Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 14
CD103+ T Lymphocyte Count Linked to the Thickness of Invasion on Acral Melanoma without E-Cadherin Involvement
Authors Abidin FAZ , Usman HA , Suryanti S, Hernowo BS
Received 2 September 2021
Accepted for publication 30 October 2021
Published 24 November 2021 Volume 2021:14 Pages 1783—1790
DOI https://doi.org/10.2147/CCID.S334984
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Fauzan Ali Zainal Abidin, Hermin Aminah Usman, Sri Suryanti, Bethy S Hernowo
Department of Anatomical Pathology, Faculty of Medicine, Universitas Padjadjaran/Dr. Hasan Sadikin General Hospital, Bandung, Indonesia
Correspondence: Hermin Aminah Usman
Department of Anatomical Pathology, Faculty of Medicine, Universitas Padjadjaran/Dr. Hasan Sadikin General Hospital, Jl Pasteur No. 39, Bandung, West Java, 40161, Indonesia
Tel +62 22-2031447
Fax +62 22-2551126
Email [email protected]
Background: Acral melanoma (AM) is a malignancy that originates from melanocytes, located in an anatomical area without sun exposure, aggressive, resistant to chemotherapy, and quickly metastasize. The invasion capability of tumor cells is the main factor for metastasis in malignancy. E-cadherin is a marker of tumor progressivity that has an important role in the process of invasion. The responsibility of E-cadherin in the invasion process of AM is not well known. CD103 is an immune component found in the tumor microenvironment that contributes to melanoma progression control, whereas E-cadherin is the ligand for CD103.
Purpose: The objective of this research was to see if there was an association between E-cadherin and CD103 immunoexpression and the thickness of invasion in AM.
Materials and Methods: This is observational cross-sectional research. Formalin-fixed paraffin-embedded (FFPE) acral melanoma tissue samples were collected during 2014– 2020 at the Department of Anatomic Pathology, Dr. Hasan Sadikin Hospital, Bandung. A total of 40 samples were collected, including 20 cases of invasive melanoma less than 4 mm thickness and 20 cases of invasive melanoma greater than 4 mm thickness. All samples were immunostained with E-cadherin and CD103. Chi-Square test was used to examine the association concerning E-cadherin and CD103 with the thickness of invasion, respectively. The p-value of 0.05 was chosen as the significance level.
Results: This study showed an insignificant association between E-cadherin immunoexpression and the thickness of invasion on AM (p = 0.4272). CD103 immunoexpression had a significant association with the thickness of invasion on AM (p = 0.0001).
Conclusion: The findings revealed that CD103 in AM is associated with the thickness of invasion, and it may play important functions throughout the invasion process despite the uninvolvement of E-cadherin.
Keywords: CD103, E-cadherin, acral melanoma, invasion
Introduction
Melanoma is a kind of malignancy that develops from melanocytes, which produce melanin pigment.1,2 Melanoma cases in Asia have a poor prognosis and often cause death because these tumors have a very high ability to metastasize.1 Based on the 2018 World Health Organization (WHO) Skin Tumor Classification, melanoma is classified based on a multidimensional pathway. This classification divides melanoma into two significant groups, melanoma-associated with cumulative sun damage (CSD) and melanoma not associated with CSD/nonCSD melanoma. Acral melanoma (AM) belongs to the nonCSD melanoma group, which has distinct pathogenesis from CSD melanoma. The prognosis for AM is worse than the CSD melanoma subtype.1–3
The thickness of invasion in melanoma is an imperative factor in staging and prognosis.3,4 Teramoto et al stated that the thickness of invasion affects the survival rate of AM patients.5 Chang et al demonstrated that the thickness of invasion was one of the predictors of lymph node metastasis in AM.6 To date, the pathogenesis of the mechanism of invasion in AM is not well understood.
E-cadherin is a cell adhesion protein molecule and is classified into the type-1 transmembrane protein class. E-cadherin is known as a marker of progression to various malignancies through the ability of tumor cells to invade.7 Generally, low E-cadherin expression is associated with melanoma progression and metastasis. However, there are inconsistencies in other melanoma research findings, showing no relationship between E-cadherin and invasion.8 There are currently no studies that associate E-cadherin expression with the thickness of invasion in AM.7,8
A subset population of CD8+ T cells stays in the tissue and does not flow in the circulation. This subset is characterized by the phenotype of CD103+ CD8+ T Cells. The CD103 integrin in the CD8+ T cell population causes these T cells to remain in peripheral tissues. CD8+ CD103+ T cells are called resident memory (TRM) cells so that CD103 can be a biological marker for TRM cells.9 Some studies state that TRM cells perform a more important part in the anti-tumor activity and have prognostic value in several malignancies. CD103’s main ligand is E-cadherin,9 so it is hypothesized that E-cadherin and CD103 have a relationship in the context of the thickness of invasion in AM. It is currently unknown the role of CD103 concerning the invasion of AM.
The purpose of this study is to look at the relationship between E-cadherin and CD103 immunoexpression and the thickness of invasion in AM. The findings of this study should provide light on the pathogenesis of immune surveillance in AM.
Materials and Methods
Tissue Specimens
The current study used a cross-sectional observational analytic design. The inclusion and exclusion criteria were met by paraffin-embedded archival pathologic specimens from 40 patients with AM who received initial surgical resection between 2014 and 2020 at Dr. Hasan Sadikin General Hospital Bandung, Indonesia. Cases of AM that had been biopsied, excisioned, or surgically diagnosed histopathologically, the limit of tumor cell invasion that could be assessed well from the surface to the most profound limit, and comprehensive patient data were the inclusion criteria. The uncertain location of the paraffin block in the original tumor and the diminished tumor mass paraffin block were the exclusion criteria.
Ethics Statement
This work was evaluated and approved by Hasan Sadikin Hospital’s Research Ethics Committee (ethical clearance number: LB.02.01/X.6.5/123/2020) and was carried out in accordance with the Helsinki Declaration. Due to the research’s use of a bio-archive containing unlinked patient data, the ethics committee waived the patients’ informed permission. All data examined is anonymous, and patient information is protected.
Clinicopathological Characteristics
The following clinicopathological characteristics were examined: age, gender, Breslow thickness, Clark level, location of the lesions, ulceration, and degree of lymphocytic infiltration. The thickness of the invasion was assessed digitally using an Olympus BX5 light microscope with an Olympus XC10 digital camera attached. The software used is dotSlide version 2.0.
Immunohistochemical Staining
The One-Step Neopoly (Detection Kit Biogear Scientific, BioVentures, Inc., Iowa, USA) was used to perform immunohistochemical staining on all samples using the label method streptavidin-biotin immunoperoxidase complex. The sample was sliced 4 μm in length, then de-waxed with xylene and rehydrated with an alcohol solution-intake of antigens utilizing decloaking equipment for 45–60 minutes at 98°C.
The primary antibodies used were E-cadherin (mouse monoclonal, HECD-1 clone, Biocare) with 1:100 dilution and CD103 antibody (rabbit monoclonal, clone EP206, Cell Marque) with a 1:50 dilution.
Scoring
E-cadherin and CD103 immunoexpression were evaluated by two pathologists using a light microscope without knowing each case’s clinical and pathological data. The criteria assessed E-cadherin immunoexpression semiquantitatively based on the intensity and percentage of tumor cells showing expression (histoscore count). Tumor cells that showed positive E-cadherin immunoexpression (positive cell distribution) were when the tumor cell displaying membranous immunostaining in the following order: <25% of tumor cells (0) and ≥25% of tumor cells (1). The intensity of E-cadherin immunoexpression was scored in the following order: absent (0), weakly positive (1), moderate positive (2), and strongly positive (3). Then, the histoscore is calculated by multiplying the positive cell distribution and intensity. E-cadherin immunoexpression ≤1 histoscore was included in the “Low” category, and >1 immunoexpression histoscore results were included in the “High” category.
CD103 immunoexpression was assessed qualitatively by digital imaging using an Olympus BX5 microscope and an Olympus XC10 camera. The number of intratumoral T lymphocytes (TIL) with membranous immunostaining was counted to assess CD103 immunoexpression. The count was done manually in 5 large visual fields (400x) that were chosen at random in locations rich in intratumor lymphocytes. The CD103 + T lymphocyte count <20 cells fell into the “Low” category, and the CD103 + T lymphocyte count ≥ 20 cells fell into the “High” category.
Statistical Data Analysis
This study is an observational analysis with a cross-sectional design. The research sample was taken by consecutive sampling. Statistical analysis was using Chi-square. The significance of the data is obtained if the p-value <0.05. The acquired data were recorded in a specific format and then analyzed using the SPSS software version 24.0 for macOS (SPSS, Chicago, Illinois, USA).
Results
Patient Characteristics
The average age of AM patients was 58 years old, with 57.5% males and 42.5% females. In this investigation, the mean of invasion thickness was high (7.85±6.39 mm) with the number of Breslow thickness category T4 was 20, T3 was 18, T2 was 2, and no T1. This study included 40 AM cases, comprising 2 cases of subungual melanoma, 5 cases of palm melanoma, and 33 cases of pedis melanoma. Figure 1 depicts E-cadherin immunoexpression with weak, moderate, and strong intensities. The histoscore findings revealed that 14 patients (35%) had low expression of E-cadherin, and 26 had high expression (65%). Figure 2 depicts the CD103 + T lymphocyte count in the low and high categories. The results of histoscore showed that the number of patients with a low CD103 count was 19 (47.5%), and the high count was 21 (52.5%). Clark Categories II–III had 19 patients (47.5%), while IV–V had 21 patients (52.5%). Brisk tumor-infiltrating lymphocyte (TIL) patients included was 27 (67.5%) and non-brisk TIL was 13 patients (32.5%). The positive category was 21 (52.5%) for patients with ulceration, and the negative one was 19 (47.5%).
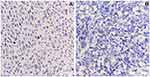 |
Figure 2 Result of CD103 immunohistochemical staining. Notes: (A) High immunoexpression of CD103 (magnification 400X). (B) Low immunoexpression of CD103 (magnification 400X). |
Table 1 shows the characteristics of the subjects.
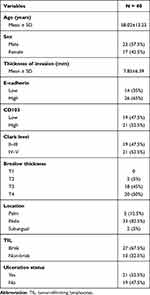 |
Table 1 Research Subject Characteristics |
Association of Clinical Patient Characteristics with the CD103 Immunoexpression
The data in Table 2 provide information regarding the association between clinical patient characteristics with the CD103 immunoexpression.
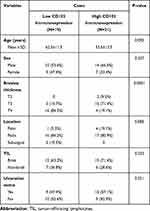 |
Table 2 Association of Clinical Patient Characteristics with the CD103 Immunoexpression |
Association of E-Cadherin Immunoexpression with the Thickness of Invasion in Acral Melanoma
The findings in Table 3 demonstrate that there is no association between E-cadherin immunoexpression and invasion thickness. It demonstrates that the statistical test result is not statistically significant (p = 0.4272).
 |
Table 3 Association of E-Cadherin Immunoexpression with the Thickness of Invasion in Acral Melanoma |
Association of CD103 Immunoexpression with the Thickness of Invasion in Acral Melanoma
The findings in Table 4 demonstrate that there is an association between CD103 immunoexpression and invasion thickness.
 |
Table 4 Association of CD103 Immunoexpression with the Thickness of Invasion in Acral Melanoma |
Discussion
AM is a subtype of melanoma with a higher incidence in Asian and African races than other types of melanoma.10−12 AM belongs to the non-CSD melanoma group, which has different pathogenesis from CSD melanoma.3 The prognosis for AM is worse than the CSD melanoma subtype.3–5
In this study, the E-cadherin association with the thickness of invasion in AM was not significant with a p-value = 0.4272. Based on Table 3, it can be seen that E-cadherin immunoexpression remains high even at invasion more than 4 mm thickness. This result is not in line with the theory that the deeper the invasion, the lower the expression of E-cadherin on the tumor cell membrane.7 The results in this study are in line with the results of research conducted by Silye et al.8 They showed that E-cadherin would remain expressed or even increased in malignant melanoma with Clark IV level and with a densely clustered growth pattern (nesting).8 Research by Jung et al and Saberko et al gave different results with this study. In their studies, it was stated that there was a significant association between E-cadherin immunoexpression and the thickness of invasion in melanoma. The E-cadherin immunoexpression is strong in lower Clark level melanoma and is weak in higher Clark level melanoma.7,13
The difference between this study and that of Jung et al and Saberko et al is the sample type. In this study, the samples used were only AM. In their study, Jung et al and Saberko et al used melanoma samples located in the anatomical area exposed to sunlight such as the head, neck, back, arms, etc (CSD melanoma). The differences in the results of these studies can be explained by the theory of tumor cell invasion patterns. The pattern of tumor cell invasion is broadly divided into collective cell invasion (CCI) and individual cell invasion (ICI). Each invasion pattern has a different morphology and molecular mechanisms.14,15 Based on the research conducted by Usman et al, it was found that AM has a different molecular pathway from CSD melanoma.11 Usman et al showed no relationship between BRAF mutations and the thickness of the invasion of AM.11 Whereas, it was known that the CSD subtype melanomas had more BRAF mutations.3,4,10 In contrast, Desai et al found that the KIT somatic mutation pathway was more important in the etiology of AM than the BRAF mutation.10
The study of Jung et al and Saberko et al demonstrated that E-cadherin immunoexpression was associated with invasion of melanoma, all of which were associated with changes in the molecular pathway of intratumor oncogenic factors.7,13 The intratumor oncogenic factor referred to is the role of the BRAF mutation that causes the MAPK pathway (RAS/RAF/MEK/ERK).3,4,11 However, the findings of this work show that there is no association between E-cadherin immunoexpression and the thickness of invasion. Thus, there may be a different molecular pathway in AM that causes E-cadherin to remain expressed.
The invasion pattern may vary according to the tumor microenvironment (TME) and molecular changes in tumor cells.14 Collective invasion is morphologically characterized by group migration of cells, whereas individual invasion of tumor cells is carried out independently. In a collective invasion pattern, tumor cells still maintain intercellular bonds following the discovery of high levels of E-cadherin in cancer cells. In a collective invasion, the cells in charge of invasion are the cells at the very end, while the other cells are the followers. The cells that are at the very end will form pseudopodia, carry out proteolytic degradation to the surrounding tissue and use the contractile apparatus to invade.14,15
The pattern of tumor cell invasion is influenced by the progressive process that occurs. The collective invasion pattern can transform into an individual invasion pattern through the epithelial-mesenchymal transition (EMT) and collective-amoeboid transition (CAT) processes. EMT occurs in a microenvironment that has a stiff matrix. CAT occurs in a microenvironment that has a soft matrix.14,16 During the EMT process, tumor cells have a mesenchymal phenotype; tumor cells are separated from the tumor mass group and migrate through the mesenchymal mechanism.17 In the CAT process, tumor cells in the tumor mass group have specific characteristics; they still maintain cell adhesion, but they are able to migrate.17
The theory of change in invasion pattern supports the results of this study. It can explain why there was no association between E-cadherin immunoexpression and the thickness of invasion. In this study, most of the tumor cells in AM still retained cell-to-cell bonds, as indicated by high E-cadherin immunoexpression in 65% of the samples in both groups of the thickness of invasion. According to the findings of this study, the type of tumor cell invasion in AM is the collective invasion. This may occur due to the oncogenic pathways and microenvironment conditions that are distinct from other subtypes of melanoma. In this study, the role of oncogenic factors did not cause the EMT process by decreasing E-cadherin. Invasion of AM probably occurs via other molecular pathways (not through BRAF mutations and MAPK pathways) but by passing through KIT/NTRK3/ALK/NF1 mutations and different signaling pathways.3,4,11
This study also examined the association of CD103 immunoexpression in TME with the thickness of invasion of AM. The findings of this investigation revealed that CD103 immunoexpression was related to the thickness of invasion. This study is in line with research by Shields et al, which used samples from melanoma cell line B16F10 grown in animal models of mice. Their study showed that high CD103 immunoexpression could slow down the growth of melanoma tumors cell lines characterized by no invasion of melanoma tumor cells.18
CD103 is an integrin that can be found on the surface of TRM CD8+ lymphocytes. The number of CD8+ T cells will affect the number of CD103+ T cells because intratumor CD103+ T cells are only found in CD8+ T cells, but CD8+ T cells do not necessarily express CD103+.9 CD103 has a vital role in immunity against tumor cells, increasing the cytotoxic function of CD8+ T cells when CD103 binds to E-cadherin as a ligand.9,18 The effect of CD103 immunoexpression on tumor prognosis has been studied in several malignancies, for example, ovarian carcinoma,19 breast carcinoma,20 and esophageal squamous cell carcinoma.21
The results of this study indicate that CD103 immunoexpression is associated with the thickness of invasion in melanoma, specifically in AM. The lower the CD103 immunoexpression, the deeper the tumor cell invasion. A low CD103+ T cell count (<20 cells) was more common at the invasion level of more than 4 mm thickness. The decrease in the number of CD103+ T cells was associated with a decrease in CD8+ T cells. In TME, the number of CD8+ T cells is influenced by the NF-kB pathway.9,11 This is based on a hypothesis that an NF-kB pathway can affect TME conditions, especially T cells.11 According to the findings of Usman et al study, there is a relationship between NF-kB immunoexpression and the thickness of invasion in AM.11 As a transcription factor, NF-kB regulates the production of anti-apoptotic, pro-proliferative, and pro-metastatic genes during tumor cell survival. In their research, Usman et al proved that NF-kB is a variable that causes a decrease in the number of CD8+ T cells.11
Regulatory T cells (Treg cells) are a factor that assists in immune evasion in various malignancies. Treg cells are responsible for regulating the amount of T lymphocytes in order to prevent autoimmune processes. The amount of Treg cells multiply as a result of NF-kB activation and cause cell death in CD8+ T cells via perforin, FasL, and granzyme B and then the overall number of CD8+ T cells will drop.22 Also, in the study of Gipsiyanti et al, it will decrease in number due to the presence of PGE2 produced by COX2 and due to increased TGF-β1 immunoexpression in AM tumor cells. This develops because PGE2 eliminates the expression of CD127 on the surface of CD8+ T lymphocytes, decreasing their role as immunological surveillance and their capacity to proliferate.12 TGF-β1, on the other hand, acts as an immunosuppressor by decreasing the activity of CD8+ T cells, NK cells, and dendritic cells.23 In the end, the number of CD103+ T cells will decrease because the total number of CD8+ T cells is reduced since it is known that only CD8+ T cells express intratumor CD103+ T cells.9,20–22
Based on these explanations, the reduction in CD103+ T cell counts in AM with deep invasion is likely due to the decreased total CD8+ T cell count by the activation of the intratumor NF-kB, PGE2 pathways of COX2, and TGB-β1.24 According to the findings of this study (Table 4), low immunoexpression of CD103 is linked with the thickness of invasion in AM, lending credence to the idea that AM is an immunogenic tumor.
In this study, the multivariate analysis could not be carried out between the variable E-cadherin immunoexpression and CD103 immunoexpression with the thickness of invasion. These results prove that there is no simultaneous connection between E-cadherin and CD103 with the thickness of invasion. The role of E-cadherin derived from intracellular oncogenic factors is unrelated and does not affect the thickness of invasion in AM. On the other hand, the role of CD103 from the TME by itself is in influencing the thickness of invasion in AM. This result reinforces the hypothesis that the pathogenesis of the invasion pathogenesis in AM is more towards cancer immunology.25 Therapeutic blockade of immune checkpoints still a reassuring management strategy for many kinds of malignancies including melanoma. Our data suggest that CD103+ lymphocyte could be critical for tumor invasion and are likely to be one of the key factors that support immunotherapy for acral melanoma.26
Conclusion
Low immunoexpression of CD103 (intratumor T cell CD103+ count <20) was associated with a thickness of invasion >4mm in AM. The findings of this work may be combined with those of earlier AM studies, which revealed the role of TME in invasion pathogenesis.
Acknowledgments
The author would like to express gratitude to the personnel of the Anatomical Pathology Installation of the Faculty of Medicine Universitas Padjadjaran/Dr. Hasan Sadikin General Hospital for their assistance during the study process. Part of the funds for this research came from the Universitas Padjadjaran 2020 Internal Grants (No. 1427/U.N. 6.3.1/LT/2020).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chopra A, Sharma R, Rao UNM. Pathology of melanoma. Surg Clin North Am. 2020;100(1):43–59. doi:10.1016/j.suc.2019.09.004
2. Bastian BC. The molecular pathology of melanoma: an integrated taxonomy of melanocytic neoplasia. Annu Rev Pathol. 2014;9:239–271. doi:10.1146/annurev-pathol-012513-104658
3. Elder DE, Bastian BC, Cree IA, Massi D, Scolyer RA. Melanocytic tumour classification and the pathway concept of melanoma pathogenesis. In: Elder D, Massi D, Scolyer RA, Willemze R, editors. WHO Classification of Skin Tumours.
4. Patterson JW, Hosler GA, Weedon D. Lentigines, nevi, and melanomas. In: Patterson JW, editor. Weedon’s Skin Pathology.
5. Teramoto Y, Keim U, Gesierich A, et al. Acral lentiginous melanoma: a skin cancer with unfavourable prognostic features. A study of the German central malignant melanoma registry (CMMR) in 2050 patients. Br J Dermatol. 2018;178(2):443–451. doi:10.1111/bjd.15803
6. Chang JM, Kosiorek HE, Dueck AC, et al. Stratifying SLN incidence in intermediate thickness melanoma patients. Am J Surg. 2018;215(4):699–706. doi:10.1016/j.amjsurg.2017.12.009
7. Jung JE, Anselmi Júnior R, Gennaro L, et al. Immunohistochemical assessment of E-cadherin, B-catenin, CEACAM-1 and PTEN: tumor progression markers in melanoma. Jornal Brasileiro de Patologia e Medicina Laboratorial. 2010;46:111–118. doi:10.1590/S1676-24442010000200007
8. Silye R, Karayiannakis AJ, Syrigos KN, et al. E-cadherin/catenin complex in benign and malignant melanocytic lesions. J Pathol. 1998;186(4):350–355. doi:10.1002/(SICI)1096-9896(199812)186:4<350::AID-PATH181>3.0.CO;2-K
9. Corgnac S, Boutet M, Kfoury M, Naltet C, Mami-Chouaib F. The emerging role of CD8+ Tissue Resident Memory T (TRM) cells in antitumor immunity: a unique functional contribution of the CD103 integrin. Front Immunol. 2018;15(9):1904. doi:10.3389/fimmu.2018.01904
10. Desai A, Ugorji R, Khachemoune A. Acral melanoma foot lesions. Part 1: epidemiology, aetiology, and molecular pathology. Clin Exp Dermatol. 2017;42(8):845–848. doi:10.1111/ced.13243
11. Usman HA, Hernowo BS, Tobing MDL, Hindritiani R. The major role of NF-κB in the depth of invasion on acral melanoma by decreasing CD8(+) T cells. J Pathol Transl Med. 2018;52(3):164–170. doi:10.4132/jptm.2018.04.04
12. Gipsyianti N, Aziz A, Hernowo BS, Usman HA. High expression of COX-2 associated with the depth of invasion on acral melanoma by increasing TGF-beta1. Clin Cosmet Investig Dermatol. 2021;14:209–216. doi:10.2147/CCID.S285564
13. Saberko S, Agus S, Yenny SW. Hubungan Ekspresi Ki-67 dan E-Cadherin dengan kedalaman invasi melanoma malignum berdasarkan clark level. Majalah Patologi Indonesia. 2019;28(3):22–28.
14. Krakhmal NV, Zavyalova MV, Denisov EV, Vtorushin SV, Perelmuter VM. Cancer invasion: patterns and mechanisms. Acta naturae. 2015;7(2):17–28.
15. Jiang WG, Sanders AJ, Katoh M, et al. Tissue invasion and metastasis: molecular, biological and clinical perspectives. Semin Cancer Biol. 2015;35(Suppl):S244–s75. doi:10.1016/j.semcancer.2015.03.008
16. Etemad-Moghadam S, Alaeddini M. Pattern of invasion in squamous cell carcinomas of the lower lip and oral cavity. J Oral Biol Craniofac Res. 2017;7(3):167–170. doi:10.1016/j.jobcr.2017.04.005
17. Alba-Castellón L, Olivera-Salguero R, Mestre-Farrera A, et al. Snail1-dependent activation of cancer-associated fibroblast controls epithelial tumor cell invasion and metastasis. Cancer Res. 2016;76(21):6205–6217. doi:10.1158/0008-5472.CAN-16-0176
18. Shields BD, Koss B, Taylor EM, et al. Loss of E-cadherin inhibits CD103 antitumor activity and reduces checkpoint blockade responsiveness in melanoma. Cancer Res. 2019;79(6):1113–1123. doi:10.1158/0008-5472.CAN-18-1722
19. Webb JR, Milne K, Watson P, Deleeuw RJ, Nelson BH. Tumor-infiltrating lymphocytes expressing the tissue resident memory marker CD103 are associated with increased survival in high-grade serous ovarian cancer. Clin Cancer Res. 2014;20(2):434–444. doi:10.1158/1078-0432.CCR-13-1877
20. Wang ZQ, Milne K, Derocher H, Webb JR, Nelson BH, Watson PH. CD103 and intratumoral immune response in breast cancer. Clin Cancer Res. 2016;22(24):6290–6297. doi:10.1158/1078-0432.CCR-16-0732
21. Chu Y, Liao J, Li J, et al. CD103(+) tumor-infiltrating lymphocytes predict favorable prognosis in patients with esophageal squamous cell carcinoma. J Cancer. 2019;10(21):5234–5243. doi:10.7150/jca.30354
22. Jang TJ. Progressive increase of regulatory T cells and decrease of CD8+ T cells and CD8+ T cells/regulatory T cells ratio during colorectal cancer development. Korean J Pathol. 2013;47(5):443–451. doi:10.4132/KoreanJPathol.2013.47.5.443
23. Chou JP, Ramirez CM, Ryba DM, Koduri MP, Effros RB. Prostaglandin E(2) Promotes features of replicative senescence in chronically activated human CD8+ T cells. PLoS One. 2014;9(6):e99432. doi:10.1371/journal.pone.0099432
24. Jayaraman P, Parikh F, Newton JM, et al. TGF-β1 programmed myeloid-derived suppressor cells (MDSC) acquire immune-stimulating and tumor killing activity capable of rejecting established tumors in combination with radiotherapy. Oncoimmunology. 2018;7(10):e1490853–e. doi:10.1080/2162402X.2018.1490853
25. Mahmoud F, Shields B, Makhoul I, et al. Immune surveillance in melanoma: from immune attack to melanoma escape and even counterattack. Cancer Biol Ther. 2017;18(7):451–469. doi:10.1080/15384047.2017.1323596
26. Edwards J, Wilmott JS, Madore J, et al. CD103+ tumor-resident CD8+ T cells are associated with improved survival in immunotherapy-naïve melanoma patients and expand significantly during anti-PD-1 treatment. Clin Cancer Res. 2018;24(13):3036–3045. doi:10.1158/1078-0432.CCR-17-2257
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

