Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
CD133, but Not CD44, May Serve as a Novel Biomarker for Differential Diagnosis Between Basal Cell Carcinoma and Trichoblastomas
Authors Bi Y, Shi X, Chen D, Zhao Y
Received 2 June 2022
Accepted for publication 20 July 2022
Published 2 August 2022 Volume 2022:15 Pages 1517—1526
DOI https://doi.org/10.2147/CCID.S373331
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Yalan Bi,1 Xiaohua Shi,2 Dian Chen,1 Yi Zhao1
1Department of Dermatology, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University, Beijing, People’s Republic of China; 2Department of Pathology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China
Correspondence: Yi Zhao, Department of Dermatology, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University, No. 168 Litang Road, Changping District, Beijing, People’s Republic of China, Tel/Fax +86 010 56119127, Email [email protected]
Purpose: To investigate the clinical value of CD133 and CD44 as putative cancer stem cell markers in distinguishing between basal cell carcinoma (BCC) and trichoblastomas (TB).
Patients and Methods: Tumor samples from 24 BCC and 23 TB patients were retrospectively retrieved for immunohistochemical staining of CD133 and CD44. The results were interpreted using a semiquantitative scoring system (H score). A receiver operating characteristic (ROC) curve was developed to identify an optimal cutoff value for differentiating between BCC and TB.
Results: Expression of CD133 was significantly higher in BCC patients than in TB patients (median H score: 30 [IQR: 12.5– 56.3] vs 0 [IQR: 0– 2], P < 0.001). However, there was no significant difference in CD44 expression between the two groups (median H score: 105 [IQR: 63.8– 155.0] vs 60 [IQR: 30– 120], P = 0.095). The ROC analysis of CD133 immunostaining yielded an area under the curve (AUC) of 0.881 (95% CI: 0.756– 1.000) for differentiating between BCC and TB by using a H score of 7 as the cut-off value (98.5% sensitivity and 87.0% specificity). By contrast, immunostaining of CD44 showed a lower diagnostic value, with an AUC of 0.642 (95% CI: 0.476– 0.808) at the optimal cut-off value of 85 (62.5% sensitivity and 73.9% specificity). The positive and negative predictive values were 88.5% and 95.2% for CD133 and 71.4% and 65.4% for CD44, respectively. Additionally, CD133 expression was significantly associated with mitotic activity in BCC patients (r = 0.549, P = 0.005).
Conclusion: Our study expanded upon previous studies of CD133 and CD44 expressions in skin tumors, suggesting that CD133, but not CD44, may serve as a novel biomarker for differential diagnosis of BCC, although future studies using a larger number of patients are needed to justify it further.
Keywords: basal cell carcinoma, BCC, trichoblastomas, TB, differential diagnosis, CD133, CD44, immunohistochemistry, receiver operating characteristic, ROC
Introduction
Basal cell carcinoma (BCC) is the most common epithelial malignancy with an increasing incidence.1 It has been historically reported to associate with male sex and middle or elder age. However, there seems to be an increasing trend toward younger and female individuals,2,3 representing a growing public healthcare burden. Surgical excision is the primary treatment for BCC. However, despite the slow-growing nature with rare metastasis, delayed or inadequate treatment of BCC may cause significant morbidity that destroys facial sensory organs (like nose, ear, lips, and eyelids). Therefore, early definitive diagnosis of BCC is of major importance.4
Diagnosis of BCC is generally straightforward on dermatopathology practice. Although substantial efforts have been made to develop novel tools, such as reflectance confocal microscopy, histopathological diagnosis is still considered golden standard.5 However, the overlapping histopathologic features of BCC with benign follicular tumors can be challenging for definitive diagnosis. The misdiagnosis may lead to either unnecessary excision or delays in workup and appropriate treatment. Differential diagnosis of BCC from its potential histologic mimics is thus critical. Trichoblastomas (TB), a benign, hair-germ tumor, is such a histologic mimic of BCC.6 Immunohistochemistry has been studied to differentiate TB from BCC. Immunohistochemical biomarkers, such as CK20, androgen receptor (AR), and PHLDA1 (a follicular stem-cell marker), have been reported to be useful in discriminating BCC from TB.7–9 However, no single biomarker appears to be sensitive or specific enough for this distinction,10 and thus identification of novel biomarkers to help distinguish between BCC and TB is of major clinical significance.
Cancer stem cells have been suggested to play a critical role in tumor initiation and maintenance in various cancers.11–15 As putative cancer stem cell markers, CD133 and/or CD44 expressions were found to be helpful in distinguishing between benign and malignant lesions.16,17 In skin cancers, both CD133 and CD44 were identified as potential cancer stem cell markers and associated with invasive cancer subtypes.18–23 In this study, we hypothesize that CD133 and CD44 may be differentially expressed in BCC and TB. Immunohistochemical staining for CD133 and CD44 was therefore performed to explore their differential diagnostic value for BCC.
Patients and Methods
Patients
This retrospective study included 24 BCC and 23 TB patients. All consecutive patients with BCC or TB were retrieved from the Beijing Tsinghua Changgung Hospital affiliated to Tsinghua University and Peking Union Medical College Hospital between January 2018 and January 2021. Electronic medical records of patients were retrospectively reviewed. Patients with previous or concurrent malignancies were excluded. We also excluded those with incomplete data or inadequate pretreated tumor samples. Hematoxylin and eosin sections were reassessed to confirm the diagnosis according to the WHO criteria.24 Histological classification of BCC was further grouped into high and low risk of recurrence. The pretreated tumor samples were used for immunohistochemical analysis.
This study was approved by the Institutional Ethical Review Board of the Tsinghua Changgung Hospital and Peking Union Medical College Hospital. The study was conducted in accordance with the Declaration of Helsinki (2013), the International Ethical Guidelines of International Organizations of Medical Sciences (CIOMS), and the Ethical review of biomedical research involving human beings in China (2016). Written informed consent was waived due to the retrospective nature of study design. All data were maintained with confidentiality to ensure the privacy of participants.
Immunohistochemistry
Tumor sections of 4-μm-thickness were used for immunohistochemical analysis. Briefly, the de-paraffinized and rehydrated sections were pretreated with Tris/EDTA buffer pH 9.0 for heat mediated antigen retrieval. Endogenous peroxidase was blocked with hydrogen peroxidase (H2O2). The sections were then incubated with rabbit anti-human CD133 monoclonal antibody (cat no. ab222782, 1:000, Abcam) or rabbit anti-human CD44 monoclonal antibody (cat no. ab189524, 1:4000, Abcam) overnight at 4°C, followed by horseradish peroxidase (HRP)-conjugated anti-rabbit/mouse IgG secondary antibodies. Immunoreactivity was visualized with 3,3’-diaminobenzidine (DAB) (Power-stainTM 1.0 polymer peroxidase system, Beijing Jinqiao Yatu Biological Technology Co., Ltd., Beijing, China). Sections from human bladder and breast cancers were stained as positive controls for CD133 and CD44, respectively. The primary antibodies were omitted to generate negative controls.
The stained slides were independently reviewed by two experienced pathologists (YL Bi and XH Shi) in a blind manner. Any disagreements were resolved by consensus. Immunoactivities of CD133 and CD44 were assessed by a semiquantitative scoring system based on staining intensity and percentage of positive cells in at least ten fields at low and high power. The staining intensity was graded as follows: 0 (negative: no staining); 1+ (weak: light yellow); 2+ (moderate: yellow brown); and 3+ (strong: brown). The percentage of positive tumor cells was estimated. A histochemical score (H score: 0–300) was then calculated by multiplying the intensity score and percentage of positive cells.
Statistical Analyses
Statistical analyses were performed with the SPSS software version 17.0 (SPSS, Chicago, IL, USA). Continuous variables were expressed as mean ± standard deviation (SD) or median and interquartile range (IQR) and compared with the Student’s t-test or Mann–Whitney U-test. Categorical variables were expressed as frequency and percentage and compared with the Chi-square test or Fisher exact test as appropriate. An area under the curve (AUC) of receiver operator characteristic (ROC) curve was calculated to identify an optimal cutoff value for differentiating between BCC and TB. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and overall accuracy of CD133 and CD44 expressions or their co-expression were calculated. The Spearman’s rank correlation test was then further performed to evaluate correlation of CD133 expression with clinicopathological characteristics in BCC patients.
Results
Clinicopathological Characteristics
Pretreated tumor samples were obtained from 47 patients, including 24 BCC and 23 TB. Clinicopathological characteristics of the patients are summarized in Table 1. The mean age was 64.1 (SD: 14.8) years for BCC patients and 52.2 (SD: 21.5) years for TB patients. There were 29 female and 18 male patients, with a female/male ratio of 1.2 (13/11) and 2.3 (16/7) in BCC and TB patients, respectively. Lesions in most patients were located on the face or scalp (BCC: 83.3% [20/24]; TB: 87.0% [20/23]) and confined to the dermis (BCC: 79.2% [19/24]; TB: 91.3% [21/23]). Almost half (45.8%) of patients in the BCC group developed skin ulcers, while few (8.7%) in the TB group. Besides, focal necrosis and atypical mitoses were also noted in most BCC patients. No patient had perineural involvement in either BCC or TB groups. Regarding histological classification of BCC, nodular type was the most common subtype, occurring in 62.5% (15/24) of patients. Six patients (25.0%) were observed to have high-risk subtypes.
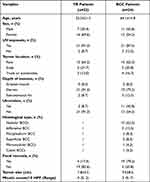 |
Table 1 Clinicopathological Characteristics of the Patients |
CD133 and CD44 Expressions and Their Differential Diagnostic Values
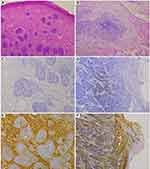
Representative imaging of CD133 and CD44 expression is shown in Figure 1. As summarized in Table 2, CD133 was positively (>1% tumor cells) stained with varying intensities in all 24 BCC patients, while only in 6 (26.1%) out of 23 TB patients. All the positively stained patients (24 BCC and 6 TB) were judged to have a moderate or strong intensity. The H score of CD133 immunostaining in BCC patients was significantly higher than in TB patients (median H score: 30 [IQR: 12.5–56.3] vs 0 [IQR: 0–2], P < 0.001, Figure 2A).
 |
Table 2 Immunohistochemical Analysis of CD133 and CD44 Expressions |
 |
Figure 2 Box plots of CD133 (A) and CD44 (B) expression based on a semiquantitative scoring system (H score). |
Unlike immunostaining pattern of CD133, CD44 was positively stained with varying intensities in both BCC and TB patients, and the staining intensity was moderate or strong in almost all patients irrespective of tumor types (BCC and TB) (Table 2). There was no significant difference in H scores between the two groups (median H score: 105 [IQR: 63.8–155.0] vs 60 [IQR: 30–120], P = 0.095, Figure 2B).
ROC analysis of CD133 immunostaining yielded an AUC of 0.881 (95% CI: 0.756–1.000) for differentiation between BCC and TB (Figure 3). The sensitivity was 98.5% and specificity was 87.0% by using a H score of 7 as the cut-off value. By contrast, immunostaining of CD44 showed a lower diagnostic value, with an AUC of 0.642 (95% CI: 0.476–0.808; 62.5% sensitivity and 73.9% specificity) at the optimal cut-off value of H score = 85. The positive and negative predictive values and overall accuracy are summarized in Table 3.
 |
Table 3 Diagnostic Value of CD133 and CD44 |
 |
Figure 3 A receiver operating characteristic curve developed to identify an optimal cutoff value for differentiating between BCC and TB. Abbreviations: BCC, basal cell carcinoma; TB, trichoblastomas. |
Besides, we also integrated CD133 and CD44 to explore their co-expression in distinguishing BCC from TB. As shown in Table 3, co-expression of CD133 and CD44 improved specificity at the cost of the sensitivity when compared with CD133 alone. The overall accuracy was 80.9%, lower than that of CD133 alone (91.9%), while higher than that of CD44 alone (68.1%).
Clinicopathological Correlation of CD133 Expression in BCC Patients
Clinicopathological significance of CD133 expression in BCC patients was further explored. CD133 expression was found to associate significantly with mitotic activity (r = 0.549, P = 0.005, Figure 4A). Besides, patients with high-risk of recurrence according to the histological subtypes showed a comparatively higher level of CD133 expression when compared with their low-risk counterparts, although the difference did not reach the statistical significance (median H score: 45 [IQR: 15.5–78.8] vs 30 [IQR: 10.0–41.3], P = 0.119, Figure 4B). There was no significant correlation of CD133 expression with other clinicopathological characteristics of the patients (data not shown).
 |
Figure 4 Clinical correlation of CD133 expression with mitotic activity (A) and risk of recurrence (B) in BCC patients. Abbreviation: BCC, basal cell carcinoma. |
Discussion
As potential biomarkers of cancer stem cells in various cancers, CD133 and CD44 have been reported to show distinct expression patterns that are associated with aggressive tumor behavior in skin cancers.19,20 In the present study, we reported, for the first time, that immunostaining of CD133, while not CD44, may be helpful in differential diagnosis between BCC and TB. The CD133 expression in BCC patients was significantly higher when compared with that in TB patients. Moreover, CD133 expression was positively correlated with mitotic activity in BCC patients.
CD133 and CD44 have been suggested as potential stem cells biomarkers in various types of solid tumors.25,26 In previous studies of skin cancer, higher expressions of both CD133 and CD44 were noted in patients with melanoma when compared with those with cutaneous squamous cell carcinomas or BCC.19,20 In melanoma patients, CD133 expression was found to be significantly associated with increased tumor invasiveness and metastasis, as well as skin ulceration.19 Meanwhile, a higher expression of CD44 was associated with tumor recurrence in melanoma and ulceration in cutaneous squamous cell carcinomas.20 The findings of this study showed a significant higher expression of CD133 in BCC patients when compared with that in TB patients. Also, the higher CD133 expression was significantly associated with increased mitotic activity in BCC patients. Conversely, we did not observe significant difference in CD44 expression between the BCC and TB groups. These results suggest that CD133 expression may provide as an additional marker for differential diagnosis between BCC and TB. Also, the high expression of CD133 may associate with the aggressive tumor behavior of BCC, although the underlying mechanisms are largely unknown.
One thing worth noting is that our study seems to report comparatively higher levels of CD133 and CD44 expressions. CD133 was found to be positively stained in 95.8% (23/24) of the BCC patients and 13.0% (3/23) of the TB patients when ROC curve was created to select the optimal threshold value. Correspondingly, CD44 was found in 62.5% (15/24) and 26.1% (9/23) of the patients, respectively. In the previous study of BCC patients, CD133 was found to be highly expressed in 13 (24%) out of 53 patients by using a mean H score of 89 as the cutoff value.19 Meanwhile, CD44 was reported to be highly expressed in 11 (19%) of 58 patients when using a mean H score of 21 as the cutoff value.20 To exclude the potential influence of threshold values selected, mean H scores of 10 and 80 were further used herein to explore the CD133 and CD44 expressions in BCC and TB patients. The results were not dramatically changed. The distinct staining properties observed may be partially explained by the fact that different study populations were included for analysis. Also, the differences in immunostaining platforms plus primary antibody clones used, and criteria for immunohistochemistry interpretation (scoring approaches and cutoff value determinations), as well as intra-observer reproducibility may matter.27,28 Nevertheless, immunohistochemistry has been widely accepted as an invaluable adjunct to morphologic diagnosis. In this study, antibodies against CD133 and CD44 were all obtained from the Abcam company (Cambridge, UK). Besides, a semiquantitative scoring system based on the staining intensity and percentage of positively stained cells was applied to evaluate CD133 and CD44 expressions. Also, to minimize the interobserver variability, two experienced pathologists (>10 years working experiences) were required to review the slides independently. Moreover, the ROC curve was used to calculate the optimal cutoff values. Taken all these together, this study expanded previous investigation to CD133 and CD144 expressions in BCC and TB. The findings of our study showed the distinct differential expressions of CD133 in BCC and TB patients. Future efforts to characterize the expression patterns of CD133 and CD44 and their differential diagnostic values are therefore warranted.
This study also has some limitations. Primarily, the retrospective nature of study design and a small number of patients analyzed, the potential selection bias and confounding effects could not be ruled out, especially for subgroup analysis of CD133 expression with respect to high- and low-risk BCC. Also, independent validation was not carried out due to the small sample size. Still, only the CD133 and standard CD44 were analyzed in this study due to the limited tumor samples. Future studies are therefore indispensable to validate the results observed and to explore the potential diagnostic and prognostic values of CD133 expressions among BCC patients with different histological subtypes.
In conclusion, our study expanded upon previous studies of CD133 and CD44 expression in patients with skin tumors, suggesting that CD133, but not CD44, may serve as a novel biomarker for differential diagnosis between BCC and TB. However, due to the small sample size and retrospective nature of study design, future studies using a larger number of patients are indispensable to justify it further.
Ethics Approval and Informed Consent
This study was approved by the Institutional Ethical Review Board of the Tsinghua Changgung Hospital and Peking Union Medical College Hospital. The study was conducted in accordance with the Declaration of Helsinki (2013), the International Ethical Guidelines of International Organizations of Medical Sciences (CIOMS), and the Ethical review of biomedical research involving human beings in China (2016). Written informed consent was waived due to the retrospective nature of study design. All data were maintained with confidentiality to ensure the privacy of participants.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lai V, Cranwell W, Sinclair R. Epidemiology of skin cancer in the mature patient. Clin Dermatol. 2018;36(2):167–176. doi:10.1016/j.clindermatol.2017.10.008
2. Flohil SC, Seubring I, van Rossum MM, Coebergh JW, de Vries E, Nijsten T. Trends in basal cell carcinoma incidence rates: a 37-year Dutch observational study. J Invest Dermatol. 2013;133(4):913–918. doi:10.1038/jid.2012.431
3. Birch-Johansen F, Jensen A, Mortensen L, Olesen AB, Kjær SK. Trends in the incidence of nonmelanoma skin cancer in Denmark 1978–2007: rapid incidence increase among young Danish women. Int J Cancer. 2010;127(9):2190–2198. doi:10.1002/ijc.25411
4. Hoorens I, Vossaert K, Ongenae K, Brochez L. Is early detection of basal cell carcinoma worthwhile? Systematic review based on the WHO criteria for screening. Br J Dermatol. 2016;174(6):1258–1265. doi:10.1111/bjd.14477
5. Patalay R. Which method is better for the diagnosis of basal cell carcinoma: biopsy vs. reflectance confocal microscopy? Br J Dermatol. 2021;184(4):590. doi:10.1111/bjd.19571
6. Cazzato G, Cimmino A, Colagrande A, et al. The multiple faces of nodular trichoblastoma: review of the literature with case presentation. Dermatopathology. 2021;8(3):265–270. doi:10.3390/dermatopathology8030032
7. Evangelista MT, North JP. Comparative analysis of cytokeratin 15, TDAG51, cytokeratin 20 and androgen receptor in sclerosing adnexal neoplasms and variants of basal cell carcinoma. J Cutan Pathol. 2015;42(11):824–831. doi:10.1111/cup.12546
8. Tebcherani AJ, de Andrade HF
9. Sellheyer K, Nelson P, Kutzner H, Patel RM. The immunohistochemical differential diagnosis of microcystic adnexal carcinoma, desmoplastic trichoepithelioma and morpheaform basal cell carcinoma using BerEP4 and stem cell markers. J Cutan Pathol. 2013;40(4):363–370. doi:10.1111/cup.12085
10. Stanoszek LM, Wang GY, Harms PW. Histologic mimics of basal cell carcinoma. Arch Pathol Lab Med. 2017;141(11):1490–1502. doi:10.5858/arpa.2017-0222-RA
11. Liu YC, Yeh CT, Lin KH. Cancer stem cell functions in hepatocellular carcinoma and comprehensive therapeutic strategies. Cells. 2020;9(6):1331. doi:10.3390/cells9061331
12. O’Flaherty JD, Barr M, Fennell D, et al. The cancer stem-cell hypothesis: its emerging role in lung cancer biology and its relevance for future therapy. J Thorac Oncol. 2012;7(12):1880–1890. doi:10.1097/JTO.0b013e31826bfbc6
13. Razmi M, Ghods R, Vafaei S, Sahlolbei M, Saeednejad Zanjani L, Madjd Z. Clinical and prognostic significances of cancer stem cell markers in gastric cancer patients: a systematic review and meta-analysis. Cancer Cell Int. 2021;21(1):139. doi:10.1186/s12935-021-01840-z
14. Yu SS, Cirillo N. The molecular markers of cancer stem cells in head and neck tumors. J Cell Physiol. 2020;235(1):65–73. doi:10.1002/jcp.28963
15. Kokuryo T, Yokoyama Y, Nagino M. Recent advances in cancer stem cell research for cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2012;19(6):606–613. doi:10.1007/s00534-012-0542-6
16. Tse GM, Tan PH, Ma TK, Gilks CB, Poon CS, Law BK. CD44s is useful in the differentiation of benign and malignant papillary lesions of the breast. J Clin Pathol. 2005;58(11):1185–1188. doi:10.1136/jcp.2005.026906
17. Lin CH, Liu CH, Wen CH, Ko PL, Chai CY. Differential CD133 expression distinguishes malignant from benign papillary lesions of the breast. Virchows Arch. 2015;466(2):177–184. doi:10.1007/s00428-014-1695-2
18. Milosevic M, Lazarevic M, Toljic B, et al. Characterization of stem-like cancer cells in basal cell carcinoma and its surgical margins. Exp Dermatol. 2018;27(10):1160–1165. doi:10.1111/exd.13755
19. Sabet MN, Rakhshan A, Erfani E, Madjd Z. Co-expression of putative cancer stem cell markers, CD133 and Nestin, in skin tumors. Asian Pac J Cancer Prev. 2014;15(19):8161–8169. doi:10.7314/apjcp.2014.15.19.8161
20. Erfani E, Roudi R, Rakhshan A, Sabet MN, Shariftabrizi A, Madjd Z. Comparative expression analysis of putative cancer stem cell markers CD44 and ALDH1A1 in various skin cancer subtypes. Int J Biol Markers. 2016;31(1):e53–61. doi:10.5301/jbm.5000165
21. Madjd Z, Erfani E, Gheytanchi E, Moradi-Lakeh M, Shariftabrizi A, Asadi-Lari M. Expression of CD133 cancer stem cell marker in melanoma: a systematic review and meta-analysis. Int J Biol Markers. 2016;31(2):e118–125. doi:10.5301/jbm.5000209
22. Kang SG, Kim CH, Kim SK, Choi BR, Cho MK. Expression of CD133, CD24, CD44 in cutaneous malignant tumors. Korean J Dermatol. 2008;46(6):742–748.
23. Xu R, Cai MY, Luo RZ, Tian X, Han JD, Chen MK. The expression status and prognostic value of cancer stem cell biomarker CD133 in cutaneous squamous cell carcinoma. JAMA Dermatol. 2016;152(3):305–311. doi:10.1001/jamadermatol.2015.3781
24. Elder DE, Scolyer RA, Willemze R. WHO Classification of Skin Tumours.
25. Senbanjo LT, Chellaiah MA. CD44: a multifunctional cell surface adhesion receptor is a regulator of progression and metastasis of cancer cells. Front Cell Dev Biol. 2017;5:18. doi:10.3389/fcell.2017.00018
26. Glumac PM, LeBeau AM. The role of CD133 in cancer: a concise review. Clin Transl Med. 2018;7(1):18. doi:10.1186/s40169-018-0198-1
27. Walker RA. Quantification of immunohistochemistry–issues concerning methods, utility and semiquantitative assessment I. Histopathology. 2006;49(4):406–410. doi:10.1111/j.1365-2559.2006.02514.x
28. Taylor CR, Levenson RM. Quantification of immunohistochemistry–issues concerning methods, utility and semiquantitative assessment II. Histopathology. 2006;49(4):411–424. doi:10.1111/j.1365-2559.2006.02513.x
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.