Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Causal Relationship Between Blood Triglyceride Levels and Age Spots: A Mendelian Randomization Analysis
Received 16 August 2023
Accepted for publication 17 October 2023
Published 31 October 2023 Volume 2023:16 Pages 3121—3128
DOI https://doi.org/10.2147/CCID.S431276
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Anne-Claire Fougerousse
Hongtao Liu,1 Shaopeng Ming2
1Clinical Medical School, Guangxi Health Science College, Nanning, 530011, People’s Republic of China; 2Anesthesiology Department, The Second Affiliated Hospital of Guangxi Medical University, Nanning, 530007, People’s Republic of China
Correspondence: Shaopeng Ming, The Second Affiliated Hospital of Guangxi Medical University, Nanning, 530007, People’s Republic of China, Email [email protected]
Objective: This study examined the association between blood triglyceride (TG) levels (TLIB) and age spots (AS).
Methods: We acquired data from the Mendelian randomization (MR) Base database and evaluated the causal association between TLIB and AS.
Results: From genome-wide association studies, we selected 33 single nucleotide polymorphisms (SNPs) that were significantly associated with TLIB and AS. The inverse variance-weighted (IVW) and weighted median estimation methods showed that TLIB had a protective effect on AS (IVW: β=− 0.214, P=0.019, odds ratio [OR]=0.807, 95% confidence interval [CI]=0.674– 0.966; weighted median: β=− 0.277, P=0.032, OR=0.758, 95% CI=0.589– 0.977). However, the MR‐Egger analysis suggested no causal association (β=− 0.234, P=0.085, OR=0.792, 95% CI=0.612– 1.024). The greater precision of the weighted median estimation and IVW suggests that our results support a potential causal association between TLIB and AS.
Conclusion: The MR analysis proved that TLIB has a protective effect against AS and that triglycerides have potential preventive and therapeutic effects against AS. However, the specific dose-effect relationship requires further study.
Keywords: age spots, triglycerides, Mendelian randomization
Introduction
Age spots (AS) are uneven patches caused by pigmentation due to skin aging.1 AS not only affects the skin’s beauty, it negatively impacts a patient’s psychological well-being and self-esteem.2 The main mechanisms underlying AS development include ultraviolet (UV) radiation, natural aging, and endocrine changes.3 The current clinical treatment of AS mainly involves destroying the skin-forming spots and then stimulating skin regeneration. The main treatment methods include laser treatment, cryotherapy, chemical stripping, and microneedle treatment;4–7 these invasive therapies often lead to skin dryness, dysplasia, and other discomfort, so we must continue exploring the mechanism of AS to develop superior treatment strategies.
Triglycerides are important lipid compounds composed of three molecules of glycerol and three molecules of fatty acids through ester bonds. Studies have shown that triglycerides are associated with skin health and can reduce UV-induced metalloproteinase-1 expression in cultured human epidermal keratinocytes.8,9 UV irradiation is also an important factor in skin aging and AS formation.10,11 However, the relationship between triglyceride levels in the blood (TLIB) and AS remains controversial; thus, more evidence is required.
Mendelian randomization (MR) has been used to evaluate causality in observational studies. This is based on the Mendelian principle of inheritance, which states that genetic variations are randomly inherited. Therefore, genetic variations associated with other diseases may also affect the variables of interest. MR evaluates the association between exposure and outcomes by comparing populations with different genetic variations; moreover, it enables better control of potential confounders than traditional observational studies, thereby improving the reliability of the conclusion.12 Therefore, our study aimed to examine the association between TLIB and AS using a two-sample MR analysis.
Materials and Methods
Acquired Genetic Variant Data
We acquired data from the MR Base (http://app.mrbase.org), a platform for MR research that compiles and summarizes data from GWAS.13,14
We used publicly available summary statistics datasets from GWAS meta-analyses of TLIB in Europe (ID: ebi-a-GCST002216, n=94,595, variants=2,410,057)15 as the exposure. We set the threshold of statistical significance as “P<5.00E−8; LD Rsq<0.1” to identify single nucleotide polymorphisms (SNPs). A total of 54 SNPs associated with triglycerides were identified. We used the same method to obtain data regarding AS in East Asian individuals (ID: ebi-a-GCST006096, n=11,253, variants= 559,843)16 as the outcome (Table 1).
 |
Table 1 Study Exposure and Outcome |
MR Analysis
Upon acquiring the data, we evaluated the association between the SNPs and triglyceride. We then separately examined the association between each SNP and AS. Finally, we used MR analysis to evaluate the causal association between triglyceride and AS. Three statistical methods were used to investigate the relationship between the TLIB and AS: MR-Egger regression, weighted median estimation, and inverse variance-weighted (IVW). MR-Egger regression is a statistical technique applied in MR studies to examine the causal associations between exposure and outcomes. This method is commonly used to identify and adjust for pleiotropic effects in which a genetic variant influences outcomes through various paths. The MR-Egger approach uses instrumental variables to estimate the causal effect of exposure on outcomes by considering any potential effects resulting from pleiotropy.17
Weighted median estimation is a statistical method that estimates the typical value of a population by computing the median score of a weighted sample. In this technique, each data point in the sample is assigned a weight that denotes its relative significance or impact on the overall estimation of population parameters. Weighted median estimation is particularly useful when outliers are present in the data distribution since it provides a more robust measure of the central tendency than traditional techniques such as the arithmetic mean.18
IVW is a statistical analysis technique commonly employed in meta-analyses to integrate the effect size estimates derived from numerous studies. In IVW, the weight assigned to each study was proportional to its inverse variance, which signified the accuracy of the effect estimate. The IVW approach constructs a pooled effect size by considering both sample size and variation in effect sizes across studies.17
Heterogeneity Test and Sensitivity Analysis
The IVW method was used to calculate the Q value19 to test for heterogeneity between SNPs. The Q_P-value was calculated to determine the heterogeneity. We also conducted a leave-one-out analysis to explore the potential influence of an individual SNP on the causal association.
Statistical Analysis
The MR analysis was completed using the “TwoSampleMR” package of the R Project (V4.2.3).20 Calculations of the causal association between TLIB and AS are reported as odds ratios (OR) and corresponding 95% confidence intervals (CI). Values of P<0.05 were considered statistically significant.
Results
Detail of SNPs
We selected 33 independent SNPs from GWAS that were significantly associated with both triglyceride and AS (Table 2, Figure 1).
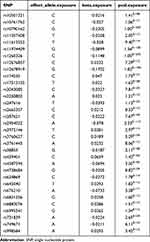 |
Table 2 Details of Each SNP Identified in the Study |
 |
Figure 1 Forest plot of SNPs associated with triglyceride and AS. Abbreviations: AS, age spots; SNPs, single nucleotide proteins. |
Mendelian Randomization
The MR-Egger regression, weighted median estimation, and IVW results are presented in Table 3 and Figure 2. The IVW and weighted median estimation methods showed that triglyceride had a protective effect on AS (IVW: β=−0.214, P=0.019, OR=0.807, 95% CI=0.674–0.966; weighted median estimation: β=−0.277, P=0.032, OR=0.758, 95% CI=0.589–0.977). On the other hand, the MR‐Egger analysis suggested no causal association between triglyceride and AS (β=−0.234, P=0.085, OR=0.792, 95% CI=0.612–1.024). However, the weighted median estimation and IVW feature greater estimation precision than the MR-Egger analysis.21 Therefore, our results support the potential causal association between TLIB and AS.
 |
Table 3 Causal Associations Between Blood Triglyceride Levels and Age Spots |
Heterogeneity Test and Sensitivity Analysis
Cochran’s Q_P=0.513 (IVW) suggested no heterogeneity. Low heterogeneity indicates the reliability of the MR results.22 The horizontal pleiotropy test showed no pleiotropy in our MR analysis (MR-Egger regression intercept=0.002, standard error=0.008, P=0.840; values of P>0.05 indicate no pleiotropy and conformation to the MR). The leave-one-out analysis showed that no individual SNP significantly impacted the causal inference (Figure 3).
Discussion
In the wake of enhanced living standards, the focus on aesthetic appeal and preservation of skin vitality has surged. The treatment of AS has emerged as a predominant aspect of anti-aging skincare, sparking the interest of dermatologists, cosmetologists, and skin beauty researchers worldwide. This has stimulated significant investment in research to elucidate the mechanism of AS formation.6,23
Our study used MR as a powerful tool to measure variation in genes of known function to examine the causal effect of modifiable exposure on disease24 and determine whether blood triglycerides potentially exert preventive and therapeutic effects on AS (Figure 2). Previous studies demonstrated that triglycerides retain water in the skin, and low triglyceride levels indicate insufficient or impaired lipid metabolism, which plays a crucial role in skin homeostasis.
Triglycerides are an integral component of sebum that are essential for forming the hydrophobic lipid film on the skin’s surface.25 This film plays a protective role by preventing excessive water evaporation and maintaining appropriate skin hydration.26 In recent years, advances in molecular technology and the theory that free radicals cause aging have been proposed; additional studies have shown that triglycerides can regulate the esterification reaction in the cell, thus reducing the accumulation of lipids in the cell and counteracting the effect of free radicals, which are among the important factors leading to AS formation.27 In addition, triglycerides can regulate cholesterol metabolism, which in turn regulates cellular changes and makes the skin healthier.9,28
These are all possible reasons why triglycerides can fight AS. Moreover, low triglyceride levels can reduce adipose tissue, which is known to exert a photoprotective effect against UV radiation. As UV radiation is a critical etiological factor in AS,29 the promotion of adipogenesis by triglycerides plays an indirect role in protecting the skin. Furthermore, triglycerides also influence cholesterol metabolism, an important regulatory pathway that controls cellular changes and skin health.30 Because of these multifaceted roles, triglycerides have been extensively used in topical skin care product formulations.31
TLIB was the exposure in this study,15 Few studies to date have examined the relationship between blood triglyceride levels and AS, which is also a highlight of this study; however, it is well known that high blood triglyceride levels may cause hyperlipidemia and do not benefit the skin’s health.32 Although we used a relatively large sample size, MR did not show a specific dose-effect relationship, which is also a study limitation. Therefore, maintaining steady-state triglyceride levels in a way that does not compromise health and can prevent senile plaques requires future investigation. Meanwhile, we found no data related to exposure and sex outcomes in the public database, which is also a disadvantage of its use. Therefore, we will conduct clinical observational studies in the future to determine the dose-effect relationship between AS and blood triglycerides to guide clinical treatment.
Conclusion
In this study, MR analysis was used to prove that blood triglycerides have a protective effect against AS and have potential preventive and therapeutic effects against it. However, the specific dose-effect relationship requires further study.
Abbreviations
AS, age spots; CI, confidence interval; GWAS, genome-wide association study; IVW, inverse variance-weighted; MR, Mendelian randomization; OR, odds ratio; SNP, single nucleotide polymorphism; TLIB, triglyceride levels in the blood.
Ethics Approval
This study was approved by the Medical Ethics Committee of Second Affiliated Hospital of Guangxi Medical University (approval no. 2023-KY (0380)).
Acknowledgments
We thank Editage for English language editing.
Author Contributions
All authors made a significant contribution to the work reported, whether in the study conception, design, and execution; data acquisition, analysis, and interpretation; participated in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; agree about the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the 2020 Guangxi Medical and Health Appropriate Technology Development and Application Program, a study of the protective effects of dexmedetomidine in perioperative patients undergoing kidney transplantation (grant no. S2020014), and the Guangxi Medical and Health Key Discipline Construction Project.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kim JC, Park TJ, Kang HY. Skin-aging pigmentation: who is the real enemy? Cells. 2022;11(16). doi:10.3390/cells11162541
2. Ortonne JP. Pigmentary changes of the ageing skin. Br J Dermatol. 1990;122(Suppl 35):21–28. doi:10.1111/j.1365-2133.1990.tb16121.x
3. Whiteman D, Green A. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 1999;341(10):766–767.
4. Katta R, Desai SP. Diet and dermatology: the role of dietary intervention in skin disease. J Clin Aesthet Dermatol. 2014;7(7):46–51.
5. Ortonne JP, Arellano I, Berneburg M, et al. A global survey of the role of ultraviolet radiation and hormonal influences in the development of melasma. J Eur Acad Dermatol Venereol. 2009;23(11):1254–1262. doi:10.1111/j.1468-3083.2009.03295.x
6. Rabe JH, Mamelak AJ, McElgunn PJ, Morison WL, Sauder DN. Photoaging: mechanisms and repair. J Am Acad Dermatol. 2006;55(1):1–19. doi:10.1016/j.jaad.2005.05.010
7. Wu SZ, Muddasani S, Alam M. A systematic review of the efficacy and safety of microneedling in the treatment of melasma. Dermatol Surg. 2020;46(12):1636–1641. doi:10.1097/DSS.0000000000002763
8. Akaza N, Akamatsu H, Numata S, Matsusue M, Matsunaga K. Fatty acid compositions of triglycerides and free fatty acids in sebum depend on amount of triglycerides, and do not differ in presence or absence of acne vulgaris. J Dermatol. 2015;41(12):1069–1076. doi:10.1111/1346-8138.12699
9. Kim EJ, Jin XJ, Kim YK, et al. UV decreases the synthesis of free fatty acids and triglycerides in the epidermis of human skin in vivo, contributing to development of skin photoaging. J Dermatol Sci. 2010;57(1):19–26. doi:10.1016/j.jdermsci.2009.10.008
10. Fitsiou E, Pulido T, Campisi J, Alimirah F, Demaria M. Cellular senescence and the senescence-associated secretory phenotype as drivers of skin photoaging. J Invest Dermatol. 2021;141(4s):1119–1126. doi:10.1016/j.jid.2020.09.031
11. Shin J, Kim JH, Kim EK. Repeated exposure of human fibroblasts to UVR induces secretion of stem cell factor and senescence. J Eur Acad Dermatol Venereol. 2012;26(12):1577–1580. doi:10.1111/j.1468-3083.2011.04223.x
12. Sekula P, Del Greco MF, Pattaro C, Köttgen A. Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol. 2016;27(11):3253–3265. doi:10.1681/ASN.2016010098
13. Hemani G, Zheng J, Elsworth B, et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife. 2018;7:e34408. doi:10.7554/eLife.34408
14. Elsworth B, Lyon M, Alexander T, et al. The MRC IEU OpenGWAS data infrastructure. bioRxiv. 2020. doi:10.1101/2020.08.10.244293
15. Willer CJ, Schmidt EM, Sengupta S, et al. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45(11):1274–1283.
16. Endo C, Johnson TA, Morino R, et al. Genome-wide association study in Japanese females identifies fifteen novel skin-related trait associations. Sci Rep. 2018;8(1):8974. doi:10.1038/s41598-018-27145-2
17. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–525. doi:10.1093/ije/dyv080
18. Yang S. The weighted kernel estimators of nonparametric regression function with censored data. Acta Mathematica Sinica. 1999;42(2). doi:10.12386/A1999sxxb0033
19. Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. BMJ. 1997;315(7121):1533–1537. doi:10.1136/bmj.315.7121.1533
20. Dessau RB, Pipper CB. ”R”-projekt til statistisk databehandling [“R”--project for statistical computing]. Ugeskr Laeger. 2008;170(5):328–330. Danish.
21. Bowden J, Smith GD, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40(4). doi:10.1002/gepi.21965
22. Bowden J, Holmes MV. Meta-analysis and Mendelian randomization: a review. Res Synth Methods. 2019;10(4):486–496. doi:10.1002/jrsm.1346
23. Gilchrest BA. Skin aging and photoaging: an overview. J Am Acad Dermatol. 1989;21(3 Pt 2):610–613. doi:10.1016/S0190-9622(89)70227-9
24. Smith GD, Ebrahim S. Mendelian randomization: prospects, potentials, and limitations. Int J Epidemiol. 2004;33(1):30–42. doi:10.1093/ije/dyh132
25. Downing DT, Stranieri AM, Strauss JS. The effect of accumulated lipids on measurements of sebum secretion in human skin. J Invest Dermatol. 1982;79(4):226–228. doi:10.1111/1523-1747.ep12500066
26. Rawlings AV. Trends in stratum corneum research and the management of dry skin conditions. Int J Cosmet Sci. 2003;25(1–2):63–95. doi:10.1046/j.1467-2494.2003.00174.x
27. Draelos ZD, Ertel K, Berge C. Niacinamide-containing facial moisturizer improves skin barrier and benefits subjects with rosacea. Cutis. 2005;76(2):135–141.
28. Baydar A, Johnston R. Novel quaternary ammonium salts derived from triglycerides and their application in skin and hair products. Int J Cosmet Sci. 1991;13(4):169–190. doi:10.1111/j.1467-2494.1991.tb00560.x
29. Takahashi Y, Fukushima Y, Kondo K, Ichihashi M. Facial skin photo-aging and development of hyperpigmented spots from children to middle-aged Japanese woman. Skin Res Technol. 2017;23(4):613–618. doi:10.1111/srt.12380
30. Ponec M, Kempenaar J, Weerheim A, de Lannoy L, Kalkman I, Jansen H. Triglyceride metabolism in human keratinocytes cultured at the air-liquid interface. Arch Dermatol Res. 1995;287(8):723–730. doi:10.1007/BF01105796
31. Del Rosso JQ. The role of skin care as an integral component in the management of acne vulgaris: part 1: the importance of cleanser and moisturizer ingredients, design, and product selection. J Clin Aesthet Dermatol. 2013;6(12):19–27.
32. Khan F, Litchfield SJ, Stonebridge PA, Belch JJ. Lipid-lowering and skin vascular responses in patients with hypercholesterolaemia and peripheral arterial obstructive disease. Vasc Med. 1999;4(4):233–238. doi:10.1177/1358836X9900400405
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


