Back to Journals » OncoTargets and Therapy » Volume 15
CD24 as a Potential Therapeutic Target in Patients with B-Cell Leukemia and Lymphoma: Current Insights
Authors Christian SL
Received 28 July 2022
Accepted for publication 10 November 2022
Published 18 November 2022 Volume 2022:15 Pages 1391—1402
DOI https://doi.org/10.2147/OTT.S366625
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Gaetano Romano
Sherri L Christian1,2
1Department of Biochemistry, Memorial University of Newfoundland, St. John’s, NL, Canada; 2Beatrice Hunter Cancer Research Institute, Halifax, NS, Canada
Correspondence: Sherri L Christian, 45 Arctic Ave, St. John’s, NL, A1C 5S7, Canada, Tel +1 709864-8550, Email [email protected]
Abstract: CD24 is a highly glycosylated glycophosphatidylinositol (GPI)-anchored protein that is expressed in many types of differentiating cells and some mature cells of the immune system as well as the central nervous system. CD24 has been extensively used as a biomarker for developing B cells as its expression levels change over the course of B cell development. Functionally, engagement of CD24 induces apoptosis in developing B cells and restricts cell growth in more mature cell types. Interestingly, CD24 is also expressed on many hematological and solid tumors. As such, it has been investigated as a therapeutic target in many solid tumors including ovarian, colorectal, pancreatic, lung and others. Most of the B-cell leukemias and lymphomas studied to date express CD24 but its role as a therapeutic target in these malignancies has, thus far, been understudied. Here, I review what is known about CD24 biology with a focus on B cell development and activation followed by a brief overview of how CD24 is being targeted in solid tumors. This is followed by an assessment of the value of CD24 as a therapeutic target in B cell leukemia and lymphoma in humans, including an evaluation of the challenges in using CD24 as a target considering its pattern of expression on normal cells.
Keywords: CD24, leukemia, lymphoma, B lymphocyte, CD24-Fc
Background
Cluster of Differentiation (CD) 24, also called heat stable antigen or nectadrin, is a highly glycosylated glycophosphatidylinositol (GPI)-anchored protein consisting of a mature peptide of 27 residues in mouse and 32 residues in humans.1–3 It was first discovered in 1978 by Springer et al through the screening of monoclonal antibodies binding to unique blood cell antigens.4 The mature peptide is derived from a precursor peptide of 76 residues in mice and 82 residues in humans. The cleavage of the N-terminal signal sequence and C-terminal glycophosphatidylinositol (GPI)-anchor sequence in addition to multiple glycosylation sites generates a mature peptide of 20 to 70 KDa.5
CD24 is located on chromosome 6q21 in humans and chromosome 10 in mice. Analysis of the evolution of CD24 suggests that it arose prior to the divergence of reptiles and avians but was lost in marsupials and monotremes.2 The consensus sequence of the mature peptide shows multiple highly conserved residues.2 These residues are sites, or potential sites, of glycosylation, which is consistent with a critical role of these post-translational modifications for the interaction of CD24 with its ligands (see below).
In a healthy individual, CD24 is expressed on and regulates many different cell types. In general, CD24 expression is dynamic with higher expression on immature cells followed by decreased expression at mature cell stages.2,6 CD24 promotes the normal maturation of B lymphocytes (B cells), T lymphocytes (T cells), neuronal cells, adipocytes, colon crypt cells, and epithelial cells in the mammary gland.7–9 CD24 acts as a co-stimulatory molecule for T cells10 and regulates dendritic cell activation in response to danger-associated molecular patterns (DAMPs).11,12 CD24 is constitutively expressed in erythrocytes where it decreases aggregation and promotes their half-life in circulation in mice13 but does not appear to be expressed in human erythrocytes.14 It is also highly expressed on developing neurons and regions of active neurogenesis in the adult brain.9 In addition, CD24 is highly expressed on many different types of cancer and can be associated with both increased or decreased cancer aggressiveness.15 Interestingly, while low levels of CD24 in combination with high levels of CD44 have been associated with breast cancer stem cells,16 high CD24 levels have also been associated with pancreatic cancer stem cells and other cancer types (please see Altevogt et al15 for a more in-depth discussion).
CD24 has multiple ligands that interact either in cis (on the same cell) or in trans (on a different cell).1 These interactions are mediated by the differential glycosylation of CD24.5,17–19 In trans, CD24 on cells from the myeloid lineage, splenic B cells activated by lipopolysaccharide, and thymocytes, as well as breast cancer cells binds to P-selectin to mediate adhesion to endothelial cells.18,20–23 Similarly, CD24 on MCF-7 breast cancer cells mediates adhesion to E-selectin.24 The L1 cell adhesion molecule (L1CAM) is the ligand for CD24 in neurons and neuroblastoma where it regulates neurite outgrowth by binding to CD24 in cis.18,25,26 In dendritic cells, CD24 acts in cis with Siglec-G (Siglec-10) to suppress DAMPs-mediated activation of these cells.11,12 Lastly, CD24 can interact with itself and so might act as its own ligand in some cases.27 However, the natural ligand for CD24 on developing B cells or B cell leukemias or lymphomas is unknown.
CD24 Expression in B Cells
B cells develop in the bone marrow in discrete stages that are defined by expression of key biomarkers, one of these being CD24 (Figure 1).28 CD24 levels are low in the earliest stages of lineage commitment and increase in mRNA and protein expression until they peak at the pre-B cell stage (Fraction C’/D).2 CD24 expression drops when B cells exit the bone marrow but rebounds at transitional stage 1, which occurs in the spleen,29 followed by a reduction in circulating mature B cells and beyond.2,28 Moreover, CD24 is highly expressed on both B1 B cells28 and regulatory B cells.30,31 In the bone marrow, CD24 is not simply a biomarker; it is a key regulator of B cell development (Figure 1). Both transgenic mice that overexpress CD24 and CD24 knock-out mice display a reduction in developing B cell numbers, particularly at the pro and pre-B cell stages.13,32 However, there are normal numbers of B cells in the periphery demonstrating that reduction in early B cell numbers is compensated by increased proliferation at later cell stages. In vitro, engaging CD24 with stimulating antibodies induces apoptosis in developing B cells and suppresses the proliferation of splenic B cells.33,34 Furthermore, apoptosis of pre-B cells in CD24 transgenic mice is observed in vivo.35 Thus, the main role for CD24 in B cell development is to control B cell survival at the pro- and pre-B cell stages.
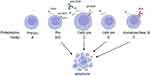 |
Figure 1 CD24 induces apoptosis in developing B cells. Shown are stages of B cell development in the bone marrow with both Philadelphia and Hardy nomenclature shown. The relative levels of CD24 are indicated by the number and size of CD24 molecules shown. Engagement of CD24 with antibody or overexpression in transgenic mice leads to increased apoptosis of B cells early in development. Indicated are the stages susceptible to CD24-mediated apoptosis. B cells continue to have low level of CD24 expression after they egress from the bone marrow with an increase in transitional splenic B cells (not shown), however, they are no longer susceptible to CD24-induced apoptosis. Also, shown is the expression of the B cell receptor (BCR) during B cell development. Created in BioRender.com. |
More recently, CD24 has been linked to extracellular vesicles (EVs) secreted from B lymphocytes36–39 and EVs that are present in other bodily fluids such as saliva,40 urine and amniotic fluid.41 EVs are nano-sized particles derived from the endocytic pathway (exosomes) or budded off the plasma membrane (microvesicles, ectosomes).42 EVs are important mediators of cell communication that can transport lipids, proteins, and nucleic acids from donor to recipient cells. Cancer cells have been shown to release more EVs than normal cells42 and CD24 on EVs from plasma has been suggested to be a marker for detection and monitoring of different cancers.43–45
CD24 in Immune Regulation and Evasion
CD24 regulates immune activation in cis and in trans. Chen et al12 demonstrated that the interaction of CD24 with Siglec-10 (Siglec-G in mouse) on dendritic cells (DCs) acts a molecular brake to limit inflammation in response to DAMPs.46 CD24 does this by interacting directly with Siglec-10 through its sialic acid modifications when DAMPs such as HMBG1, HSP70, or HSP90 are present.12 This binding activates inhibitory signaling downstream of Siglec-10, which, through the immunoreceptor tyrosine-based inhibitory motif (ITIMs), activates the Shp-1 phosphatase.47 This in turn downregulates signaling downstream of Toll-like receptors (TLR) to limit DC activation.12 Interestingly, CD24 specifically interacts with DAMPS but not pathogen-associated molecular patterns (PAMPs) to specifically inhibit the overactivation of the immune system in response to signals of injury but not infection.11
Acute graft-vs-host (GVHD) disease can occur post hematopoietic transplantation whereby the donor immune cells mount an immune response against the host. High dose chemotherapy and/or total body irradiation causes damage to the host and this conditioning it is thought to be the first step in the development of GVHD.48 In this situation, CD24 on T cells interacts with Siglec-G expressed on DCs to reduce T cell activation and inflammatory cytokine release in response to the DAMPs released by the injured tissue.49 The soluble CD24-Fc fusion protein (CD24-Fc) is able to recapitulate the function of CD24 by interacting with Siglec-G and activating its inhibitory signaling function to limit immune activation.47 In a pre-clinical model of GVHD, CD24-Fc was able to significantly mitigate the effects of GVHD and improve survival.49
CD24-Fc has been further investigated as a non-chemical inhibitor of immune activation in vivo to target chronic inflammation (Table 1). In a simian immunodeficiency virus (SIV) model, injection of CD24-Fc reduced chronic inflammation and improved overall survival of these animals.50 Similarly, in a human clinical trial, injection of CD24-Fc reduced chronic inflammation associated with SARS-CoV-2 infection.51 In a mouse model of sepsis, bacterial sialidases inhibit the sialic acid-mediated interaction between CD24 and Siglec-G, which exacerbates inflammation that is not accompanied by changes in bacterial load.17 Therefore, in the absence of the limiting effect of CD24 on DCs, inflammation itself causes severe tissue damage and subsequent death in this mouse model of sepsis. Overactivation of the immune system, called a cytokine storm, can be a major cause of mortality in sepsis and viral infection. Thus, limiting inflammation via CD24-Fc-mediated activation of Siglec-10/G can ameliorate the immune mediated damage occurring in response to pathogenic infection.
 |
Table 1 CD24-Targeted Therapies for Chronic Inflammation |
Metaflammation is the low-level chronic inflammation caused by obesity. It has recently been demonstrated that metaflammation is regulated by interactions of CD24 with Siglec-E.52 Acting as an innate immune check point inhibitor, CD24 represses the metabolic disorders associated with obesity, including dyslipidemia, insulin resistance and nonalcoholic steatohepatitis. Similar to the interaction of CD24 with Siglec-G, CD24 promotes the activation of Shp-1 phosphatase by Siglec-E to downregulate immune cell activation. CD24-Fc treatment of mice ameliorated these diet-induced metabolic dysfunctions and decreased expression of inflammatory cytokines. Similarly, the group showed that CD24-Fc decreased expression of inflammatory cytokines in healthy human subjects.52
Recently, EVs have been loaded with CD24 by transfecting a HEK-293-derived cell-line (293-TREx™) for human studies or a NIH/3T3 cell line (Expi293™) for mouse studies with plasmids encoding the human or murine CD24, respectively, under control of tetracycline.53 The resulting EVs, called EXO-CD24, were then administered via inhalation to either mice challenged with LPS or SARS-CoV-2- infected humans. A significant reduction in inflammatory cytokines, including IL-17A, TNF-α, and IL-6 among others, was observed in both situations as well as a reduction in systemic C-reactive protein (CRP). This observed decrease in inflammatory markers supports the idea that EXO-CD24 is mediating a reduction in a cytokine storm response. While EXO-CD24 appeared to be safe in humans, the efficacy of this treatment remains to be determined. In any case, EXO-CD24 is presumably activating the Siglec-10/G inhibitory cascade to block NF-κB activation similar to CD24-Fc.53 These data further suggest that uncoupling viral clearance response from the DAMP response by activation of Siglec-10/G by EXO-CD24 can potentially reduce the overall degree of the inflammatory cytokine response. This uncoupling could, therefore, reduce mortality from viral infections normally due to cytokine storm; similar to the effects of CD24-Fc.
In cancer, CD24 expression on some tumors serves as a “don’t eat me” signal (ie an immune evasion strategy). Specifically, the presence of CD24 impairs phagocytosis of ovarian and triple negative breast cancer cells by macrophages.14 In the absence of CD24, macrophage activity caused a significant decrease in tumor volume in vivo. These data suggest that CD24, in concert with Siglec-10, acts as a mechanism to regulate self/non-self-interactions by the innate immune system.54 It is proposed that interfering with the CD24/Siglec-10 interaction via monoclonal antibody (mAb) treatment may improve the innate immune response to cancer by removing this innate immune checkpoint.14
CD24 in B-Cell Leukemias and Lymphomas
In Canada, leukemia is the most diagnosed cancer in children at 32% but is one of the rarer cancers in adults at 2.4–3.4%.55 Lymphomas are the third most common cancer in children (13%) following central nervous system cancers (17%) while lymphomas make up approximately 5% of cancers in Canadian adults. Similar prevalence of leukemias and lymphoma exist globally.56
Leukemias and lymphomas arise from B cells at many different stages of development (Figure 2). These neoplasms can be classified based on cytomorphological features as well as expression of distinct cell surface receptors linked to the stage from which it derived.57 Furthermore, molecular characterization of leukemias have been used to further delineate the origin of different leukemias and lymphomas.58,59 However, characterization of the leukemic cell of origin is not simple as leukemic cells will continue to acquire additional mutations while it develops.58,60 Nevertheless, B cell derived leukemias and lymphomas share many of the same features as the cells they arise from; including susceptibility to pro-apoptotic stimuli and expression of key B cell markers. For example, expression of the pan B-cell marker CD20 on many B cell leukemias and lymphomas has made this a valuable therapeutic target for mAb therapy Rituximab.61 Activation of pro-apoptotic signaling pathways in response to Rituximab along with activation of complement, recruitment of NK cells to induce antibody-dependent cellular cytotoxicity (ADCC), and phagocytosis all contribute to the anti-tumor effects of the mAb therapy. Targeting surface receptors in this manner has the advantage over general chemotherapeutics in that only cells expressing the receptor will be targeted. However, unless the target is only expressed on cancer cells, and not normal cells, both cell types will be affected by the therapy thereby reducing the targeted advantage of the therapy.
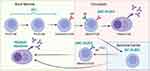 |
Figure 2 B cell leukemia and lymphoma can derive from their normal B cell counterparts. Shown are the major stages of B cell development prior to (in the bone marrow) and in response to antigen stimulation (in the circulation and germinal centre). Shown is a simplified schematic of the relationship between some B cell leukemia and lymphoma and stage of B cell development. Because mutations continue to accumulate after the initial tumorigenic event, leukemia and lymphoma clones may not very closely reflect their cell of origin. In addition, some leukemia and lymphomas may resemble multiple stages of B cell development when examining their gene expression profiles. Most B-acute lymphoblastic leukemia (ALL) arises from pro- and pre-B cell stages,60 while diffuse large B-cell lymphoma (DLBCL), which can be divided into activated B-cell like (ABC) and germinal center B-cell like (GC), arise from more mature B cell stages.58 Similarly, evidence suggests that chronic lymphocytic leukemia (CLL) and multiple myeloma arise from mature cell stages.59,104 Created in BioRender.com. |
CD24 expression on hematological malignancies has been well documented (Table 2). While it is expressed on most subtypes of leukemias and lymphomas, CD24 is notably low or absent in a subset of B-ALL with KMT2A rearrangements.62,63 In general, high CD24 expression has been associated with poor prognosis except in the case of multiple myeloma.64–70 For chronic lymphocytic leukemia, high CD24 expression was associated with progressive vs stable disease.71 Even though CD24 has been found to be expressed in most B cell malignancies to date, the protein expression levels do not always differentiate healthy from cancer patients due to high variability in expression.66 It is possible that combining CD24 expression with expression of other surface antigens will increase the diagnostic or prognostic potential of CD24.71 However, this remains to be determined in a clinical setting. Interestingly, the presence of CD24 in pediatric B-ALL was associated with sensitivity to radiotherapy72, suggesting that CD24 could be used as a prognostic marker if radiotherapy is advised.
 |
Table 2 Expression of CD24 in Hematological Cancers |
Targeting CD24 for Cancer Treatment
CD24 is highly expressed in multiple tumors and has, thus, been targeted in preclinical trials for lymphoma65,69,73, as well as lung,14,74–76 colorectal,75,77,78 bladder,79 pancreatic,14,74 ovarian,14,74,80 liver,75,81–83 breast,84 and neuroendocrine14 cancers in addition to osteosarcoma85 and multiple myeloma86 (Table 3). Similar to CD20 targeting, the anti-CD24 mAb can bind directly and cluster CD24 to induce apoptosis, recruit NK cells via their Fc receptors to induce ADCC, induce complement-mediated cytotoxicity, as well as the recruitment of phagocytes to the tumor cells.87 In addition, conjugation of a toxin to the mAb can allow for directed interaction and killing of the cancer cell by the toxin. Furthermore, bispecific antibodies targeting both CD24 and the Natural Killer cell receptor, NKG2D, have been shown to enhance ADCC in liver cancer.83 However, in most studies the mechanism of action has not been directly determined. Nevertheless, all the published data show thus far show that targeting CD24 via mAb in the cancer types tested reduces the growth of cancer cells in animal models (Table 3). With respect to B cell leukemia and lymphoma, naturally EBV-transformed B cells and Burkitt’s lymphoma (BL) have been tested in preclinical animal models.73,88 In the EBV-transformed cells, unconjugated mAb was found to improve survival of SCID mice to approximately 40% at 150 days with treatment by i.v. infusion at days 30–42 days post tumor cell injection.88 In the BL situation, the direct cytotoxic effects of the anti-CD24 SWA11 antibody conjugated to Ricin-A (SWA11.dgA) were shown against BL cell lines.73 Furthermore, injection of SCID mice with disseminated BL disease approximately doubled the tumor-free survival time when injected 4 days after tumor establishment and cured all mice when injected 1 day after tumor establishment.73 To the best of my knowledge, no recent reports on targeting CD24 in leukemia or lymphoma have been published. Therefore, more remains to be done to determine if targeting CD24 is effective in pre-clinical models of B cell leukemia and lymphoma.
 |
Table 3 CD24-Targeted mAb-Based Therapeutic Studies Showing a Reduction in Tumor Growth in vivo |
The biggest challenge in targeting CD24 as a direct cancer target is that CD24 is also expressed in cells of the immune system and the nervous system (see above). However, the observation that anti-CD24 mAb does not bind to human erythrocytes14 is reassuring as this treatment should, thus, not result in hemolytic anemia in humans. One major limitation of the pre-clinical models tested to date is that they are, necessarily, performed in immunodeficient mice (Table 3). Furthermore, the mAbs used are specific to human CD24 and do not cross-react with murine CD24. Therefore, it is not possible to truly appreciate the potential side-effects of this treatment as the binding of the mAb to CD24 on host immune cells and neuronal cells does not occur in the pre-clinical models used. The most likely side-effects of mAb treatment would be immunosuppression and disruption of cognitive function consistent with inhibition of immune cell development1,3,13,32 and neurogenesis.89–91 Moreover, it has been shown that the lack of CD24 on DCs causes the rapid homeostatic proliferation of T cells when lymphopenia was induced.92 This rapid and massive expansion of T cells causes death of the immunocompetent mice. Therefore, off-target killing of DCs in secondary lymphoid organs upon injection of an anti-CD24 mAb is likely to induce an expansion of T cells that will be severely detrimental to the human host.
In addition, transport of the mAb to the bone marrow via the circulation will cause the killing of developing B cells; resulting in a severe reduction in circulating B cells. Moreover, mAb against CD24 prevents co-stimulation of T cells to further induce immunosuppression.93,94 This immunosuppression may be similar to that induced by current chemotherapeutic strategies and could be mitigated by avoidance of pathogenic organisms. Thus, the issue of immunosuppression is much less of a concern than the expansion of T cells covered above.
In cancer treatment, CD24 could also be targeted as an innate immune checkpoint inhibitor to promote phagocytosis of tumor cells by macrophages.14 The use of innate immune checkpoint inhibitors to target the phagocytic inhibitory ligand CD47, which binds to signal-regulatory protein-α (SIRPα) on macrophages have gone through clinical trials for both solid and hematological cancers.95 Similarly, targeting the interaction of TIGIT on NK cells or its ligands CD112 (also called PVRL2 and nectin-2) and CD155 (also called PVR) have been tested in clinical trials.95 However, response rates thus far to these checkpoint inhibitors have been variable. Nevertheless, further clinical trials are in progress to test the effectiveness of these and other innate checkpoint inhibitors, often in combination with other treatments.
Chimeric antigen receptor (CAR) T cell therapy targeting CD19 has been proven to be very successful in relapsed or refractory B-ALL.96 Using similar logic, Klapdor et al97 have generated third generation CD24-CAR NK cells to target ovarian cancer showing that CD24+ ovarian cell lines and primary tumor cells were selectively killed by the CD24-NK-CAR cells. However, a dual-CAR cell targeting both CD24 and Mesothelin, an ovarian cancer specific antigen, did not enhance killing over the CD24-targeted CAR alone. Thus, CD24-NK-CAR could potentially be trialed against CD24+ B-cell neoplasms, with the caveat that they may also target normal CD24-expressing cells, similar to the cytotoxic antibody situation described above. Application of bispecific T-cell engagers (BiTEs) may also be an immunotherapeutic strategy to pursue as these molecules can also act to engage CD24-directed T cell-mediated killing of leukemia and lymphomas.98
Future Directions
While CD24 is a promising target for B-cell leukemias and lymphomas, questions still remain on the viability of this therapy given the expression on many normal cell types. Thus, identification of a therapeutic window may be difficult. The variable glycosylation of CD24 on different cells may be an unexpected benefit to this conundrum. The development of anti-CD24 mAbs that target tumor-specific forms of CD24 would generate the specificity that is needed for high efficacy of this treatment. Multiple versions of anti-CD24 mAb have been generated with some binding only to the carbohydrates (eg BA-199 and SN3b100) and some to the peptide backbone (eg SWA11101). The generation of a mAb with specificity towards the tumor-specific carbohydrate-peptide conjugate may further improve tumor-specific targeting.
Lastly, there has been a lack of pre-clinical in vivo studies directly assessing efficacy of targeting CD24 in B cell leukemias and lymphomas. Even though expression data on CD24 in these cancers is promising, the in vivo data are necessary to move forward with using CD24 as a target in B cell neoplasms. Once these models are tested, further studies on safety will be necessary.
Conclusion
Overall, the outlook for CD24 as a target in the treatment of B cell leukemias and lymphomas is promising. However, care will need to be taken to limit or avoid off-target effects on normal CD24-expressing cells.
Acknowledgments
No funding to declare. Thank you to Modeline Longjohn and Hong-Dien Phan for helpful comments on the manuscript.
Disclosure
The author reports no conflict of interest in this work.
References
1. Ayre DC, Christian SL. CD24: a rheostat that modulates cell surface receptor signaling of diverse receptors. Front Cell Dev Biol. 2016;4(December):1–6. doi:10.3389/fcell.2016.00146
2. Ayre DC, Pallegar NK, Fairbridge NA, Canuti M, Lang AS, Christian SL. Analysis of the structure, evolution, and expression of CD24, an important regulator of cell fate. Gene. 2016;590(2):324–337. doi:10.1016/j.gene.2016.05.038
3. Fang X, Zheng P, Tang J, Liu Y. CD24: from A to Z. Cell Mol Immunol. 2010;7(2):100–103. doi:10.1038/cmi.2009.119
4. Springer T, Galfrè G, Secher DS, Milstein C. Monoclonal xenogeneic antibodies to murine cell surface antigens: identification of novel leukocyte differentiation antigens. Eur J Immunol. 1978;8(8):539–551. doi:10.1002/EJI.1830080802
5. Motari E, Zheng X, Su X, et al. Analysis of recombinant CD24 glycans by MALDI-TOF-MS reveals prevalence of sialyl-T antigen. Am J Biomed Sci. 2009;1(1):1–11. doi:10.5099/aj090100001
6. Tan Y, Zhao M, Xiang B, Chang C, Lu Q. CD24: from a hematopoietic differentiation antigen to a genetic risk factor for multiple autoimmune diseases. Clin Rev Allergy Immunol. 2016;50(1):70–83. doi:10.1007/s12016-015-8470-2
7. Smith NC, Fairbridge NA, Pallegar NK, Christian SL. Dynamic upregulation of CD24 in pre-adipocytes promotes adipogenesis. Adipocyte. 2015;4(2):89–100. doi:10.4161/21623945.2014.985015
8. Smith NC, Swaminathan V, Pallegar NK, Cordova C, Buchanan SC, Christian SL. CD24 is required for regulating gene expression, but not glucose uptake, during adipogenesis. Adipocyte. 2018;7(4):248–260. doi:10.1080/21623945.2018.1525251
9. Gilliam DT, Menon V, Bretz NP, Pruszak J. The CD24 surface antigen in neural development and disease. Neurobiol Dis. 2017;99:133–144. doi:10.1016/j.nbd.2016.12.011
10. Li O, Zheng P, Liu Y. CD24 expression on T cells is required for optimal T cell proliferation in lymphopenic host. J Exp Med. 2004;200(8):1083–1089. doi:10.1084/jem.20040779
11. Liu Y, Chen GY, Zheng P. CD24-Siglec G/10 discriminates danger- from pathogen-associated molecular patterns. Trends Immunol. 2009;30(12):557–561. doi:10.1016/j.it.2009.09.006
12. Chen GY, Tang J, Zheng P, Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323(5922):1722–1725. doi:10.1126/science.1168988
13. Nielsen PJ, Lorenz B, Muller AM, et al. Altered erythrocytes and a leaky block in B-cell development in CD24/HSA-deficient mice. Blood. 1997;89(3):1058–1067. doi:10.1182/blood.V89.3.1058
14. Barkal AA, Brewer RE, Markovic M, et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature. 2019;572(7769):392–396. doi:10.1038/s41586-019-1456-0
15. Altevogt P, Sammar M, Hüser L, Kristiansen G. Novel insights into the function of CD24: a driving force in cancer. Int J Cancer. 2021;148(3):546–559. doi:10.1002/IJC.33249
16. Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100(7):3983–3988. doi:10.1073/PNAS.0530291100/ASSET/C251EFA2-0831-42E3-931C-CFA289B5DAF4/ASSETS/GRAPHIC/PQ0530291004.JPEG
17. Chen GY, Chen X, King S, et al. Amelioration of sepsis by inhibiting sialidase-mediated disruption of the CD24-SiglecG interaction. Nat Biotechnol. 2011;29(5):428–435. doi:10.1038/nbt.1846
18. Sammar M, Aigner S, Altevogt P. Heat-stable antigen (mouse CD24) in the brain: dual but distinct interaction with P-selectin and L1. Biochim Biophys Acta. 1997;1337(2):287–294. doi:10.1016/S0167-4838(96)00177-X
19. Lieberoth A, Splittstoesser F, Katagihallimath N, et al. Lewis(x) and alpha2,3-sialyl glycans and their receptors TAG-1, contactin, and L1 mediate CD24-dependent neurite outgrowth. J Neurosci. 2009;29(20):6677–6690. doi:10.1523/JNEUROSCI.4361-08.2009
20. Aigner S, Ramos CL, Hafezi-Moghadam A, et al. CD24 mediates rolling of breast carcinoma cells on P-selectin. FASEB J. 1998;12(12):1241–1251. doi:10.1096/fasebj.12.12.1241
21. Aigner S, Sthoeger ZM, Fogel M, et al. CD24, a mucin-type glycoprotein, is a ligand for P-selectin on human tumor cells. Blood. 1997;89(9):3385–3395. doi:10.1182/blood.V89.9.3385
22. Aigner S, Ruppert M, Hubbe M, et al. Heat stable antigen (mouse CD24) supports myeloid cell binding to endothelial and platelet P-selectin. Int Immunol. 1995;7(10):1557–1565. doi:10.1093/intimm/7.10.1557
23. Sammar M, Aigner S, Hubbe M, et al. Heat-stable antigen (CD24) as ligand for mouse P-selectin. Int Immunol. 1994;6(7):1027–1036. doi:10.1093/intimm/6.7.1027
24. Myung JH, Gajjar KA, Pearson RM, Launiere CA, Eddington DT, Hong S. Direct measurements on CD24-mediated rolling of human breast cancer MCF-7 cells on E-selectin. Anal Chem. 2011;83(3):1078–1083. doi:10.1021/AC102901E
25. Kadmon G, von Bohlen Und Halbach F, Horstkorte R, Eckert M, Altevogt P, Schachner M. Evidence for cis interaction and cooperative signalling by the heat-stable antigen nectadrin (murine CD24) and the cell adhesion molecule L1 in neurons. Eur J Neurosci. 1995;7(5):993–1004. doi:10.1111/j.1460-9568.1995.tb01087.x
26. Kleene R, Yang H, Kutsche M, Schachner M. The neural recognition molecule L1 is a sialic acid-binding lectin for CD24, which induces promotion and inhibition of neurite outgrowth. J Biol Chem. 2001;276(24):21656–21663. doi:10.1074/jbc.M101790200
27. Kadmon G, Eckert M, Sammar M, Schachner M, Altevogt P. Nectadrin, the heat-stable antigen, is a cell adhesion molecule. J Cell Biol. 1992;118(5):1245–1258. doi:10.1083/jcb.118.5.1245
28. Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19(1):595–692. doi:10.1146/annurev.immunol.19.1.595
29. Pieper K, Grimbacher B, Eibel H. B-cell biology and development. J Allergy Clin Immunol. 2013;131(4):959–971. doi:10.1016/J.JACI.2013.01.046
30. Evans JG, Chavez-Rueda KA, Eddaoudi A, et al. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol. 2007;178(12):7868–7878. doi:10.4049/jimmunol.178.12.7868
31. Iwata Y, Matsushita T, Horikawa M, et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117(2):530–541. doi:10.1182/BLOOD-2010-07-294249
32. Hough MR, Chappel MS, Sauvageau G, Takei F, Kay R, Humphries RK. Reduction of early B lymphocyte precursors in transgenic mice overexpressing the murine heat-stable antigen. J Immunol. 1996;156(2):479–488.
33. Chappel MS, Hough MR, Mittel A, Takei F, Kay R, Humphries RK. Cross-linking the murine heat-stable antigen induces apoptosis in B cell precursors and suppresses the anti-CD40-induced proliferation of mature resting B lymphocytes. J Exp Med. 1996;184(5):1639–1649. doi:10.1084/jem.184.5.1639
34. Suzuki T, Kiyokawa N, Taguchi T, Sekino T, Katagiri YU, Fujimoto J. CD24 induces apoptosis in human B cells via the glycolipid-enriched membrane domains/rafts-mediated signaling system. J Immunol. 2001;166(9):5567–5577. doi:10.4049/jimmunol.166.9.5567
35. Lu L, Chappel MS, Humphries RK, Osmond DG. Regulation of cell survival during B lymphopoiesis: increased pre-B cell apoptosis in CD24-transgenic mouse bone marrow. Eur J Immunol. 2000;30(9):2686–2691. doi:10.1002/1521-4141(200009)30:9<2686::AID-IMMU2686>3.0.CO;2-F
36. Ayre DC, Elstner M, Smith NC, Moores ES, Hogan AM, Christian SL. Dynamic regulation of CD24 expression and release of CD24-containing microvesicles in immature B cells in response to CD24 engagement. Immunology. 2015;146(2):217–233. doi:10.1111/imm.12493
37. Ayre DC, Chute IC, Joy AP, et al. CD24 induces changes to the surface receptors of B cell microvesicles with variable effects on their RNA and protein cargo. Sci Rep. 2017;7(1):8642. doi:10.1038/s41598-017-08094-8
38. Phan HD, Longjohn MN, Gormley DJB, et al. CD24 and IgM stimulation of B cells triggers transfer of functional B cell receptor to B cell recipients via extracellular vesicles. J Immunol. 2021;207(12):3004–3015. doi:10.4049/jimmunol.2100025
39. Oksvold MP, Kullmann A, Forfang L, et al. Expression of B-cell surface antigens in subpopulations of exosomes released from B-cell lymphoma cells. Clin Ther. 2014;36(6):847–862.e1. doi:10.1016/J.CLINTHERA.2014.05.010
40. Yu Y, Gool E, Berckmans RJ, et al. Extracellular vesicles from human saliva promote hemostasis by delivering coagulant tissue factor to activated platelets. J Thromb Haemost. 2018;16(6):1153–1163. doi:10.1111/jth.14023
41. Keller S, Rupp C, Stoeck A, et al. CD24 is a marker of exosomes secreted into urine and amniotic fluid. Kidney Int. 2007;72(9):1095–1102. doi:10.1038/sj.ki.5002486
42. Yáñez-Mó M, Siljander PRM, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4(1):27066. doi:10.3402/jev.v4.27066
43. Ekström K, Crescitelli R, Pétursson HI, Johansson J, Lässer C, Bagge RO. Characterization of surface markers on extracellular vesicles isolated from lymphatic exudate from patients with breast cancer. BMC Cancer. 2022;22(1). doi:10.1186/S12885-021-08870-W
44. Rupp AK, Rupp C, Keller S, et al. Loss of EpCAM expression in breast cancer derived serum exosomes: role of proteolytic cleavage. Gynecol Oncol. 2011;122(2):437–446. doi:10.1016/J.YGYNO.2011.04.035
45. Li Y, Zhou J, Zhuo Q, et al. Malignant ascite-derived extracellular vesicles inhibit T cell activity by upregulating Siglec-10 expression. Cancer Manag Res. 2019;11:7123–7134. doi:10.2147/CMAR.S210568
46. Bianchi ME, Manfredi AA. Dangers in and out. Science. 2009;323(5922):1683–1684. doi:10.1126/SCIENCE.1172794/ASSET/DDDEC913-7E4D-4F71-AC70-B93990BD37DB/ASSETS/GRAPHIC/1683-1.GIF
47. Toubai T, Rossi C, Oravecz-Wilson K, et al. Siglec-G represses DAMP-mediated effects on T cells. JCI Insight. 2017;2(14). doi:10.1172/JCI.INSIGHT.92293
48. Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373(9674):1550–1561. doi:10.1016/S0140-6736(09
49. Toubai T, Hou G, Mathewson N, et al. Siglec-G–CD24 axis controls the severity of graft-versus-host disease in mice. Blood. 2014;123(22):3512–3523. doi:10.1182/BLOOD-2013-12-545335
50. Tian RR, Zhang MX, Zhang LT, et al. CD24 and Fc fusion protein protects SIVmac239-infected Chinese rhesus macaque against progression to AIDS. Antiviral Res. 2018;157:9–17. doi:10.1016/j.antiviral.2018.07.004
51. Song NJ, Allen C, Vilgelm AE, et al. Treatment with soluble CD24 attenuates COVID-19-associated systemic immunopathology. J Hematol Oncol. 2022;15(1). doi:10.1186/s13045-021-01222-y
52. Wang X, Liu M, Zhang J, et al. CD24-Siglec axis is an innate immune checkpoint against metaflammation and metabolic disorder. Cell Metab. 2022;34(8):1088–1103.e6. doi:10.1016/j.cmet.2022.07.005
53. Shapira S, Ben Shimon M, Hay-Levi M, et al. A novel platform for attenuating immune hyperactivity using EXO-CD24 in COVID-19 and beyond. EMBO Mol Med. 2022;14(9):e15997. doi:10.15252/emmm.202215997
54. Chen GY, Brown NK, Zheng P, Liu Y. Siglec-G/10 in self–nonself discrimination of innate and adaptive immunity. Glycobiology. 2014;24(9):800. doi:10.1093/GLYCOB/CWU068
55. Canadian Cancer Statistics Advisory Committee in collaboration with the Canadian Cancer Society. Statistics Canada, the public health agency of Canada. Can Cancer Stat. 2021;2021:1.
56. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/CAAC.21660
57. De Leval L, Jaffe ES. Lymphoma classification. Cancer J. 2020;26(3):176–185. doi:10.1097/PPO.0000000000000451
58. Johnsen HE, Bergkvist KS, Schmitz A, et al. Cell of origin associated classification of B-cell malignancies by gene signatures of the normal B-cell hierarchy. Leuk Lymphoma. 2014;55(6):1251–1260. doi:10.3109/10428194.2013.839785
59. Chiorazzi N, Ferrarini M. Cellular origin(s) of chronic lymphocytic leukemia: cautionary notes and additional considerations and possibilities. Blood. 2011;117(6):1781–1791. doi:10.1182/BLOOD-2010-07-155663
60. Campos-Sanchez E, Toboso-Navasa A, Romero-Camarero I, Barajas-Diego M, Sanchez-Garcia I, Cobaleda C. Acute lymphoblastic leukemia and developmental biology: a crucial interrelationship. Cell Cycle. 2011;10(20):3473. doi:10.4161/CC.10.20.17779
61. Salles G, Barrett M, Foà R, et al. Rituximab in B-cell hematologic malignancies: a review of 20 years of clinical experience. Adv Ther. 2017;34(10):2232–2273. doi:10.1007/S12325-017-0612-X
62. Schwartz S, Rieder H, Schläger B, Burmeister T, Fischer L, Thiel E. Expression of the human homologue of rat NG2 in adult acute lymphoblastic leukemia: close association with MLL rearrangement and a CD10−/CD24−/CD65s+/CD15+ B-cell phenotype. Leukemia. 2003;17(8):1589–1595. doi:10.1038/sj.leu.2402989
63. Pui CH, Yang JJ, Hunger SP, et al. Childhood acute lymphoblastic leukemia: progress through collaboration. J Clin Oncol. 2015;33(27):2938–29948. doi:10.1200/JCO.2014.59.1636
64. Qiao LY, Li HB, Zhang Y, Shen D, Liu P, Che YQ. CD24 contributes to treatment effect in ABC-DLBCL patients with R-CHOP resistance. Pharmgenomics Pers Med. 2021;14:591–599. doi:10.2147/PGPM.S310816
65. Higashi M, Momose S, Takayanagi N, et al. CD24 is a surrogate for ‘immune-cold’ phenotype in aggressive large B-cell lymphoma. J Pathol Clin Res. 2022;8(3):340–354. doi:10.1002/CJP2.266
66. Coustan-Smith E, Song G, Clark C, et al. New markers for minimal residual disease detection in acute lymphoblastic leukemia. Blood. 2011;117(23):6267–6276. doi:10.1182/blood-2010-12-324004
67. Foa R, Migone N, Fierro MT, et al. Genotypic characterization of common acute lymphoblastic leukemia may improve the phenotypic classification. Exp Hematol. 1987;15(9):942–945.
68. Ludwig WD, Reiter A, Löffler H, et al. Immunophenotypic features of childhood and adult acute lymphoblastic leukemia (ALL): experience of the German multicentre trials ALL-BFM and GMALL. Leuk Lymphoma. 1994;13(s1):71–76. doi:10.3109/10428199409052679
69. Freile JÁ, Ustyanovska Avtenyuk N, Corrales MG, et al. CD24 is a potential immunotherapeutic target for mantle cell lymphoma. Biomedicines. 2022;10(5):1175. doi:10.3390/BIOMEDICINES10051175
70. Gross Even-Zohar N, Pick M, Hofstetter L, et al. CD24 is a prognostic marker for multiple myeloma progression and survival. J Clin Med. 2022;11(10):2913. doi:10.3390/JCM11102913
71. Huang PY, Best OG, Almazi JG, et al. Cell surface phenotype profiles distinguish stable and progressive chronic lymphocytic leukemia. Leuk Lymphoma. 2014;55(bretz):2085–2092. doi:10.3109/10428194.2013.867486
72. Uckun FM, Song CW. Lack of CD24 antigen expression in B-lineage acute lymphoblastic leukemia is associated with intrinsic radiation resistance of primary clonogenic blasts. Blood. 1993;81(5):1323–1332. doi:10.1182/blood.V81.5.1323.1323
73. Schnell R, Katouzi AA, Linnartz C, et al. Potent anti-tumor effects of an anti-CD24 ricin A-chain immunotoxin in vitro and in a disseminated human Burkitt’s lymphoma model in SCID mice. Int J Cancer. 1996;66(4):526–531. doi:10.1002/(SICI)1097-0215(19960516)66:4<526::AID-IJC17>3.0.CO;2-5
74. Bretz NP, Salnikov AV, Perne C, et al. CD24 controls Src/STAT3 activity in human tumors. Cell Mol Life Sci. 2012;69(22):3863–3879. doi:10.1007/s00018-012-1055-9
75. Chen Z, Wang T, Tu X, et al. Antibody-based targeting of CD24 enhances antitumor effect of cetuximab via attenuating phosphorylation of Src/STAT3. Biomed Pharmacother. 2017;90:427–436. doi:10.1016/j.biopha.2017.03.094
76. Zangemeister-Wittke U, Lehmann HP, Waibel R, Wawrzynczak EJ, Stahel RA. Action of a CD24-specific deglycosylated ricin-A-chain immunotoxin in conventional and novel models of small-cell-lung-cancer xenograft. Int J Cancer. 1993;53(3):521–528. doi:10.1002/ijc.2910530327
77. Sagiv E, Memeo L, Karin A, et al. CD24 is a new oncogene, early at the multistep process of colorectal cancer carcinogenesis. Gastroenterology. 2006;131(2):630–639. doi:10.1053/j.gastro.2006.04.028
78. Shapira S, Shapira A, Starr A, et al. An immunoconjugate of anti-CD24 and Pseudomonas exotoxin selectively kills human colorectal tumors in mice. Gastroenterology. 2011;140(3):935–946. doi:10.1053/j.gastro.2010.12.004
79. Overdevest JB, Thomas S, Kristiansen G, Hansel DE, Smith SC, Theodorescu D. CD24 offers a therapeutic target for control of bladder cancer metastasis based on a requirement for lung colonization. Cancer Res. 2011;71(11):3802–3811. doi:10.1158/0008-5472.CAN-11-0519
80. Salnikov AV, Bretz NP, Perne C, et al. Antibody targeting of CD24 efficiently retards growth and influences cytokine milieu in experimental carcinomas. Br J Cancer. 2013;108(7):1449–1459. doi:10.1038/bjc.2013.102
81. He H, Tu X, Zhang J, et al. A novel antibody targeting CD24 and hepatocellular carcinoma in vivo by near-infrared fluorescence imaging. Immunobiology. 2015;220(12):1328–1336. doi:10.1016/j.imbio.2015.07.010
82. Sun F, Wang Y, Luo X, et al. Anti-CD24 antibody-nitric oxide conjugate selectively and potently suppresses hepatic carcinoma. Cancer Res. 2019;79(13):3395–3405. doi:10.1158/0008-5472.CAN-18-2839
83. Han Y, Sun F, Zhang X, et al. CD24 targeting bi-specific antibody that simultaneously stimulates NKG2D enhances the efficacy of cancer immunotherapy. J Cancer Res Clin Oncol. 2019;145(5):1179–1190. doi:10.1007/s00432-019-02865-8
84. Chan SH, Tsai KW, Chiu SY, et al. Identification of the novel role of CD24 as an oncogenesis regulator and therapeutic target for triple-negative breast cancer. Mol Cancer Ther. 2019;18(1):147–161. doi:10.1158/1535-7163.MCT-18-0292
85. Zhang YH, Wang ZY, Hao FY, Zhang L. Cluster of differentiation 24 monoclonal antibody induces apoptosis in the osteosarcoma cells. Eur Rev Med Pharmacol Sci. 2014;18(14):2038–2041.
86. Gao M, Bai H, Jethava Y, et al. Identification and characterization of tumor-initiating cells in multiple myeloma. J Natl Cancer Inst. 2020;112(5):507–515. doi:10.1093/jnci/djz159
87. Suzuki M, Kato C, Kato A. Therapeutic antibodies: their mechanisms of action and the pathological findings they induce in toxicity studies. J Toxicol Pathol. 2015;28(3):133–139. doi:10.1293/TOX.2015-0031
88. Durandy A, Brousse N, Rozenberg F, Basile GDS, Fischer AM, Fischer A. Control of human B cell tumor growth in severe combined immunodeficiency mice by monoclonal anti-B cell antibodies. J Clin Invest. 1992;90(3):945–952. doi:10.1172/JCI115971
89. Gao X, Wang H, Gao YY, et al. Elevated hippocampal CD24 in astrocytes participates in neural regeneration possibly via activating SHP2/ERK pathway after experimental traumatic brain injury in mice. Am J Transl Res. 2020;12(10):6395–6408.
90. Wang H, Zhou XM, Xu WD, et al. Inhibition of elevated hippocampal CD24 reduces neurogenesis in mice with traumatic brain injury. J Surg Res. 2020;245:321–329. doi:10.1016/j.jss.2019.07.082
91. Chen XX, Tao T, Gao S, et al. Knock-down of CD24 in astrocytes aggravates oxyhemoglobin-induced hippocampal neuron impairment. Neurochem Res. 2022;47(3):590–600. doi:10.1007/s11064-021-03468-x
92. Li O, Chang X, Zhang H, et al. Massive and destructive T cell response to homeostatic cue in CD24-deficient lymphopenic hosts. J Exp Med. 2006;203(7):1713–1720. doi:10.1084/jem.20052293
93. Liu Y, Jones B, Brady W, Janeway CA, Linley PS. Co‐stimulation of murine CD4 T cell growth: cooperation between B7 and heat‐stable antigen. Eur J Immunol. 1992;22(11):2855–2859. doi:10.1002/eji.1830221115
94. Liu Y, Jones B, Aruffo A, Sullivan KM, Linsley PS, Janeway CA. Heat-stable antigen is a costimulatory molecule for CD4 T cell growth. J Exp Med. 1992;175(2):437–445. doi:10.1084/jem.175.2.437
95. Lentz RW, Colton MD, Mitra SS, Messersmith WA. Innate immune checkpoint inhibitors: the next breakthrough in medical oncology? Mol Cancer Ther. 2021;20(6):961–974. doi:10.1158/1535-7163.MCT-21-0041
96. Davila ML, Brentjens RJ. CD19-targeted CAR T cells as novel cancer immunotherapy for relapsed or refractory B-cell acute lymphoblastic leukemia. Clin Adv Hematol Oncol. 2016;14(10):802–808.
97. Klapdor R, Wang S, Morgan M, et al. Characterization of a novel third-generation anti-CD24-CAR against ovarian cancer. Int J Mol Sci. 2019;20(3):660. doi:10.3390/IJMS20030660
98. Deak D, Pop C, Zimta AA, et al. Let’s talk about BiTEs and other drugs in the real-life setting for B-cell acute lymphoblastic leukemia. Front Immunol. 2019;10:2856. doi:10.3389/fimmu.2019.02856
99. Mehmet H, Larkin M, Tang PW, Lebien TW, Feizi T. Monoclonal antibody BA-1 to the human B lymphocyte marker CD24 recognizes a sialic acid (N-acetylneuraminic acid) dependent epitope in multi-valent display on peptide. Clin Exp Immunol. 1990;81(3):489–495. doi:10.1111/j.1365-2249.1990.tb05361.x
100. Kristiansen G, Machado E, Bretz N, et al. Molecular and clinical dissection of CD24 antibody specificity by a comprehensive comparative analysis. Lab Invest. 2010;90(7):1102–1116. doi:10.1038/labinvest.2010.70
101. Weber E, Lehmann HP, Beck-Sickinger AG, et al. Antibodies to the protein core of the small cell lung cancer workshop antigen cluster-w4 and to the leucocyte workshop antigen CD24 recognize the same short protein sequence leucine-alanine-proline. Clin Exp Immunol. 1993;93(2):279–285. doi:10.1111/j.1365-2249.1993.tb07980.x
102. Cossman J, Neckers LM, Hsu SM, Longo D, Jaffe ES. Low-grade lymphomas. Expression of developmentally regulated B-cell antigens. Am J Clin Pathol. 1984;115(1):117.
103. Ashihara K, Terai Y, Tanaka T, et al. Pharmacokinetic evaluation and antitumor potency of liposomal nanoparticle encapsulated cisplatin targeted to CD24-positive cells in ovarian cancer. Oncol Lett. 2020;19(3):1872–1880. doi:10.3892/ol.2020.11279
104. Barwick BG, Gupta VA, Vertino PM, Boise LH. Cell of origin and genetic alterations in the pathogenesis of multiple myeloma. Front Immunol. 2019;10(MAY):1121. doi:10.3389/FIMMU.2019.01121/BIBTEX
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
