15. Cappelli C, Boggi U, Mazzeo S, et al. Contrast enhancement pattern on multidetector CT predicts malignancy in pancreatic endocrine tumours. Eur Radiol. 2015;25(3):751–759. doi:10.1007/s00330-014-3485-2
16. Chou WC, Chen JS, Hung YS, et al. Plasma chromogranin A levels predict survival and tumor response in patients with advanced gastroenteropancreatic neuroendocrine tumors. Anticancer Res. 2014;34(10):5661–5669.
17. Fuksiewicz M, Kowalska M, Kolasinska-Cwikla A, et al. Prognostic value of chromogranin A in patients with GET/NEN in the pancreas and the small intestine. Endocr Connect. 2018;7(6):803–810. doi:10.1530/EC-18-0059
18. Li Y, Bi X, Zhao J, et al. CEA level, radical surgery, CD56 and CgA expression are prognostic factors for patients with locoregional gastrin-independent GNET. Medicine. 2016;95(18):e3567. doi:10.1097/MD.0000000000003567
19. Massironi S, Rossi RE, Casazza G, et al. Chromogranin A in diagnosing and monitoring patients with gastroenteropancreatic neuroendocrine neoplasms: a large series from a single institution. Neuroendocrinology. 2014;100(2–3):240–249. doi:10.1159/000369818
20. Rossi RE, Garcia-Hernandez J, Meyer T, et al. Chromogranin A as a predictor of radiological disease progression in neuroendocrine tumours. Ann Transl Med. 2015;3(9):118. doi:10.3978/j.issn.2305-5839.2015.04.23
21. Lanier LL, Testi R, Bindl J, Phillips JH. Identity of Leu-19 (CD56) leukocyte differentiation antigen and neural cell adhesion molecule. J Exp Med. 1989;169(6):2233–2238. doi:10.1084/jem.169.6.2233
22. Wang XH, Lu X, He B, et al. Clinicopathologic features of primary renal neuroendocrine carcinoma. Zhonghua Bing Li Xue Za Zhi. 2018;47(11):851–856. doi:10.3760/cma.j.issn.0529-5807.2018.11.007
23. Simtniece Z, Vanags A, Strumfa I, et al. Morphological and immunohistochemical profile of pancreatic neuroendocrine neoplasms. Pol J Pathol. 2015;66(2):176–194. doi:10.5114/pjp.2015.53015
24. Liu B, Kudo A, Kinowaki Y, et al. A simple and practical index predicting the prognoses of the patients with well-differentiated pancreatic neuroendocrine neoplasms. J Gastroenterol. 2019;54(9):819–828. doi:10.1007/s00535-019-01570-0
25. Naito Y, Kinoshita H, Okabe Y, et al. CD56 (NCAM) expression in pancreatic carcinoma and the surrounding pancreatic tissue. Kurume Med J. 2006;53(3–4):59–62. doi:10.2739/kurumemedj.53.59
26. Gradiz R, Silva HC, Carvalho L, Botelho MF, Mota-Pinto A. MIA PaCa-2 and PANC-1 - pancreas ductal adenocarcinoma cell lines with neuroendocrine differentiation and somatostatin receptors. Sci Rep. 2016;6:21648. doi:10.1038/srep21648
27. Konukiewitz B, Jesinghaus M, Steiger K, et al. Pancreatic neuroendocrine carcinomas reveal a closer relationship to ductal adenocarcinomas than to neuroendocrine tumors G3. Hum Pathol. 2018;77:70–79. doi:10.1016/j.humpath.2018.03.018
28. Zeromski J, Nyczak E, Dyszkiewicz W. Significance of cell adhesion molecules, CD56/NCAM in particular, in human tumor growth and spreading. Folia Histochem Cytobiol. 2001;39(Suppl 2):36–37.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

