Back to Journals » Research Reports in Clinical Cardiology » Volume 10
Cavotricuspid isthmus-dependent atrial flutter: clinical perspectives
Authors Bun SS , Lațcu DG, Wedn AM , Hasni K, Saoudi N
Received 27 January 2019
Accepted for publication 3 April 2019
Published 26 April 2019 Volume 2019:10 Pages 7—17
DOI https://doi.org/10.2147/RRCC.S171326
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Kones
Sok-Sithikun Bun, Decebal Gabriel Lațcu, Ahmed Mostfa Wedn, Karim Hasni, Nadir Saoudi
Department of Cardiology, Princess Grace Hospital, Monaco (Principality), Monaco, Monaco
Abstract: The precise circuit of cavotricuspid isthmus (CTI)-dependent atrial flutters (AFLs) has been well characterized, but the recent arrival of ultrahigh-resolution mapping systems has further improved our understanding of this “old” arrhythmia. CTI-dependent AFL may be the arrhythmia for which the electrocardiograph (ECG) correlation with the mechanism may be the highest. Once the diagnosis is made (predominantly based upon the surface ECG), the therapeutic options are precisely defined, with radiofrequency catheter ablation representing an efficient strategy with a high success rate and few complications. This article will focus on the clinical perspectives for CTI-dependent AFL.
Keywords: typical atrial flutter, cavotricuspid isthmus-dependent, catheter ablation
Introduction
Since its first description more than a century ago, our understanding of cavotricuspid isthmus (CTI)-dependent atrial flutter (AFL) has significantly improved, using recent advanced ultrahigh-resolution (UHR) mapping systems. Our knowledge of CTI-dependent AFL has evolved from a relatively simple and unique electrocardiograph (ECG) pattern corresponding to a right atrial (RA) macroreentry to different forms of atrial tachycardias (ATs) propagating through the CTI (or even short-circuiting with epicardial connections).1 Once the diagnosis of CTI-dependent AFL is made (mainly based upon the surface ECG), the therapeutic strategy is well standardized, and radiofrequency (RF) catheter ablation (or with cryotherapy) may be performed with high success rate and low complications and recurrence rate. A close follow-up of the patient will be suggested to detect the occurrence of atrial fibrillation (AF).
Definition and classification of CTI-dependent flutters
The term flutter was first used in 1887 by Mac William who described the visual phenomena resulting from “faradic stimulation of the auricles which sets them into a rapid flutter”.2 The first ECG recording of AFL (with characteristic sawtooth waves in the inferior leads) appeared 23 years later with Jolly and Ritchie, using the Cambridge model of Einthoven’s string galvanometer.3
Lewis was the first to explain the mechanism of this arrhythmia by a single-wave circus movement.4 The macroreentrant mechanism was later proven by detailed mapping in the operating room, the use of steerable multipolar catheters, transient tachycardia entrainment and systems that allowed sequential or simultaneous recording of a large number of endocardial points acquired during the arrhythmia.
AFL classically refers to the ECG pattern of an undulating wave with no electrical silence in at least one lead of the surface ECG. In 1970, a classification of AFL was proposed by Puech and Grolleau based upon the ECG morphology.5 The most type of AFL was called “common” if negative biphasic flutter waves with a sawtooth pattern were present in the inferior ECG leads, and preceding the positivity in V1; AFL was named “atypical” or “rare” if a sawtooth pattern was observed in the frontal plane but now best seen in lead I.
In 2001, an international group of experts proposed the definition of AFLs as follows: AFL refers to the ECG aspect of a regular AT with a rate ≥240 beats/min lacking an isoelectric baseline between deflections.6 Of note, all experts agreed to the fact that neither rate nor lack of isoelectric baseline was specific for the tachycardia mechanism. AFL is named typical if the inferior pivot point is the CTI, i.e. the area bounded anteriorly by the inferior part of the tricuspid valve and posteriorly by the inferior vena cava (IVC) orifice.
This article will focus on the clinical features of CTI-dependent AFL. Non-CTI-dependent AFL will be excluded from this review.
Typical AFLs
Counterclockwise (CCW) typical flutter
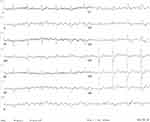
This is the most frequent form of AFL. The mechanism is a macroreentrant circuit confined within the RA, with a descending wavefront in the lateral wall and an ascending wavefront on the septum with passive activation of the left atrium (LA). The tricuspid valve represents the anterior bound of the circuit, whereas the posterior one is a combination of anatomic obstacles (orifices of superior vena cava superiorly, IVC inferiorly, and Eustachian ridge posteriorly) and anatomo-functional barriers (region of the crista terminalis, see below).7–9 The characteristic “sawtooth” pattern is usually present in the inferior ECG leads. In lead V1, the flutter wave shows an initial isoelectric line followed by a positive component which typically falls later than the negative component of the inferior leads (Figure 1). This gives an “overall impression of an upright flutter wave in V1 which becomes inverted by V6”.10
 | Figure 1 Typical counterclockwise atrial flutter with variable atrioventricular transmission and alternating right and left bundle branch block. |
This classical presentation may manifest some morphological variations, giving rise to a classification into 3 types of ECG patterns for CCW CTI-dependent flutters, based on the presence and type of the initial positive deflection.11 It was reported that the presence of a terminal positive component of the F-wave in CCW CTI-dependent AFL may identify patients with a high likelihood of heart disease and a higher incidence of AF and LA enlargement.
CCW lower loop reentry
In 1999, Cheng et al described the circuit of lower loop reentry as a variant of typical flutter.12 The circuit is located in the lower RA, but is also CTI dependent. It is characterized by: 1) an early breakthrough in the lower RA, 2) a wavefront collision in the high lateral RA or septum, and 3) a conduction through the CTI. The LA and the septum are activated in a similar sequence to CCW typical AFL, giving negative F waves in the inferior ECG leads.
In one study including 12 patients with positive flutter wave in the inferior ECG leads, it was found that the CTI–dependent AFL involved a reentrant circuit around the IVC, but now with a CW rotation (CW lower loop reentry). In all but 1 patient, entrainment pacing confirmed that the whole reentrant circuit was totally located in the lower RA.13
Dual-loop reentry during typical flutter
The circuit is composed by a CCW loop around the tricuspid valve, but sharing a common anterior channel with a CW loop around a lateral atriotomy scar. This may occur after postatrial septal defect surgical closure.14 RF delivery within the CTI transforms the tachycardia without any pause to a second tachycardia with different axis and morphology, but nearly the same cycle length owing to rotation around the periatriotomy loop alone. This second tachycardia requires ablation of a second isthmus: between a natural obstacle and one end of the atriotomy. This tachycardia may also be observed without any atriotomy incision, but in the presence of an unexpected scar located on the lateral wall.15
Clockwise typical flutter
In approximatively 10–30% of typical AFLs, the reentrant circuit and the anatomical/functional barriers are identical within the RA, but propagates in a CW direction around the tricuspid valve in a left anterior oblique perspective.16 In the initial series, the classic “sawtooth” pattern was observed in 14 of 18 out of CW AFL. They are frequently associated with a “positive flutter wave in the inferior leads“, but the early description also reported a shorter plateau phase, a widening of the negative component of the F-wave, and a negative and frequently bifid F-wave in V1. A positive F-wave in V6 follows after the negative one in V1 (Figure 2).
Intraisthmus reentry
This form of CTI-dependent AFL has been recently described.17 Surface ECGs show typical CCW pattern in the majority of the patients. Fractionated potentials covering about 34–71% of the tachycardia cycle length are always recorded within the CTI. The ablation may be successfully performed in the area with maximal fractionated potentials duration. Although still debated, the circuit is confined within the CTI itself and bounded by the medial part of the CTI and the CS ostium on the septal side. Interestingly, in the initial description, some parts of the circuits considered could be located outside the CTI region and could occur in the presence of a proven complete bidirectional CTI block.18
ECG modifications of typical AFLs
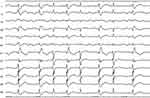
In the era of AF ablation, significant modification of inter- and intra-atrial propagation after circumferential pulmonary vein isolation (and even more after extensive lesions) is almost always accompanied by a flutter wave distortion that is also encountered during sinus rhythm.19 These previous atrial lesions make very challenging any attempt of location of the site/chamber of origin based on the flutter wave morphology (Figure 3).20 Recently, even biatrial circuits have been reported using UHR mapping system, and including the CTI as part of this biatrial circuit.21
CTI as a zone of slow conduction/role of the crista terminalis/ECG correlation
Previous studies demonstrated that CTI was a zone of slow conduction using an electroanatomical noncontact mapping system.22 More recently, an UHR mapping system revealed that approximatively 58% of the patients had a slowing of the conduction impulse in the CTI.23 In contrast, another study showed that the conduction velocity (CV) was not decreased in the CTI, in comparison with the other RA structures, using the same technology.24 In our series of patients, CTI was constantly a zone of slow conduction in all patients.25 32 patients were mapped either during ongoing CCW (n=25), or CW (n=3) AFL, or during coronary sinus pacing at 400 ms (n=1), 500 ms (n=1), or 600 ms (n=3). CTI CV was significantly lower (0.56±0.18 m/s) in comparison with the lateral CV (1.31±0.29 m/s; p<0.0001) and the septal border CV of the CTI (1.29±0.31 m/s; p< 0.0001). In our population of CTI ablation using UHR mapping system, only one patient experienced an AFL recurrence of 32 (3.1%) after a mean follow-up of 20±13 months. Nine patients of 32 (28%) presented an AF recurrence during the follow-up.
The transverse conduction block in the crista terminalis has been reported to be a major determinant in the arrhythmogenesis of CTI-dependent AFL.26,27 Recent data with UHR showed that the crista terminalis was inconstantly observed during AFL, and that a more posterior line of the block may also be involved in 16 of 22 patients.
Our group published that there was an excellent correlation between the plateau phase on the surface ECG that can be measured in the inferior ECG leads and the extra isthmus conduction time either CW or CCW, once the line of block has been created within the CTI.28 Nevertheless, a recent study by Sau et al showed that the CV within the CTI was not correlated to the sawtooth pattern on the surface ECG. There is still room for further studies for ECG analyses in AFL.
Epidemiology/clinical presentation
AFL in not as frequent as AF (less than one-tenth as often as AF).29 The MESA database reported an overall incidence of AFL of about 88 for 100,000 person-years with 80,000 new AFL cases in the USA annually.30 The incidence is approximatively 2.5 times higher in men than in women, and dramatically increases with age, as compared to AF (5/100,000 before the age of 50 vs 587/100,000 in those older than 80 years old).31
Identified risk factors are chronic pulmonary disease, heart failure, previous stroke, and myocardial infarction. The associated conditions are thyrotoxicosis, pericardial disease, valvular heart disease, post-open-heart surgery, and congenital heart disease. AFL may occur in the follow-up of patients having been repaired for a congenital heart defect (Mustard, Senning, or Fontan).
Clinical presentation
AFL may usually manifest with paroxysmal palpitations or short-breathing. Symptoms are more marked when AFL is paroxysmal, and when the ventricular rate answer is fast. Rarely, it may be revealed by the presence of a tachycardia-induced cardiomyopathy, and the ablation will then allow the left ventricular function to recover once the sinus rhythm has been restored. The characteristics of these patients have been reported in a French cohort (103 of 1,269 patients referred for AFL ablation): they were found to be younger, with a lower prevalence of ischemic cardiomyopathy, and a lower use of AAD, in comparison with patients with systolic dysfunction unrelated to AFL.32 The tolerance may be lower in case of 1:1 atrioventricular conduction (8%). Factors favoring the occurrence of 1:1 conduction are: younger age, history of AF, absence of structural heart disease, and obviously the presence of Ic AAD.33
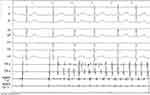
The key for diagnosis will be the 12-lead ECG. As the atrioventricular transmission is almost always 2:1, manoeuvers to increase the degree of AV block such as carotid massage may be required. In other cases, the adenosine injection (Figure 4), by the shortening of the atrial refractory periods, may also induce AF (Figure 5). Sometimes, the diagnosis may be established using the pulsed doppler on the mitral valve (Figure 6). A recurrence of CTI-dependent AFL may manifest with a prolonged cycle length (related to the lengthening of the conduction within the CTI), which can be recorded on the 12-lead ECG.
 | Figure 4 Example of cavotricuspid isthmus-dependent atrial flutter with 1:1 atrioventricular conduction, unmasked by the adenosine injection. |
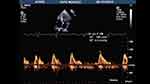 | Figure 6 Recording during echocardiographic examination with the pulsed doppler on the mitral valve, suggesting the presence of an atrial flutter by the absence of individualized A waves. |
AFL and AF are often considered as “fellow-travelers”. In the study published by Peyrol et al, patients presenting with isolated AFL (n=44) were compared to patients presenting with combined AFL and AF (n=32): they had more frequently a prior history of cardiac surgery (presence of an atriotomy) and were less exposed to the use of AAD in comparison with patients with both AFL and AF.34 Interestingly, data from the recent Danish nationwide cohort study revealed a higher mortality risk after CTI ablation compared to patients undergoing an initial AF ablation. The authors reported a higher rate of heart failure and renewed (non-AF) arrhythmia management in AFL.35 In another national cohort study from Taïwan, the net clinical outcomes with anticoagulation were observed in solitary AFL with a CHA2DS2-VASc score ≥4. Solitary AFL without anticoagulation had better clinical outcomes than patients with combined AFL and AF.36
Therapeutic options
As suggested by the recent international guidelines, medical therapy is limited for CTI AFL, and catheter ablation is an efficient strategy, which may be proposed as a first-line therapy if nontolerated.
For acute therapy, intravenous beta-blockers or calcium antagonists are recommended for acute control in patients with AFL who are hemodynamically stable at first intention (synchronized direct current cardioversion if hemodynamically unstable).
For chronic therapy, CTI ablation is recommended with a low incidence of complications in patients with recurrent or poorly tolerated typical AFL (cardioversion with AAD for patients with infrequent AFL recurrences or refusing ablation).37
Not surprisingly, class Ic AAD should not be used in the absence of atrioventricular blocking agents because of the risk of slowing atrial rate, leading to 1:1 conduction.
Catheter ablation
The procedure is well standardized, and the endpoints have been described three decades ago. After the initial attempts at direct current fulguration,38 CTI-dependent AFL are easily amenable to RF catheter ablation with a high success rate, independent of the direction of the rotation, with the same endpoints.39–41 Careful confirmation of CTI dependency of the circuit is always the first step of the procedure, using entrainment-guided mapping techniques. Performing a continuous line of ablation across the CTI has become the standard therapeutic approach. A complete corridor (line of double potentials) may be recorded all along the line.42 The first endpoint is obviously the arrhythmia interruption, but should be associated with the presence of a persisting complete CTI bidirectional conduction block, which should be assessed by pacing techniques.43 More recently, some authors have proposed an ablation technique targeting preferentially high-voltage electrograms within the CTI, corresponding anatomically to muscle bundles.44
This therapeutical approach is now proposed as a first-line therapy with high success rate,45 rare complications, and uncommon late recurrences in experienced hands, even in elderly patients.46
In a pooled population of patients experiencing AF and/or AFL with a prolonged follow-up, it was reported that those who benefited from a CTI ablation (37% with a history of AF) had a better survival rate than other patients.47 From this study, the authors concluded that among patients with atrial tachyarrhythmias, those with AFL who undergo CTI ablation independently have a lower risk of stroke and/or death of any cause, whether a history of AF is present or not.
For AF ablation procedures, the CTI ablation is recommended only if the patient had a history of CTI-dependent AFL, or if induced during the procedure.48 Figure 7 shows an example of ectopies originating from the right superior pulmonary vein, and inducing a CTI-dependent CCW AFL. The CTI line was performed during the same procedure, on top of the PVI.
Technology used
Different technologies have been used and validated for CTI ablation. A 8-mm closed-tip catheter, or a 4 mm-catheter, may be used, either with or without irrigation.49 Remote magnetic navigation,50 a gold-tip catheter,51 as well as cryotherapy have also been used to perform the CTI line.52 More recently, contact-force guided catheters may also be used for CTI-dependent AFL with a good efficacy and safety profile.53,54 Some authors also used mini electrodes at the 8-mm tip of the ablation catheter for better discrimination of the local electrograms within the CTI.55
Recurrences after CTI ablation
Although rare, the recurrences may be seen, either with a CCW or CW rotation.56 The latter patients were younger in our experience, with a shorter plateau duration on the surface ECG. The other form of recurrence may be the occurrence of an intraisthmus reentry. Careful entrainment mapping just outside the CS ostium can facilitate the diagnosis of this unusual variant.57 Finally, patients may elicit a recurrence in the presence of an endocardial block. This may be explained by the possibility to observe an endo-epicardial breakthrough that may represent a shortcut despite obvious endocardial conduction block.58 If confirmed with an UHR mapping system, the target will then become the endocardial breakthrough of the circuit (Figure 8).
Thromboembolic therapy management
Lone AFL has a risk of stroke at least as high as lone AF and carries a higher risk for subsequent development of AF than in the general population.59
The international guidelines then recommend that intravenous anticoagulation may be considered in case of emergency cardioversion, continued for 4 weeks after sinus rhythm has been established. Stroke prevention is recommended with the same indications as in AF among patients with typical AFL and associated episodes of AF.
Question remains for patients presenting for isolated AFL documented before the apparition of AF.60 New AF occurs in ≥25% after RF ablation of isolated typical AFL after a mean follow-up of 2.5±1.8 years (in a cohort of 315 patients).61 Obstructive sleep apnea and LA enlargement were independently associated with the development of new AF. Interestingly, most AF episodes will occur in the 2 years after CTI ablation.62
A HATCH score >2 may be used to better identify the patients most likely to develop new AF during the follow-up.63 A recent nationwide cohort study (219,416 individuals) compared the rate of ischemic strokes, heart failure hospitalization, and all-cause mortality among AF, AFL, and matched control cohorts over a decade. AF and AFL cohorts exhibited higher rates of heart failure hospitalization and all-cause mortality in comparison to the control cohort. Interestingly, the incidence of ischemic strokes was only significantly higher in the AFL group at CHA2DS2-VASc of 5–9 compared with that in the control group. The authors raise the question of a possible overtreatment (with anticoagulation) in patients with lone AFL, if we follow the recommendation of the international guidelines.64
Conclusion
CTI-dependent flutter is an “old” arrhythmia and may be one of the best examples of good ECG correlation with the endocavitary mechanism of the arrhythmia. The treatment is well standardized (after confirmation of the isthmus-dependency of the circuit), i.e. the realization of a complete CTI line of the block. It is associated with a high success rate and low complication. Recent advances in UHR mapping system are still helping us to evolve our knowledge of this arrhythmia.
Abbreviation list
AAD, antiarrhythmic drug; AF, atrial fibrillation; AFL, atrial flutter; AT, atrial tachycardia; CCW, counterclockwise; CS, coronary sinus; CTI, cavotricuspid isthmus; CV, conduction velocity; CW, clockwise; ECG, electrocardiographic; EP, electrophysiological; IVC, inferior vena cava; LA, left atrium; PVI, pulmonary vein isolation; RA, right atrium/atrial; RF, radiofrequency; UHR, ultrahigh resolution.
Disclosure
Drs Bun and Latcu received some consultant fees from Boston Scientific. The authors report no other conflicts of interest in this work.
References
1. Bun SS, Latcu DG, Marchlinski F, Saoudi N. Atrial flutter: more than just one of a kind. Eur Heart J. 2015;36(35):2356–2363. doi:10.1093/eurheartj/ehv118
2. McWilliam JA. Fibrillar contraction of the heart. J Physiol. 1887;8:296–310. doi:10.1113/jphysiol.1887.sp000261
3. Jolly WA, Ritchie WT. Auricular flutter and fibrillation. Heart. 1910;2:177.
4. Lewis T. Observations upon flutter and fibrillation; Part IX. Lewis T. Flutter. Heart. 1921;8:341–345.
5. Puech P, Latour H, Grolleau R. [Flutter and his limits]. Arch Mal Cœur Vaiss. 1970;63(1):116–144.
6. Saoudi N, Cosio F, Waldo A, et al. Classification of atrial flutter and regular atrial tachycardia according to electrophysiologic mechanism and anatomic bases: a statement from a joint expert group from the Working Group of Arrhythmias of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. J Cardiovasc Electrophysiol. 2001;12(7):852–866. Review.
7. Matsuo K, Uno K, Khrestian CM, Waldo AL. Conduction left-to-right and right-to-left across the crista terminalis. Am J Physiol Heart Circ Physiol. 2001;280:H1683–H1691. doi:10.1152/ajpheart.2001.280.4.H1683
8. Olgin JE, Kalman JM, Fitzpatrick AP, Lesh MD. Role of right atrial endocardial structures as barriers to conduction during human type I atrial flutter. Activation and entrainment mapping guided by intracardiac echocardiography. Circulation. 1995;92:1839–1848. doi:10.1161/01.CIR.92.7.1839
9. Friedman PA, Luria D, Fenton AM, et al. Global right atrial mapping of human atrial flutter: the presence of posteromedial (sinus venosa region) functional block and double potentials: a study in biplane fluoroscopy and intracardiac echocardiography. Circulation. 2000;101(13):1568–1577.
10. Medi C, Kalman JM. Prediction of the atrial flutter circuit location from the surface electrocardiogram. Europace. 2008;10(7):786–796. doi:10.1093/europace/eun106
11. Milliez P, Richardson AW, Obioha-Ngwu O, Zimetbaum PJ, Papageorgiou P, Josephson ME. Variable electrocardiographic characteristics of isthmus-dependent atrial flutter. J Am Coll Cardiol. 2002;40(6):1125–1132.
12. Cheng J, Cabeen WR, Scheinman MM. Right atrial flutter due to lower loop reentry. Circulation. 1999;99:1700–1705.
13. Zhang S, Younis G, Hariharan R, et al. Lower loop reentry as a mechanism of clockwise right atrial flutter. Circulation. 2004;109(13):1630–1635. doi:10.1161/01.CIR.0000124221.84399.48
14. Shah D, Jaïs P, Takahashi A, et al. Dual-loop intra-atrial reentry in humans. Circulation. 2000;101(6):631–639.
15. Bun SS, Latcu DG, Delassi T, Jamili ME, Amoura AA, Saoudi N. Ultra-high-definition mapping of atrial arrhythmias. Circ J. 2016;80(3):579–586. doi:10.1253/circj.CJ-16-0016
16. Saoudi N, Nair M, Abdelazziz A, et al. Electrocardiographic patterns and results of radiofrequency catheter ablation of clockwise type I atrial flutter. J Cardiovasc Electrophysiol. 1996;7(10):931–942.
17. Yang Y, Varma N, Badhwar N, et al. Prospective observations in the clinical and electrophysiological characteristics of intra-isthmus reentry. J Cardiovasc Electrophysiol. 2010;21(10):1099–1106. doi:10.1111/j.1540-8167.2010.01778.x
18. Saoudi N, Latcu DG. Intra-isthmus reentry: another form of typical atrial flutter? J Cardiovasc Electrophysiol. 2010;21(10):1107–1108. doi:10.1111/j.1540-8167.2010.01819.x
19. Van Beeumen K, Houben R, Tavernier R, Ketels S, Duytschaever M. Changes in P-wave area and P-wave duration after circumferential pulmonary vein isolation. Europace. 2010;12(6):798–804. doi:10.1093/europace/eup410
20. Bochoeyer A, Yang Y, Cheng J, et al. Surface electrocardiographic characteristics of right and left atrial flutter. Circulation. 2003;108(1):60–66. doi:10.1161/01.CIR.0000079140.35025.1E
21. Kitamura T, Martin R, Denis A, et al. Characteristics of single-loop macroreentrant biatrial tachycardia diagnosed by ultrahigh-resolution mapping system. Circ Arrhythm Electrophysiol. 2018;11(2):e005558. doi:10.1161/CIRCEP.117.005558
22. Chen J, Hoff PI, Erga KS, Rossvoll O, Ohm OJ. Three-dimensional noncontact mapping defines two zones of slow conduction in the circuit of typical atrial flutter. Pacing Clin Electrophysiol. 2003;26(1 Pt 2):318–322.
23. Pathik B, Lee G, Sacher F, et al. New insights into an old arrhythmia high-resolution mapping demonstrates conduction and substrate variability in right atrial macro–re-entrant tachycardia. JACC Clin Electrophysiol. 2017;3(9):971–986. doi:10.1016/j.jacep.2017.01.019
24. Sau A, Sikkel MB, Luther V, et al. The sawtooth EKG pattern of typical atrial flutter is not related to slow conduction velocity at the cavotricuspid isthmus. J Cardiovasc Electrophysiol. 2017;28(12):1445–1453. doi:10.1111/jce.13323
25. Bun SS, Latcu DG, Delassi T, Al Amoura A, Enache B, Saoudi N. Typical atrial flutter circuit characterization using ultra-high density mapping. Europace. 2017;19(suppl_3):iii272 (P1386). doi:10.1093/europace/euw315
26. Saoudi N, Ercyies D, Anselme F. Why do patients develop atrial flutter? Is this crista terminalis geometry? Pacing Clin Electrophysiol. 2009;32(7):866–867. doi:10.1111/j.1540-8159.2009.02401.x
27. Morita N, Kobayashi Y, Horie T, et al. The undetermined geometrical factors contributing to the transverse conduction block of the crista terminalis. Pacing Clin Electrophysiol. 2009;32(7):868–878. doi:10.1111/j.1540-8159.2009.02402.x
28. Latcu DG, Bun SS, Arnoult M, Ricard P, Rinaldi JP, Saoudi N. New insights into typical atrial flutter ablation: extra-isthmus activation time on the flutter wave is predictive of extra-isthmus conduction time after isthmus block. J Interv Card Electrophysiol. 2013;36(1):19–25. doi:10.1007/s10840-012-9729-7
29. Lee KW, Yang Y, Scheinman MM. Atrial flutter: a review of its history, mechanisms, clinical features, and current therapy. Curr Probl Cardiol. 2005;30(3):121–167. Review. doi:10.1016/j.cpcardiol.2004.07.001
30. DeStefano F, Eaker ED, Broste SK, et al. Epidemiologic research in an integrated regional medical care system: the Marshfield Epidemiologic Study Area. J Clin Epidemiol. 1996;49(6):643–652.
31. Granada J, Uribe W, Chyou PH, et al. Incidence and predictors of atrial flutter in the general population. J Am Coll Cardiol. 2000;36(7):2242–2246.
32. Brembilla-Perrot B, Ferreira JP, Manenti V, et al. Predictors and prognostic significance of tachycardiomyopathy: insights from a cohort of 1269 patients undergoing atrial flutter ablation. Eur J Heart Fail. 2016;18(4):394–401. doi:10.1002/ejhf.482
33. Brembilla-Perrot B, Laporte F, Sellal JM, et al. 1:1 atrial-flutter. Prevalence and clinical characteristics. Int J Cardiol. 2013;168(4):3287–3290. doi:10.1016/j.ijcard.2013.04.047
34. Peyrol M, Sbragia P, Bonello L, Lévy S, Paganelli F. Characteristics of isolated atrial flutter versus atrial flutter combined with atrial fibrillation. Arch Cardiovasc Dis. 2011;104(10):530–535. doi:10.1016/j.acvd.2011.07.003
35. Skjøth F, Vadmann H, Hjortshøj SP, Riahi S, Lip GYH, Larsen TB. Disease progression after ablation for atrial flutter compared with atrial fibrillation: a nationwide cohort study. Int J Clin Pract. 2018;72(11):e13258. doi:10.1111/ijcp.13258
36. Chen YL, Lin YS, Wang HT, Liu WH, Chen HC, Chen MC. Clinical outcomes of solitary atrial flutter patients using anticoagulation therapy: a national cohort study. Europace. 2018.
37. Katritsis DG, Boriani G, Cosio FG, et al. Executive Summary: European Heart Rhythm Association Consensus Document on the Management of Supraventricular Arrhythmias: endorsed by Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardiaca y Electrofisiologia (SOLAECE). Arrhythm Electrophysiol Rev. 2016;5(3):210–224. doi:10.15420/aer.2016:5.3.GL1
38. Saoudi N, Mouton-Schleiffer D, Letac B. Direct catheter fulguration of atrial flutter. Lancet. 1987;2(8558):568–569.
39. Poty H, Saoudi N, Nair M, Anselme F, Letac B. Radiofrequency catheter ablation of atrial flutter. Further insights into the various types of isthmus block: application to ablation during sinus rhythm. Circulation. 1996;94(12):3204–3213.
40. Poty H, Saoudi N, AbdelAzziz A, Nair M, Letac B. Radiofrequency catheter ablation of type I flutter. Prediction of late success by electrophysiologic criteria. Circulation. 1995;92:1389–1392.
41. Anselme F, Saoudi N, Poty H, Douillet R, Cribier A. Radiofrequency catheter ablation of common atrial flutter: significance of palpitations and quality-of-life evaluation in patients with proven isthmus block. Circulation. 1999;99(4):534–540.
42. Tai CT, Haque A, Lin YK, et al. Double potential interval and transisthmus conduction time for prediction of cavotricuspid isthmus block after ablation of typical atrial flutter. J Interv Card Electrophysiol. 2002;7(1):77–82.
43. Saoudi N, Ricard P, Rinaldi JP, Yaïci K, Darmon JP, Anselme F. Methods to determine bidirectional block of the cavotricuspid isthmus in radiofrequency ablation of typical atrial flutter. J Cardiovasc Electrophysiol. 2005;16(7):801–803. doi:10.1111/j.1540-8167.2005.40624.x
44. Mechulan A, Gula LJ, Klein GJ, et al. Further evidence for the “muscle bundle” hypothesis of cavotricuspid isthmus conduction: physiological proof, with clinical implications for ablation. J Cardiovasc Electrophysiol. 2013;24(1):47–52. doi:10.1111/j.1540-8167.2012.02415.x
45. Da Costa A, Thévenin J, Roche F, et al. Loire-Ardèche-Drôme-Isère-Puy-de-Dôme Trial of Atrial Flutter Investigators. Results from the Loire-Ardèche-Drôme-Isère-Puy-de-Dôme (LADIP) trial on atrial flutter, a multicentric prospective randomized study comparing amiodarone and radiofrequency ablation after the first episode of symptomatic atrial flutter. Circulation. 2006;114(16):1676–1681. doi:10.1161/CIRCULATIONAHA.106.638395
46. Brembilla-Perrot B, Sellal JM, Olivier A, et al. Risk and outcome after ablation of isthmus-dependent atrial flutter in elderly patients. PLoS One. 2015;10(5):e0127672. doi:10.1371/journal.pone.0127672
47. Clementy N, Desprets L, Pierre BL, et al. Outcomes after ablation for typical atrial flutter (from the Loire Valley Atrial Fibrillation Project). Am J Cardiol. 2014;114(9):1361–1367. doi:10.1016/j.amjcard.2014.07.066
48. Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. Europace. 2018;20(1):157–208. doi:10.1093/europace/eux275
49. Kasai A, Anselme F, Teo WS, Cribier A, Saoudi N. Comparison of effectiveness of an 8-mm versus a 4-mm tip electrode catheter for radiofrequency ablation of typical atrial flutter. Am J Cardiol. 2000;86(9):1029–1032, A10.
50. Vollmann D, Lüthje L, Seegers J, Hasenfuss G, Zabel M. Remote magnetic catheter navigation for cavotricuspid isthmus ablation in patients with common-type atrial flutter. Circ Arrhythm Electrophysiol. 2009;2(6):603–610. doi:10.1161/CIRCEP.109.884411
51. Knecht S, Burch F, Reichlin T, et al. First clinical experience of a dedicated irrigated-tip radiofrequency ablation catheter for the ablation of cavotricuspid isthmus-dependent atrial flutter. Clin Res Cardiol. 2018;107(4):281–286. doi:10.1007/s00392-017-1180-4
52. Saygi S, Bastani H, Drca N, et al. Impact of cavotricuspid isthmus morphology in CRYO versus radiofrequency ablation of typical atrial flutter. Scand Cardiovasc J. 2017;51(2):69–73. doi:10.1080/14017431.2016.1259496
53. Squara F, Latcu DG, Massaad Y, Mahjoub M, Bun SS, Saoudi N. Contact force and force-time integral in atrial radiofrequency ablation predict transmurality of lesions. Europace. 2014;16(5):660–667. doi:10.1093/europace/euu068
54. Venier S, Andrade JG, Khairy P, et al. Contact-force-guided vs. contact-force-blinded catheter ablation of typical atrial flutter: a prospective study. Europace. 2017;19(6):1043–1048. doi:10.1093/europace/euw137
55. Mol D, Berger WR, Khan M, et al. Additional diagnostic value of mini electrodes in an 8-mm tip in cavotricuspid isthmus ablation. JAFIB. 2018;11(3).
56. Bun SS, Latcu DG, Prévôt S, et al. Characteristics of recurrent clockwise atrial flutter after previous radiofrequency catheter ablation for counterclockwise isthmus-dependent atrial flutter. Europace. 2012;14(9):1340–1343. doi:10.1093/europace/eus068
57. Latcu DG, Bun SS, Saoudi N. Intra-isthmus reentry: diagnosis at-a-glance. Europace. 2014;16(2):251. doi:10.1093/europace/eut293
58. Pathik B, Lee G, Sacher F, et al. Epicardial-endocardial breakthrough during stable atrial macroreentry: evidence from ultra-high-resolution 3-dimensional mapping. Heart Rhythm. 2017;14(8):1200–1207. doi:10.1016/j.hrthm.2017.04.043
59. Halligan SC, Gersh BJ, Brown RD
60. Chao TF, Fauchier L. Stroke prevention in patients with atrial flutter: many questions still unanswered. Europace. 2018.
61. Voight J, Akkaya M, Somasundaram P, et al. Risk of new-onset atrial fibrillation and stroke after radiofrequency ablation of isolated, typical atrial flutter. Heart Rhythm. 2014;11(11):1884–1889. doi:10.1016/j.hrthm.2014.06.038
62. Moubarak G, Pavin D, Laviolle B, et al. Incidence of atrial fibrillation during very long-term follow-up after radiofrequency ablation of typical atrial flutter. Arch Cardiovasc Dis. 2009;102(6–7):525–532. doi:10.1016/j.acvd.2009.04.002
63. Chen K, Bai R, Deng W, et al. HATCH score in the prediction of new-onset atrial fibrillation after catheter ablation of typical atrial flutter. Heart Rhythm. 2015;12(7):1483–1489. doi:10.1016/j.hrthm.2015.04.008
64. Lin YS, Chen YL, Chen TH, et al. Comparison of clinical outcomes among patients with atrial fibrillation or atrial flutter stratified by CHA2DS2-VASc score. JAMA Network Open. 2018;1(4):e180941. doi:10.1001/jamanetworkopen.2018.0941
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.