Back to Journals » OncoTargets and Therapy » Volume 12
CD5-negative chronic lymphocytic leukemia/small lymphocytic lymphoma in a patient with gastrointestinal mantle cell lymphoma: an unusual case report
Authors Chen D , Zhan Y, Peng J, Yao F
Received 1 November 2018
Accepted for publication 12 March 2019
Published 17 April 2019 Volume 2019:12 Pages 2937—2941
DOI https://doi.org/10.2147/OTT.S193014
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Takuya Aoki
Dangui Chen,1 Yang Zhan,1 Jun Peng,2 Fusheng Yao1
1Department of Hematology, Anqing Municipal Hospital, Anqing Hospital Affiliated to Anhui Medical University, Anqing, People’s Republic of China; 2Department of Pathology, Anqing Municipal Hospital, Anqing Hospital Affiliated to Anhui Medical University, Anqing, People’s Republic of China
Abstract: Richter’s syndrome, the development of high-grade non-Hodgkin lymphoma in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), may be triggered by viral infections (eg, Epstein–Barr virus infection). Herein, we report an unusual case of CD5-negative CLL/SLL patient with gastrointestinal mantle cell lymphoma (MCL) and hepatitis B virus infection. CLL/SLL was diagnosed based on lymph node immunohistochemistry and bone marrow pathology. This patient was treated with seven cycles of multi-agent chemotherapy. During treatment, the hepatitis B viruses were activated. Then, after 20 months of antiviral treatment with entecavir, he developed abdominal discomfort and abdominal lymphadenopathy and was diagnosed with MCL based on intestinal biopsy. This work indicates that the hepatitis B virus in patients with CLL/SLL may accelerate the progress or transformation to MCL.
Keywords: Richter’s syndrome, chronic lymphocytic leukemia/small lymphocytic lymphoma, mantle cell lymphoma, hepatitis B virus infection
Introduction
Chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) is common leukemia in adults and accounts for approximately 30% and 7% of lymphoid and nodal lymphomas, respectively.1 Second malignancies are frequent complications in CLL/SLL patients, and this process is commonly referred to as Richter’s syndrome (RS). About 2–8%, 0.5%, and 0.1% of CLL/SLL patients progress to diffuse large B cell lymphoma, Hodgkin’s lymphoma, and multiple myeloma, respectively.2 Studies show that RS is commonly associated with Epstein–Barr virus (EBV),2–4 karyotypic changes,5 and gene mutations.6–8 CLL/SLL usually expresses CD5 antigen, but 7–20% of CLL/SLL patients are CD5 negative.9 Primary gastrointestinal mantle cell lymphoma (MCL) is a rare and progressive disorder that accounts for only 1–4% of primary gastrointestinal lymphoma.10 Here, we reported an unusual case that a 61-year-old patient previously diagnosed as CD5-negative CLL/SLL developed MCL after chemotherapy and antiviral treatment.
Case report
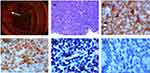
A 61-year-old man with fever and lymph node enlargement for one month was admitted to our hospital. He had a fever (>38.5 °C) for one month, but no night sweats or weight loss. Physical examination showed that superficial lymphadenopathy and splenomegaly. Laboratory examination results were white blood cells 5.78×10^9/L (45.2% lymphocytes), hemoglobin 120 g/L, and platelets 113×10^9/L. Hepatitis B virus (HBV) examination results were hepatitis B virus surface antigen (HBsAg, –), hepatitis B surface antibody (HBsAb, +), hepatitis B e antigen (HBeAg, –), hepatitis B e antibody (HBeAb, +), and hepatitis B core antibody (HBcAb, +). Hepatitis B virus-deoxyribonucleic acid (HBV-DNA) and EBV were negative. Bone marrow pathology indicated that CD20, PAX-5, CD23, SIg, and Bcl-2 were positive; SOX-11, CD3, CD5, MPO, CD34, CD10, Bcl-6, MUM-1, LEF-1 or CyclinD1 were negative, and Ki-67 staining revealed a proliferative index of 10% (Figure 1). Immunohistochemistry (IHC) of cervical lymph node showed that the lymphocytes were mature, small, and positive for CD20, PAX-5, CD21, CD23, and Bcl-2, but negative for CD3, CD5, CyclinD1, SOX-11, CD10 or Bcl-6, Ki-67 was 15% (Table 1). Flow cytometry showed that lymphocytes accounted for 68.94% nuclear cells (35.11% of B lymphocytes); CD19, CD20, and CD23 were positive, CD22 was weakly positive; CD10, CD5, FMC-7, κ, and λ were negative. Fluorescence in situ hybridization of bone marrow did not find abnormal Bcl-2 (18q21), Bcl-6(3q27), CEP8/MYC/IGH (11q13/14q32), and API2/MALT1 (11q22/18q21). IgVH, IgDH, and IgK were rearranged. Karyotype analysis showed 46, XY [20]. The above examinations supported the diagnosis of CLL/SLL (CD5 negative). He was subsequently treated with seven cycles of multi-agent chemotherapy, including cyclophosphamide, vincristine, and prednisone (COP * 1), and rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (RCHOP * 6). After that his superficial lymphadenopathy disappeared, but was found with HBsAg (+), HBsAb (–), HBeAg (–), HBeAb (+) and HBcAb (+), and HBV-DNA rose to 2.656×10^5 copy/mL, and EBV was still negative (Figure 2). Then, after 20 months of the antiviral treatment without chemotherapy, he was found HBV-DNA negative. After the treatment, the patient had diarrhea. Computed tomography scan showed that abdominal lymphadenopathy and thickening of the intestinal wall. Electrocolonoscopy found total colonic lesions. IHC of intestinal biopsy showed positive CD5, CyclinD1, CD20, SOX-11, and CD21, and negative cytokeratin (CK), LEF-1, CD23, Bcl-6, MUM1, CD10, and CD3, Ki-67 staining revealed a proliferative index of 45% (Figure 3), t(11;14) of intestinal biopsy was detected by fluorescence in situ hybridization. This patient was diagnosed as MCL based on these results. At present, the patient is receiving further treatment.
 | Table 1 Characteristics of the patient |
Discussion
It is important to distinguish CLL/SLL from other small B-cell lymphoma, such as MCL, which is an aggressive and incurable non-Hodgkin lymphoma (NHL). The complete remission rate of MCL treated by chemotherapy is low, and the median overall survival of MCL is 4–5 years.11 Our patient was diagnosed as gastrointestinal MCL after chemotherapy and antiviral treatment with entecavir. Although the presence of negative EBV during the treatment period was different from previous studies,2–4 our patient was found positive HBV-DNA after chemotherapy, and then he accepted 20 months of antiviral treatment. A recent study also shows that antiviral treatment can lead to a complete remission of hepatitis C virus (HCV)-associated low-grade NHL, suggesting a causative role of HCV in these tumors.12 Meta-analyses further show HBV-infected patients have two- to three- fold higher risk of developing B-NHL.13–15 HBV is a hepatotropic virus, but can also infect lymphocytes and the lymphoid system that has been shown as an important reservoir of HBV.16 Like the scenario of EBV-driven lymphoma, HBV also directly infects B-cells, leading to the genetic alterations that contribute to tumor development.17 One possible mechanism is that like in HBV-induced hepatocellular carcinomas,18 HBV DNA can integrate into the B-cell genome, directly activate oncogenes or repress tumor suppressors, leading to tumor development and progression.
Gene mutation is another important cause for RS.6–8 As reported, the HBV-associated gene expression signature is contributed by the enrichment of genes regulated by BCL6, FOXO1, and ZFP36L1, which contributed to HBV-related lymphomagenesis.17 In this study, we detected IgVH, IgDH, and IgK rearrangement in our patient. The underlying molecular mechanisms of RS are largely unknown, and no reliable markers are available that may predict which CLL/SLL patients are prone to RS. Thus, more clinical data need to be further analyzed.
Conclusion
Viral or other infectious complications mimicking RS in the context of CLL/SLL with HBV infection are an essential differential diagnosis to RS. Experiences from this patient suggest we also should pay attention to other viral infections, such as HBV, especially for EBV negative patients.
Ethical approval and consent
Ethical approval for the publication of this case was obtained from the ethical committee of Anqing Municipal Hospital. The patient provided written informed consent to publish the case report and accompanying images.
Acknowledgment
The authors thank the patient and his family members for their participation in this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wu J, Zhang Y, Sun L, Zhai Q. Cyclin D1 expression by histiocytes may mimic cyclin D1-positive proliferation centres of chronic lymphocytic leukaemia/small lymphocytic lymphoma. Pathol Res Pract. 2018;214(1):72–75. doi:10.1016/j.prp.2017.11.010
2. Chen YA, Wang RC, Yang Y, Chuang SS. Epstein-Barr virus-positive diffuse large B cell lymphoma arising from a chronic lymphocytic leukemia: overlapping features with classical Hodgkin lymphoma. Pathol Int. 2016;66(7):393–397. doi:10.1111/pin.12417
3. Jain P, Burger JA, Khoury JD. CLL progression after one cycle of FCR: richter’s transformation versus EBV-associated lympho-proliferation. Am J Hematol. 2017;92(10):1113–1114. doi:10.1002/ajh.24841
4. Foo WC, Huang Q, Sebastian S, Hutchinson CB, Burchette J, Wang E. Concurrent classical Hodgkin lymphoma and plasmablastic lymphoma in a patient with chronic lymphocytic leukemia/small lymphocytic lymphoma treated with fludarabine: a dimorphic presentation of iatrogenic immunodeficiency-associated lymphoproliferative disorder with evidence suggestive of multiclonal transformability of B cells by Epstein-Barr virus. Hum Pathol. 2010;41(12):1802–1808. doi:10.1016/j.humpath.2010.04.019
5. Strati P, Abruzzo LV, Wierda WG, O’Brien S, Ferrajoli A, Keating MJ. Second cancers and Richter transformation are the leading causes of death in patients with trisomy 12 chronic lymphocytic leukemia. Clin Lymphoma Myeloma Leuk. 2015;15(7):420–427. doi:10.1016/j.clml.2015.02.001
6. Fulop Z, Csernus B, Timar B, Szepesi A, Matolcsy A. Microsatellite instability and hMLH1 promoter hypermethylation in Richter’s transformation of chronic lymphocytic leukemia. Leukemia. 2003;17(2):411–415. doi:10.1038/sj.leu.2402792
7. Mao Z, Quintanilla-Martinez L, Raffeld M, et al. IgVH mutational status and clonality analysis of richterʼs transformation. Am J Surg Pathol. 2007;31(10):1605–1614. doi:10.1097/PAS.0b013e31804bdaf8
8. Rossi D, Berra E, Cerri M, et al. Aberrant somatic hypermutation in transformation of follicular lymphoma and chronic lymphocytic leukemia to diffuse large B-cell lymphoma. Haematologica. 2006;91(10):1405–1409.
9. Demir C, Kara E, Ekinci Ö, Ebinç S. Clinical and laboratory features of CD5-negative chronic lymphocytic leukemia. Medl Sci Monit Inter Medl J Exp Clin Res. 2017;23:2137–2142.
10. Ghimire P, Wu GY, Zhu L. Primary gastrointestinal lymphoma. World J Gastroenterol. 2011;17(6):384–389.
11. Vose JM. Mantle cell lymphoma: 2017 update on diagnosis, risk-stratification, and clinical management. Am J Hematol. 2017;92(8):806–813. doi:10.1002/ajh.24797
12. Maciocia N, O’Brien A, Ardeshna K. Remission of follicular lymphoma after treatment for hepatitis C virus infection. N Engl J Med. 2016;375(17):1699. doi:10.1056/NEJMc1513288
13. Wang C, Xia B, Ning Q, et al. High prevalence of hepatitis B virus infection in patients with aggressive B cell non-Hodgkin’s lymphoma in China. Ann Hematol. 2018;97(3):453–457. doi:10.1007/s00277-017-3188-2
14. Engels EA, Cho ERJee SH. Hepatitis B virus infection and risk of non-Hodgkin lymphoma in South Korea: a cohort study. Lancet Oncology. 2010;11(9):827–834. doi:10.1016/S1470-2045(10)70167-4
15. Dalia S, Chavez J, Castillo JJ, Sokol L. Hepatitis B infection increases the risk of non-Hodgkin lymphoma: a meta-analysis of observational studies. Leuk Res. 2013;37(9):1107–1115. doi:10.1016/j.leukres.2013.06.007
16. Marcucci F, Mele A. Hepatitis viruses and non-Hodgkin lymphoma: epidemiology, mechanisms of tumorigenesis, and therapeutic opportunities. Blood. 2011;117(6):1792–1798. doi:10.1182/blood-2010-06-275818
17. Ren W, Ye X, Su H, et al. Genetic landscape of hepatitis B virus-associated diffuse large B-cell lymphoma. Blood. 2018;131(24):2670–2681. doi:10.1182/blood-2017-11-817601
18. Sung WK, Zheng H, Li S, et al. Genome-wide survey of recurrent HBV integration in hepatocellular carcinoma. Nat Genet. 2012;44(7):765–769. doi:10.1038/ng.2295
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.