Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
CD207 Expression Level is a New Prognostic Marker for Condyloma Acuminatum
Authors Zhang D , Qu Y, Sui C, Li M, Yuan Y, Wang N, Ma W
Received 5 April 2023
Accepted for publication 16 May 2023
Published 23 June 2023 Volume 2023:16 Pages 1607—1613
DOI https://doi.org/10.2147/CCID.S412162
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Dong Zhang,1 Yan Qu,2 Changlin Sui,2 Meiling Li,1 Yanmei Yuan,1 Ningning Wang,1 Weiyuan Ma1
1Department of Dermatology, Affiliated Hospital of Weifang Medical University, Weifang, Shandong, People’s Republic of China; 2Department of Dermatology, Yantai Yuhuangding Hospital, Yantai, Shandong, People’s Republic of China
Correspondence: Weiyuan Ma, Department of Dermatology, Affiliated Hospital of Weifang Medical University, Weifang, Shandong, People’s Republic of China, Email [email protected]
Background: Condyloma cuminata (CA) is a sexually transmitted disease caused by human papillomavirus (HPV) infection, which is prone to recurrence and difficult to cure in the short term. CD207 is a C-type lectin receptor that is specifically expressed on the surface of Langerhans cells (LCs) and is considered as an LC-specific immunohistochemical marker. The main purpose of this study is to explore the correlation between the expression of CD207 in CA skin lesions and the duration of CA disease course and frequency of recurrence, in order to provide new prognostic markers for CA to clinicians.
Materials and Methods: A total of 40 male patients with CA and their skin lesions were collected, as well as 40 healthy male penile tissue samples. The skin lesions of CA were clinically and histologically confirmed by acetic acid test. The expression of CD207 in epidermal tissues was detected using immunohistochemistry. The difference in the number of CD207 positive cells between CA skin lesions and healthy skin controls was compared, and the association between the number of CD207 positive cells in CA skin lesions and the duration of disease course and the frequency of recurrence was determined through Spearman correlation analysis.
Conclusion: In CA skin lesions, CD207 positive cells were found to have morphological abnormalities and the number of cells was significantly reduced compared to healthy skin, suggesting that there may be antigen presentation dysfunction in CA skin lesions, which may be the reason for the prolonged and unresolved condition of the disease. The fewer CD207 positive cells in CA skin lesions, the longer the disease course and the more frequent the recurrence, therefore, the expression level of CD207 can be used as a new prognostic marker for predicting the outcome of CA.
Keywords: condyloma acuminata, CD207, langerhans cells, prognosis, biomarker
Introduction
Condyloma Acuminata (CA) is a common sexually transmitted disease caused by human papillomavirus (HPV) infection. It is characterized by wart-like growths on the skin and mucous membranes of the genital and anal areas. CA is prone to recurrence after treatment and is difficult to cure in the short term. Currently, there is a lack of effective biomarkers in clinical practice that can predict its prognosis.1,2
HPV differs from many other viral infections in that it primarily enters the body through infection of the skin or mucous membranes and only replicates (reproduces) within terminally differentiated skin or mucosal epithelial cells. This means that the immune response at these sites is particularly important for resisting infection.3,4 Langerhans cells (LCs) are a member of the dendritic cell (DC) family, located in the stratified squamous epithelium and mucosal tissue of the skin. They were the first known professional antigen-presenting cells (APCs). LCs play an important role in viral infections of the skin and mucous membranes because they are located in the outer layer of the skin and mucous membranes, which are the entry points for viruses such as human immunodeficiency virus (HIV), herpes simplex virus (HSV), varicella-zoster virus (VZV), and human papillomavirus (HPV).LCs can form a tight network structure between each other and with the surrounding keratinocytes through the adhesion molecule E-cadherin, which allows them to resist and monitor the invasion of pathogenic microorganisms.5 CD207 protein (also known as langerin protein) is encoded by the CD207 gene and is a type of C-type lectin receptor that is specifically expressed on the surface of LCs. It is one of the specific immunohistochemical markers of LCs.6
This study compared the differences in the number of CD207-expressing cells between the skin lesions of male patients with CA and a healthy control group using immunohistochemical techniques. The study also investigated the correlation between the number of CD207-positive cells in skin lesions and the duration of CA, as well as the frequency of CA recurrence.7,8 These findings provide a new biomarker for clinical doctors to predict the prognosis of CA.
Materials and Methods
This study was approved by the Clinical Medical Ethics Committee of the Affiliated Hospital of Weifang Medical University, and written informed consent was obtained from each patient before inclusion. The skin lesions of 40 male patients with CA were collected from outpatient clinics of the Department of Dermatology and Venereology at Weifang Medical University Affiliated Hospital between May 2021 and December 2021, and met the diagnostic criteria for CA.9 Exclusion criteria: females (to avoid the influence of sex hormone fluctuations on skin lesions), recurrent CA, tissue pathology not consistent with the diagnosis of CA, concomitant with other local infections or sexually transmitted diseases, concomitant with chronic severe infections (such as HIV, etc.), concomitant with severe systemic diseases (such as diabetes, organ transplantation, malignant tumors, autoimmune diseases, etc.), recent use of glucocorticoids or immunosuppressants.
The CA group consisted of 40 patients aged between 16 and 35 years old, with a mean age of (24.15±5.49) years old, including 15 patients (37.5%) under the age of 21, 17 patients (42.5%) aged between 21 and 30, and 8 patients (20.0%) over the age of 30. The duration of the disease in the patients ranged from 5 days to 4.1 weeks. The locations of the lesions were as follows: glans penis in 9 cases (22.5%), urethral meatus in 2 cases (5.0%), penile shaft and adjacent areas in 12 cases (30.0%), inner and outer prepuce in 11 cases (27.5%), and perianal skin and/or rectum in 6 cases (15%). The 40 male participants in the healthy control group were matched in age with the CA group and had normal foreskin specimens collected after circumcision, with no verrucous changes in tissue pathology and negative HPV nucleic acid detection. All patients received electrosurgical treatment, which completely removed the warts using an electrosurgical device, reaching a depth of the superficial dermis and expanding 2–3mm around the wart. If a recurrence occurred during follow-up, the patient received treatment again until they were considered cured after 12 weeks without recurrence.
Immunohistochemistry
Before the initial treatment, a sterile surgical scissors was used to cut a skin lesion of approximately 0.4*0.2*0.2cm3 in size, and 30% ferric chloride solution was used to stop bleeding by compression with a cotton swab. The specimen was immediately placed in 10% neutral formaldehyde solution and fixed for 12 hours, followed by ethanol dehydration, xylene transparency, and paraffin embedding to make a wax block. The wax block was sliced into 4μm thickness sections, baked at 70°C for 60 minutes, deparaffinized with xylene, and sequentially treated with 100%, 90%, 80%, 70% ethanol and distilled water. Tris-EDTA antigen repair solution (pH=8.0) was used for high pressure antigen repair. 3% H2O2 was soaked for 5 minutes to block endogenous peroxidase, followed by incubation with goat serum for 10 minutes and then addition of CD207 antibody (mouse anti-human monoclonal antibody, product number: MAB-0633, clone number: 12D6, Fuzhou Maixin Biotechnology Development Co., Ltd.), incubated at 37°C for 1 hour. The tissue sections were washed repeatedly with phosphate-buffered saline (PBS), and then reacted with enhanced reagent and horseradish peroxidase-labeled sheep anti-mouse IgG polymer (universal two-step immunohistochemistry test kit, product number: PV-9000, Beijing Zhongshan Golden Bridge Biotechnology Co, Ltd. at 37°C for 20 minutes. After repeated washing with PBS, DAB color development was performed (DAB color development kit, product number: ZLI-9018, Beijing Zhongshan Golden Bridge Biotechnology Co, Ltd.). The sections were counterstained with hematoxylin for 5 minutes, blued with ammonia water, dehydrated with ethanol, and then made transparent with xylene before being sealed with neutral resin. The morphology of CD207 positive cells in the epidermis of CA skin lesions and the number of CD207 positive cells in each high-power field (400X) were observed under a microscope.
Detection of HPV Genotypes by PCR Hybridization Method
Before the initial treatment, collect the exfoliated cells from the surface of the lesion using a specialized brush. Incubate the exfoliated cells in Tris-TEDA buffer (pH=8.0) containing proteinase K at 55°C for 10 minutes, then centrifuge at 4000rpm for 1 minute to collect the supernatant as the PCR template. Use an HPV genotyping test kit (catalog number: PW33221, Chaozhou Hybribio Biochemistry Ltd.) for PCR amplification and hybridization detection of HPV genomic DNA. Mix 2μL of PCR template with 23μL of PCR amplification reagent and perform amplification in a PCR tube through denaturation, annealing, and extension (40 cycles). Take 25μL of PCR product for hybridization detection and color development using a medical nucleic acid molecular rapid hybridization instrument (model HHM-2, Chaozhou Hybribio Biochemistry Ltd.) according to the instructions of the kit.
Statistical Methods
Data were analyzed using the SPSS statistical package, version 19.0 (SPSS Inc., Chicago, IL, USA) for Windows. Data were presented as mean±SD. Differences between groups were compared using an independent-samples t-test. Statistical significance was assumed as a P-value <0.05. The correlation between levels of CD207-positive cells in CA lesions and duration of CA and recurrence frequency was analyzed by Spearman’s rank correlation coefficient analysis; a P value <0.05 was considered statistically significant.
Results
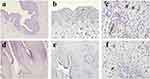
LCs in CA Lesions Undergo Morphological Changes
The histopathological presentation of normal skin tissue shows no obvious abnormalities (Figure 1a), while the histopathological presentation of condyloma acuminatum shows papillary growth of the epidermis with visible koilocytes (Figure 1d). In healthy foreskin tissue epidermis, a relatively large number of CD207-positive cells can be seen, mainly located in the upper part of the basal layer and the middle part of the spinous layer, with a relatively uniform distribution (Figure 1b); under high magnification, these cells have clear structural contours, with round or irregular-shaped cytoplasm and numerous slender dendritic processes, and secondary protrusions can be seen (Figure 1c). The number of CD207-positive cells in the epidermis of CA lesions is significantly reduced compared to normal foreskin tissue, mainly located in the lower part of the spinous layer (Figure 1e), and even disappeared in some patients’ lesions; under high magnification, the structural contours of these cells are not clear, with round cytoplasm, significantly reduced or even absent protrusions (Figure 1f). This indicates that there are obvious morphological abnormalities of CD207-positive cells in CA lesions.
The Number of CD207-Positive Cells is Downregulated in Condyloma Acuminatum Lesions
In each 400-fold field of view, there were approximately 25.30±7.60 CD207-positive cells in the epidermis of healthy foreskin tissue, while there were approximately 4.90±3.24 CD207-positive cells in the epidermis of condyloma acuminatum lesions. It shows that the number of CD207-positive cells in CA lesions is significantly lower than that in healthy foreskin tissue (t=5.28, P<0.05).
The Number of CD207 Positive Cells is Negatively Correlated with the Course of Acuteness Condyloma Acuminata and the Number of Recurrences
After follow-up, the course of the 40 patients with CA ranged from 6 to 56 weeks, with an average of 28.35±13.87 weeks, and the number of recurrences ranged from 2 to 25 times, with an average of 8.80±5.90 times. Spearman’s rank correlation coefficient analysis showed that the number of CD207 positive cells was significantly negatively correlated with the length of CA course (r=−0.75, P<0.05) and the number of CA recurrences (r=−0.74, P<0.05). The results are shown in Figure 2. This suggests that in the male population, the more CD207 cells there are in the initial lesions of CA, the better the prognosis, and CD207 may become a new prognostic marker for CA.
The Number of CD207-Positive Cells is Not Related to HPV Typing
Among the 40 CA patients tested, HPV subtypes included 6, 11, 16, 18, 31, 43, 51, 52, 53, and 55. Among them, 29 cases were low-risk types (72.5%) and 11 cases were high-risk types (27.5%). The number of CD207-positive cells in CA skin lesions caused by low-risk HPV types was 4.93±3.28, and in lesions caused by high-risk HPV types was 4.82±3.13, with no statistical difference between the two groups (t=0.10, P>0.05).
Discussion
Condyloma acuminatum, also known as genital warts, are a common sexually transmitted infection caused by human papillomavirus (HPV) infection. HPV is a DNA virus with over 100 known types, of which around 30 can infect human skin and mucous membranes. Genital warts are mostly caused by HPV types 6 and 11, but a minority of cases may be caused by other types such as HPV 16 and 18.10 The virus is primarily transmitted through sexual contact. The occurrence and recurrence of genital warts are related to persistent infection with human papillomavirus (HPV).11 After HPV infection, the immune system produces an immune response against the virus, and most infections are cleared within several months. However, in some patients, the virus persists and leads to the occurrence and recurrence of genital warts. This may be related to a decrease in the patient’s immune function, especially a decrease in cell-mediated immunity and defects in the body’s immune surveillance function. Therefore, maintaining a good immune status and enhancing immune function are important for preventing and treating CA.12,13 LC is a special type of dendritic cell that originates from hematopoietic stem cells in bone marrow. As LC is located in the epidermis and mucous membranes of the body, which are targets of invasion for some viruses, such as Human Immunodeficiency Virus, Herpesvirus, and Human Papillomavirus, LC plays an important role in viral infections of the skin and mucous membranes. LC can form a tight network structure between themselves and the surrounding keratinocytes through the adhesion molecule E-cadherin, to resist and monitor the invasion of pathogenic microorganisms. Once foreign antigens and pathogens breach the physical and biochemical barriers of the body and come into contact with LC dendrites, LC will quickly mature and undergo a series of phenotypic changes, such as upregulation of the major histocompatibility complex (MHC) class I and II molecules, as well as the co-stimulatory molecules CD80, CD86, CD40, and the acquired expression of CD83 on the surface of LC. The upregulated expression of the lymph node homing receptor CCR7 mediates the migration of LC to the local lymph node via afferent lymphatics, where they continue to mature and secrete corresponding chemokines and cytokines, and their antigen-presenting ability is continually enhanced. Therefore, LC not only plays a role as a “sentinel” cell in defending against pathogenic microorganisms, but also serves as an important bridge connecting innate immunity and adaptive immunity.14,15 In the normal immune state, when the skin is infected with a virus, the number and density of LCs in the epidermis increase in response to the infection, forming an expanding network through their dendritic structures to recognize viral antigens.16,17 When antigens bind to the surface receptor Langerin (CD207) of LCs and enter the cell, they combine with Birbeck granules and are expressed on the surface of LCs through the special structure channel of CD1a, and the antigen is presented to T helper cells, which are activated by co-stimulatory factors, thereby initiating an immune response.18,19
In this study, we found that the number of LCs in the condyloma acuminatum lesions decreased instead of increasing, and their morphology changed significantly, with a smaller size, a rounder shape, and fewer or even absent branches. This morphological change affects the structure of the LCs’ expanding network, thereby affecting their function. In terms of distribution, the distribution of LCs in CA tissue is biased toward the lower epidermal layer compared to normal tissue, while the viral antigens of HPV are only expressed in the upper layer of terminally differentiated skin or mucosal epithelial cells, making it difficult for LCs to effectively contact antigens and further weaken their antigen presentation ability. Therefore, we speculate that LCs undergo abnormal changes in morphology, number, distribution, and so on in CA, which affects their ability to effectively present HPV antigens in the epidermis to immune-active cells, leading to the persistence of HPV infection and easy recurrence.
From the process of LC uptake, processing, and presentation of antigens described above, we can see that CD207 plays an important role. CD207 protein (also known as langerin protein) is encoded by the CD207 gene and is a type of C-type lectin receptor that is specifically expressed on the surface of LCs. CD207 protein contains extracellular neck region, transmembrane region, intracellular portion, and carbohydrate recognition domain (CRD), which can preferentially bind to glycosylated compounds containing mannose, N-acetylglucosamine, and fucose, thereby playing a role in recognizing and engulfing antigens into LC cells.20,21 Currently, research has found that CD207 plays an important role in a series of non-classical antigen processing processes, such as forming Birbeck granules and processing and transporting them to local lymphoid tissue, and inducing LC maturation.22 This study found that the number of CD207-positive LCs in CA tissue was significantly reduced, indicating that there are abnormalities in the multiple functions of LCs in processing and presenting HPV antigens. Spearman correlation analysis showed that the fewer CD207-positive cells in CA lesions, the longer the patient’s course and the more frequent the recurrence. We believe that this may be due to LC dysfunction.
Conclusion
In summary, there are significant abnormalities in the number and morphology of CD207-positive LC in condyloma acuminata lesions. The number of CD207-positive cells in the lesions could potentially serve as a new biomarker for determining the length of the disease course and the frequency of recurrence.
Ethics Approval and Consent to Participate
This study was conducted with approval from the Clinical Medical Ethics Committee of the Affiliated Hospital of Weifang Medical University. This study was conducted in accordance with the declaration of Helsinki. Written informed consent has been obtained prior to the commencement of the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Research reported in this publication was supported by Traditional Chinese Medicine Science and Technology Project of Shandong Province (No. 2021Q093), Doctoral Startup Fund of Affiliated Hospital of Weifang Medical University (No. 2021BKQ02) and Weifang Science and Technology Development Program (No. 2021YX036).
Disclosure
The authors have no relevant financial or non-financial interests to disclose.
References
1. Clark DP. Condyloma acuminatum. Dermatol Clin. 1987;5(4):779–788. doi:10.1097/SGA.0b013e3181b85d4e
2. Oriel JD. Genital warts. Sex Transm Dis. 1981;8(4 suppl):326–329.
3. Arany I, Evans T, Tyring SK. Tissue specific hpv expression and downregulation of local immune responses in condylomas from hiv seropositive individuals. Sex Transm Infect. 1998;74(5):349–353. doi:10.1136/sti.74.5.349
4. Dunne EF, Park IU. Hpv and hpv-associated diseases. Infect Dis Clin North Am. 2013;27(4):765–778. doi:10.1016/j.idc.2013.09.001
5. Bennett CL, Ambler CA. Editorial: langerhans cells and how skin pathology reshapes the local immune environment. Front Immunol. 2019;10:139. doi:10.3389/fimmu.2019.00139
6. Raaby L, Rosada C, Langkilde A, et al. Langerhans cell markers cd1a and cd207 are the most rapidly responding genes in lesional psoriatic skin following Adalimumab treatment. Exp Dermatol. 2017;26(9):804–810. doi:10.1111/exd.13304
7. Mcardle JP, Muller HK. Quantitative assessment of langerhans’ cells in human cervical intraepithelial neoplasia and wart virus infection. Am J Obstet Gynecol. 1986;154(3):509–515. doi:10.1016/0002-9378(86)90592-2
8. Morelli AE, Ronchetti RD, Secchi AD, et al. Assessment by planimetry of langerhans’ cell density in penile epithelium with human papillomavirus infection: changes observed after topical treatment. J Urol. 1992;147(5):1268–1273. doi:10.1016/s0022-5347(17)37538-9
9. Stone KM. Human papillomavirus infection and genital warts: update on epidemiology and treatment. Clin Infect Dis. 1995;20(Suppl 1):S91–97. doi:10.1093/clinids/20.supplement_1.s91
10. Castellsague X. Natural history and epidemiology of hpv infection and cervical cancer. Gynecol Oncol. 2008;110(3 Suppl 2):S4–7. doi:10.1016/j.ygyno.2008.07.045
11. Chelimo C, Wouldes TA, Cameron LD, et al. Risk factors for and prevention of human papillomaviruses (hpv), genital warts and cervical cancer. J Infect. 2013;66(3):207–217. doi:10.1016/j.jinf.2012.10.024
12. Ao C, Zeng K. The role of regulatory t cells in pathogenesis and therapy of human papillomavirus-related diseases, especially in cancer. Infect Genet Evol. 2018;65:406–413. doi:10.1016/j.meegid.2018.08.014
13. Park IU, Introcaso C, Dunne EF. Human papillomavirus and genital warts: a review of the evidence for the 2015 centers for disease control and prevention sexually transmitted diseases treatment guidelines. Clin Infect Dis. 2015;61(Suppl 8):S849–855. doi:10.1093/cid/civ813
14. Feng JY, Peng ZH, Tang XP, et al. Immunohistochemical and ultrastructural features of langerhans cells in condyloma acuminatum. J Cutan Pathol. 2008;35(1):15–20. doi:10.1111/j.1600-0560.2007.00763.x
15. Pan YZ, Wang HL, Wang F, et al. Changes in distribution and ultrastructure of langerhans cells in condyloma acuminatum tissues, and analysis of the underlying mechanism. Dermatologica Sinica. 2013;31(3):120–125. doi:10.1016/j.dsi.2012.12.003
16. Da Silva DM, Woodham AW, Skeate JG, et al. Langerhans cells from women with cervical precancerous lesions become functionally responsive against human papillomavirus after activation with stabilized poly-i:C. Clin Immunol. 2015;161(2):197–208. doi:10.1016/j.clim.2015.09.003
17. Ruco LP, Uccini S, Baroni CD. The langerhans’ cells. Allergy. 1989;44(Suppl 9):27–30. doi:10.1111/j.1398-9995.1989.tb04312.x
18. Koch S, Kohl K, Klein E, et al. Skin homing of langerhans cell precursors: adhesion, chemotaxis, and migration. J Allergy Clin Immunol. 2006;117(1):163–168. doi:10.1016/j.jaci.2005.10.003
19. Maarifi G, Czubala MA, Lagisquet J, et al. Langerin (cd207) represents a novel interferon-stimulated gene in langerhans cells. Cell Mol Immunol. 2020;17(5):547–549. doi:10.1038/s41423-019-0302-5
20. Stambach NS, Taylor ME. Characterization of carbohydrate recognition by langerin, a c-type lectin of langerhans cells. Glycobiology. 2003;13(5):401–410. doi:10.1093/glycob/cwg045
21. Van Dalen R, La Cruz Diaz JS D, Rumpret M, et al. Langerhans cells sense staphylococcus aureus wall teichoic acid through langerin to induce inflammatory responses. mBio. 2019;10(3):
22. Thornton SM, Samararatne VD, Skeate JG, et al. The essential role of anxa2 in langerhans cell birbeck granules formation. Cells. 2020;9(4):974. doi:10.3390/cells9040974
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.