Back to Journals » Research and Reports in Urology » Volume 14
Catheter-Associated Urinary Tract Infections: Current Challenges and Future Prospects
Authors Werneburg GT
Received 10 January 2022
Accepted for publication 27 March 2022
Published 4 April 2022 Volume 2022:14 Pages 109—133
DOI https://doi.org/10.2147/RRU.S273663
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Guglielmo Mantica
Glenn T Werneburg
Department of Urology, Glickman Urological and Kidney Institute, Cleveland Clinic Foundation, Cleveland, OH, USA
Correspondence: Glenn T Werneburg, Department of Urology, Glickman Urological and Kidney Institute, Cleveland Clinic Foundation, Cleveland, OH, USA, Tel +1 216-444-2200, Email [email protected]
Abstract: Catheter-associated urinary tract infection (CAUTI) is the most common healthcare-associated infection and cause of secondary bloodstream infections. Despite many advances in diagnosis, prevention and treatment, CAUTI remains a severe healthcare burden, and antibiotic resistance rates are alarmingly high. In this review, current CAUTI management paradigms and challenges are discussed, followed by future prospects as they relate to the diagnosis, prevention, and treatment. Clinical and translational evidence will be evaluated, as will key basic science studies that underlie preventive and therapeutic approaches. Novel diagnostic strategies and treatment decision aids under development will decrease the time to diagnosis and improve antibiotic accuracy and stewardship. These include several classes of biomarkers often coupled with artificial intelligence algorithms, cell-free DNA, and others. New preventive strategies including catheter coatings and materials, vaccination, and bacterial interference are being developed and investigated. The antibiotic pipeline remains insufficient, and new strategies for the identification of new classes of antibiotics, and rational design of small molecule inhibitor alternatives, are under development for CAUTI treatment.
Keywords: catheter-associated urinary tract infection, biofilm, bacterial interference, bacterial competition, chaperone-usher, machine learning
Introduction and Overview
Urinary tract infections (UTI) are among the most common bacterial infections, and affect about 150 million individuals annually worldwide.1 UTIs are grouped into uncomplicated and complicated infections.2 Uncomplicated UTIs occur in female individuals who are not immunocompromised, have no foreign bodies in the urinary tract and have not undergone urinary tract manipulation recently, and have no anatomical or neurologic abnormalities in the urinary tract. Complicated UTIs occur in the context of a structural or functional abnormality. Examples include patients with urinary tract obstruction or urinary retention (due to neurological disease, for example), immunocompromised state, pregnancy, or those with an indwelling foreign body such as a stone, ureteral stent, or urinary catheter.3,4 UTIs in men are considered complicated regardless of clinical scenario. In the United States, up to 80% of complicated urinary tract infection are attributable to indwelling urinary catheters.5 These infections are termed catheter-associated urinary tract infections and are the focus of this review.
Catheter-associated urinary tract infections (CAUTI) are urinary tract infections occurring in an individual whose urinary bladder is catheterized or has been catheterized within the past 48 hours. CAUTIs are the most common nosocomial infections, and account for 1 million cases per year in the United States.6 They are the most common cause of secondary bloodstream infections. 3–10% of residents in long-term care facilities are managed with chronic indwelling catheters.7,8 The associated costs of preventable CAUTI are estimated to range from $115 million to $1.82 billion annually.9 Risk factors for CAUTI include age, female gender, diabetes, and prolonged catheterization time.10 The duration of catheterization is the most important factor in the development of bacteriuria, with a risk of 3–7% daily.8 A mean of 3.2 urinary tract infections per 1000 catheter days was reported in long-term care facilities in a US study11 In the intensive care unit (ICU), where infection rates are 3–5 times higher than other hospital patient care areas, the incidence of CAUTI is 7.78 per 1000 catheter days.12 CAUTIs in ICUs are associated with increased lengths of stay, higher health-care financial expenditure, and antibiotic overuse.13,14
UTIs may be caused by both Gram-negative and Gram-positive bacteria, as well as fungi. Uropathogenic Escherichia coli (UPEC) is the most common pathogen for both non-complicated and complicated UTI, making up 75% and 65% of infections, respectively.2 In complicated UTI, wherein CAUTIs make of the majority of cases, the overall most common causative organisms after UPEC include Enterococcus spp. (11%), Klebsiella pneumoniae (8%), Candida spp (7%), Staphylococcus aureus (3%), Proteus mirabilis (2%), Pseudomonas aeruginosa (2%), and Group B Streptococcus (2%). The cornerstone for CAUTI is antibiotics. However, the abiotic surface of the catheter is subject to biofilm formation, and thus often resistant to antibiotic penetration. Further, antibiotic treatment has known collateral damage in that it selects for resistant bacterial strains and alters the vaginal and gut microbiota, which in turn may open additional niches for colonization by resistant organisms. Pili, adhesive virulence-associated factors that contribute to antibiotic evasion, may also serve to facilitate bacterial colonization of the intracellular niche.15 The rates of antibiotic resistance are increasing, and in 2013, the CDC declared that the human race is now in a “post-antibiotic era”, and in 2014, the World Health Organization warned that the antibiotic resistance phenomenon is becoming dire.16,17 Thus, CAUTI preventive strategies and alternative treatments to antibiotics treatment are critically needed. In this review, we first discuss the molecular mechanisms associated with CAUTI. Then, the current CAUTI management strategies along with their challenges and future prospects will be discussed as they pertain to prevention, diagnosis, and treatment.
Mechanisms of Catheter-Associated Urinary Tract Infection
UTIs generally occur through urethral contamination with rectal flora, followed by microbial migration to the bladder, adhesion, and colonization.2 Invasion of the bladder is then mediated by pili and adhesins, and neutrophil infiltration commences. Bacteria then multiply and form biofilms, and bacterial proteases and toxins trigger epithelial damage. These fundamental steps of infection are the same in the presence and absence of a urinary catheter.
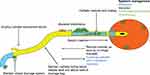
Urinary catheters provide a direct conduit from the ambient environment to the urinary bladder (Figure 1). This conduit, while critical for urinary drainage in some patients, also provides a channel for rectal and periurethral microbe ascension to the bladder where they may establish a foothold for infection. Catheters bypass the urethral sphincters, reduce the turbulence associated with spontaneous voiding, and serve as a nidus for infection, thus increasing the risk for UTI. In addition, catheters may also irritate and traumatize the uroepithelium, thereby disrupting the physiologic mucopolysaccharide coating, and rendering it susceptible to bacterial adhesion and entry.2,18 The strong immune response to catheterization leads to fibrinogen accumulation on the catheter, thus providing an optimal environment for adherence by uropathogens that express fibrinogen-binding proteins. For example, Enterococcus faecalis is unable to grow in urine or bind catheter material in vitro, but is able to grow in the context of fibrinogen-supplemented urine and adhere to a fibrinogen-coated catheter.19
Adherence is a key initial step in urinary tract infection.2 In uncomplicated UTI, bacteria may adhere directly to the uroepithelium of the bladder, allowing them to gain a foothold for infection. However, in the context of a urinary catheter, whether it be a urethral catheter or suprapubic tube, UTIs may be initiated upon bacterial adherence to the catheter, with subsequent biofilm formation.20
Biofilms are communities of microbes and their metabolic products adherent to one another and to a surface, such as a catheter. Specifically, biofilms consist of scaffolds that include extracellular DNA, exopolysaccharides, and microbial surface structures including pili and flagella.2 Biofilms allow for bacterial evasion of antibiotics and host responses, and for infection persistence.21 Biofilms serve as reservoirs for microbial seeding of the urinary tract, and are central to the pathogenesis of CAUTI.22 Biofilm formation is initiated within minutes of catheterization.20 Biofilms then progress as a function of indwelling time.
Microbial species utilize varied mechanisms for biofilm formation. For example, uropathogenic Escherichia coli (UPEC), the most common causative organism for CAUTI, utilize pili, antigen 43, and curli to promote interbacterial attachment, bacterial-surface adhesion, and subsequent biofilm formation.2 Specifically, UPEC catheter biofilm formation is dependent on type 1 pili UPEC biofilm formation (Figure 1), and is regulated by oxidative stress, iron sensing, and quorum sensing.23–26 Pseudomonas aeruginosa exhibits biofilm formation on urinary catheters through a number of mechanisms including alginate production, quorum sensing and surface hydrophobicity modulation.27 Biofilms contribute to the pathogenicity of P. aeruginosa, and lead to persistent or recurrent infections. Proteus mirabilis is a urease-producing species, and its hydrolysis of urea increases the urinary pH, thus generating calcium crystals and magnesium ammonium phosphate precipitates. Together, this leads to the formation of crystalline biofilms on catheters.2 An understanding of these mechanisms and others has provided a framework for the development of novel inhibitors and antibiotics to prevent or treat CAUTI, which will be discussed in subsequent sections.
Prevention: Current Strategies and Considerations
General Prevention Strategies
There are multiple fundamental interventions that reduce the risk of CAUTI (Figure 1).28 The duration of catheterization is the most important factor in the development of bacteriuria with a risk of 3–7% daily, and UTI risk of 0.3% per catheter day.8 Thus, the most important intervention is to minimize the use of indwelling catheters and remove them as soon as medically feasible. Thus, daily assessment of catheter presence and consideration for removal is paramount. Alternative bladder drainage strategies such as clean intermittent catheterization must also be considered. External catheters are also a viable alternative to indwelling catheters, and are recommended by the CDC.29 For men, condom catheters may be utilized, and recent advances have led to a viable external alternative for women.30 In order to minimize catheter use, the infrastructure and program at the hospital level should be in place. Sufficient staffing and staff education, with access to necessary equipment should be ensured.8,31 Electronic medical record (EMR) documentation of catheter insertion and removal dates as well as indications and reminders for removal are important. Implementation of “bundles” in the EMR, which include catheter insertion, management, and surveillance guidelines, was associated with a 37% reduction of CAUTI rate in the ICU setting in developing countries.32 In a US-based study, bundle implementation consisting of catheter removal prompts and reminders, nurse-initiated catheter discontinuation protocols, catheter alternatives, portable ultrasound bladder scanners, and insertion care and maintenance recommendations, reduced catheter use and CAUTI rate.31,33 A national prevention program that implemented these interventions resulted in a decrease in adjusted CAUTI rates from 2.40 to 2.05 per catheter day (p = 0.009) and catheter use in non-intensive care unit settings was reduced from 20.1% to 18.8%.28
Insertion Technique and Drainage Considerations
Aseptic catheter insertion technique is an important element of catheter management to reduce CAUTI. Hand hygiene should be performed before and after catheter insertion. The Infectious Diseases Society of America (IDSA) provides guidelines designed to reduce CAUTI following catheter insertion.29 First, a closed drainage system should be employed.34 If breaks occur in aseptic technique, the closed drainage system, or leaks, the catheter and drainage bag should be replaced using aseptic technique and new equipment. The drainage bag should be kept below the level of the bladder and connection tubing.14,35 Positioning of the tubing above the bladder or below the drainage bag level is associated with an increased risk of bacteriuria.36 Precautions should be taken to minimize urethral trauma during insertion, and catheter insertion should only be performed by trained personnel, with adequate lubricant, and with the smallest caliber catheter necessary for its purpose.35 Catheter securement should be performed to minimize urethral traction and trauma.29 In a randomized controlled clinical trial, the StatLock catheter securement device was associated with a 45% reduction in CAUTI versus other securement techniques, but due to prohibitively small sample size, this difference was not statistically significant.37
Catheter Exchange Timing
Timing of catheter exchange is debated. In clinical practice, catheters are generally removed and replaced at least every 4 weeks. In cases of recurrent infection or obstruction, exchange may be performed more frequently, on an individualized basis. The practice of routine catheter exchange is informed by evidence that increased indwelling time is associated with a higher CAUTI risk.38 Additionally, microbial biofilms form soon after catheter insertion, and progress over the course of indwelling time, starting with a lag phase, and then rapid progression at about 3 weeks.20 Despite these findings, due to lack of conclusive clinical trials, the IDSA guidelines do not recommend changing urinary catheters at set time intervals, but rather only for causes including infection, obstruction, or breaks in the closed drainage system.29
Catheter Materials and Coatings
In those who require urinary catheters, catheter selection is an additional consideration in CAUTI rate-reduction (Figure 1).29 Catheter materials have been examined, but there appears to be no difference between CAUTI rates associated with latex versus silicone, the two most commonly used catheter materials.8 Thus, materials are generally chosen based on clinical indication (eg, latex allergy) rather than differential CAUTI prevention. There has been considerable investigation regarding catheter coatings designed to prevent microbial colonization and biofilm formation, termed “antifouling”.39 Hydrogel and PTFE (Teflon)-coated catheters have been assessed. Hydrogels are a group of polymers that are insoluble and hydrophilic. When fully swollen, hydrogels are made up of about 90% water. This provides a hydration layer, which can resist nonspecific adherence. Clinical efficacy, however, is more variable. For example, in a clinical trial comparing silicone, siliconized latex, and hydrogel catheters, the pure silicone had mildest degree of urethral inflammation, while the hydrogel catheters prevented encrustation.40 In another study, hydrogel had the lowest degree of mucosal irritation and bacterial adherence versus PTFE-coated and silicone catheters.41 Silver-alloy coated catheters have variable clinical data. In a large study that randomized subjects to silver-coated catheters versus non-silver-coated silicone urethral catheters, rates of bacteriuria in men who did not receive antibiotics were higher in the silver-coated group relative to the non-silver-coated group (29% versus 8%). Additionally, Staphylococcus spp were more frequently isolated from the silver-coated catheter group relative to controls. Conversely, in a Cochrane review of hospitalized individuals requiring short-term catheterization, it was found that silver alloy indwelling catheters were associated with a risk reduction in CAUTI.42 Additional studies are warranted to further assess the efficacy of silver catheters relative to controls, particularly with longer indwelling times, and whether they are cost effective. Current evidence suggests that silver-coated catheters are unlikely to be cost-effective,39,42,43 and this must be weighed against their benefits.
Nitrofurazone-impregnated catheters are commercially available. Nitrofurazone inhibits replication of DNA, thus reducing bacterial growth and biofilm formation.39,44 The in vitro data are promising: nitrofurazone-impregnated catheters inhibited growth of all tested multidrug resistant and susceptible strains except vancomycin-resistant Enterococcus faecium45 Clinical results, however, have been variable. In a randomized clinical trial in the kidney transplant population, there was no difference in asymptomatic bacteriuria between groups with nitrofurazone-impregnated silicone catheters versus non-impregnated catheters, and there was a higher incidence of side effects in the nitrofurazone-impregnated group.46 Another trial that included catheters indwelling less than 1 week showed a lower rate of bacteriuria in the nitrofurazone-impregnated group versus the group with uncoated catheters.47 A Cochrane review of clinical trials concluded that there was a small reduction in CAUTI in the context of nitrofurazone catheters, but this may not be clinically significant. Further, it was concluded that they are more expensive and cause more discomfort than standard catheters.48 Nitrofurazone catheters remain commercially available, but because of mixed results and an FDA discontinuation of approval on over-the-counter topical nitrofurazone due to its implication as a carcinogen, interest has moved toward other antimicrobial coatings and other technologies.
Antibiotic Prophylaxis
There is sparse evidence regarding antibiotic prophylaxis in the context of a long-term indwelling catheter. However, studies have assessed the utility of prophylaxis in the context of short-term catheters. A Cochrane review in 2005 concluded that there is weak evidence for antibiotic prophylaxis to reduce the incidence of CAUTI following abdominal surgery in female patients wherein a catheter had remained in place for 24 hours, compared to antibiotic administration only as clinically indicated.49 The review also indicated that those who received antibiotics during the first three postoperative days or from postoperative day 2 until the time of catheter removal had lower rates of bacteriuria and other signs of CAUTI. The review also provided weak evidence that prophylactic antibiotics were associated with reduced bacteriuria in non-surgical patients. However, these latter findings of the study only assessed bacteriuria incidence, and whether or not this correlated with symptomatic infection was not assessed. In a more recent Cochrane review in 2013, there was evidence that in surgical patients who undergo catheterization from 24 hours to two weeks, there was a lower rate of febrile mortality associated with receiving prophylactic antibiotics.50 The same review also concluded that in non-surgical patients, there was limited evidence that prophylactic antibiotics reduced bacteriuria. Again, whether this translated to symptomatic UTI was not assessed.
The utility of prophylaxis at the time of catheter removal remains controversial.51 A meta-analysis in 2013 concluded that those who undergo short-term catheterization might benefit from antibiotic prophylaxis at the time of catheter removal, as they had an absolute risk reduction of 5.8% relative to controls.52 The number needed to treat to prevent one CAUTI was 17.
After radical prostatectomy, a urinary catheter is generally placed and left in place for approximately 7 days. About 40% of patients who undergo prostatectomy have bacteriuria at the time of catheter removal.53 In a prospective randomized trial, antibiotic prophylaxis with ciprofloxacin at the time of catheter removal following prostatectomy was not associated with a reduction in CAUTI or Clostridium difficile infection.54 However, in clinical practice, antibiotics are generally given at the time of catheter removal following radical prostatectomy, and a recent cross-sectional study showed that 60% of urologists routinely prescribed antibiotics prior to catheter removal.55 In a clinically integrated, cluster, randomized trial, patients were allocated to 1- or 3-day course of ciprofloxacin at the time of catheter removal.56 There were 0 CAUTIs in the 1 day regimen and 3 (0.7%) CAUTIs in the 3-day regimen group. It was concluded that the 1 day regimen was noninferior to the 3 day regimen, and that if surgeons choose to prescribe empiric antibiotic prophylaxis at the time of catheter removal, the duration should not exceed one day. Exciting surgical advances in prostatectomy such as the robotic single-port transvesical approach are associated with reduced length of catheterization following surgery.57 The possibility that this approach translates to reduced CAUTI risk is plausible, and warrants investigation.
Cranberry, Methenamine, D-Mannose, Probiotics
The use of cranberry in UTI treatment and prophylaxis has been well studied, and is currently included in the American Urological Association (AUA) recurrent uncomplicated UTI in women guidelines.58 The mechanism of action of cranberry is likely based on the disruption of adherence of bacteria to the uroepithelium.59 Specifically, proanthocyanins may disrupt P pilus-mediated adherence. Fructose, another component of cranberry juice, has been shown to inhibit type 1 pilus-mediated E. coli adherence to urothelial cell receptors in vivo.60 A limitation of cranberry as treatment, and its associated studies, is the variable level of anti-adherence components in different formulations (juice, capsules, powder). In part because of this variability, its clinical efficacy has been debated.61 There are currently limited data on the use of cranberry to prevent CAUTI. A Cochrane review in 2012 concluded that there is no benefit associated with cranberry juice consumption in CAUTI reduction, based on a combination of two studies with a total of 353 participants.62 In 2015, Foxman et al reported the results of a randomized controlled clinical trial in women who underwent elective benign gynecologic surgery, wherein cranberry tablets reduced the rate of CAUTI by about 50% relative to placebo controls.63 Another study showed no difference in rates of bacteriuria in those who received cranberry capsules versus placebo in a population in women patients with hip fracture and indwelling urinary catheter.64 A recent review reached similar conclusions to that of the 2012 Cochrane review. There remains limited literature on the utility of cranberry juice and cranberry products on the prevention of CAUTI, and additional investigation is warranted.65
Methenamine is an antiseptic, approved by the US FDA as prophylaxis against recurrent UTIs in those age 6 and older. Its efficacy has been demonstrated in this population.66 Because methenamine is an antiseptic rather than antimicrobial compound, it does not select for antibiotic resistant organisms. Methenamine is hydrolyzed into ammonia and formaldehyde, which has nonspecific bactericidal properties and denatures proteins and nucleic acids of bacteria. Of note, this reaction only occurs in an acidic environment, at a pH below approximately 6. Thus, the product is formulated in two salt formulations (hippurate and mandelate) that promote acidification, and treatment regimens frequently include urine acidifying agents such as ascorbic acid.67 There is evidence for efficacy of methenamine as CAUTI prophylaxis in individuals with indwelling urinary catheters. Methenamine has been shown to delay the onset of bacteriuria, and reduce the incidence of CAUTI in a catheterized population68,69 Similarly, methenamine reduced CAUTI and bacteriuria in those who underwent elective gynecologic surgery and had perioperative urinary catheters.70 Of note, methenamine is contraindicated in those with renal impairment, renal failure, or severe dehydration, as well as those with severe hepatic disease.
D-mannose is an inert monosaccharide that is metabolized and excreted in the urine, and inhibits bacterial adhesion to the uroepithelium70 D-mannose blocks bacterial adhesion in vitro, and antagonizes invasion and biofilm formation.71 FimH, the terminal and adhesive subunit on type 1 pili (the bacterial surface appendages on Escherichia coli responsible for adhesion to the bladder) ordinarily binds to mannosylated uroplakins of the uroepithelium20 D-mannose competes for FimH’s binding site, disrupting adhesion. Despite the detailed mechanistic understanding regarding D-mannose, there is minimal evidence for its utility in CAUTI prevention. In a study including patients with multiple sclerosis and recurrent UTIs, D-mannose intake twice daily for 16 weeks was associated with a decrease in UTIs in both catheterized and noncatheterized subgroups, and there were no adverse events reported.72 Of note, the study was small in size, and only included 12 patients in the catheterized subgroup.
Probiotics, specifically Lactobacillus spp, are gaining increased attention as prophylaxis against recurrent UTIs. A systematic review concluded that probiotics can be beneficial in preventing recurrent UTIs in women and have a favorable safety profile, but additional research is needed to confirm these results.73 However, there have been few studies in patients with indwelling catheters. A randomized controlled clinical trial of 207 patients with neurogenic bladder and stable bladder drainage management (60% of patients had an indwelling catheter) demonstrated no difference in UTI associated with probiotics versus placebo.74 A small case series in patients with neurogenic lower urinary tract dysfunction managed with indwelling catheters demonstrated that there was a significant change in the microbial composition of catheter biofilms with probiotic administration.75 However, these changes were transient. Subsequent investigations are warranted to better understand how such targeted alteration of catheter biofilm communities with probiotics may serve as an adjunct to CAUTI prevention and/or treatment.
Catheter Irrigation/Washout
A Cochrane review analyzed the available evidence regarding catheter irrigation/washout in prevention of blockage and infection in those with indwelling catheters.76 The authors concluded that 7 trials included were limited and generally of poor quality, and the evidence was not substantial enough to make recommendation regarding benefit and/or risks of washout. The trials reviewed were also heterogenous in that they used different irrigation solutions such as saline or acidic solution, and different protocols. A subsequent randomized controlled clinical trial of 60 comatose patients in intensive care units demonstrated that daily bladder irrigation with normal saline was effective in reducing CAUTI risk.77 There is limited evidence for the use of gentamicin or betadine irrigation for UTI prevention. For example, a retrospective study found that intravesical gentamicin instillation reduced symptomatic UTI episodes, oral antibiotic use, and the proportion of resistant organisms.78 Another study demonstrated that daily intravesical povidone-iodine bladder irrigation was associated with a significant reduction in symptomatic UTI in patients with neurogenic bladder and recurrent UTI who performed clean intermittent catheterization79 Randomized clinical trials are needed to confirm these findings and determine whether they are generalizable to those with indwelling catheters.
Asymptomatic Bacteriuria
Current guidelines advise against screening for or treating asymptomatic bacteriuria.80 Two notable exceptions include pregnant individuals and those who will undergo urologic surgery with risk of mucosal trauma or upper tract manipulation. The IDSA specifically recommends against screening for or treatment of asymptomatic bacteriuria in those with spinal cord injury, and short- (<30 days) or long-term indwelling catheters. The AUA guidelines also recommend against treatment of asymptomatic bacteriuria.58,80 As will be discussed in the Diagnosis section, the signs and symptoms of UTI in those with spinal cord injury or other neurologic pathology are often subtle and different from those without neurologic pathology. Thus, clinicians should carefully assess signs and symptoms and maintain a high index of suspicion in this population.
Prevention: Challenges and Future Prospects
Despite the above measures, CAUTIs remain prevalent and costly. Antibiotic resistance continues to increase, and the World Health Organization recognizes resistance as one of the biggest threats to global public health,81 with antibiotic usage a primary contributor. In addition, antibiotic usage has collateral damage including dysregulation of the healthy gut microbiome, which can lead to pathogen overgrowth and toxin translocation82 Thus alternatives to traditional antibiotics are needed. This has led to intense interest in non-antibiotic prevention measures against CAUTI. This section discusses opportunities for CAUTI prevention in different stages of development and clinical testing.
Catheter Design and Coating
Many novel catheter fabrications are under development. Few have made it to clinical trials, and even fewer are commercially available (these are discussed above). In this section, we discuss new technologies and their evidence for reduction of CAUTI. Thus far, the data associated with these technologies has largely been generated in in vitro experiments and animal models.
Biofilm Target Locations and Hydrodynamics
A recent study designed to understand the progression of biofilms on urinary catheters sheds light on the importance of targeting specific areas with anti-bacterial coatings and impregnation strategies.20 In particular, the balloon portion, distal third, and the intraluminal aspect of the catheters consistently exhibited biofilm predominance, but all aspects of the catheters were susceptible to biofilms. Catheter biofilms consistently harbored uropathogens, regardless of infection status. Antibiotic resistance genes were detected in half of the samples. The importance of the intraluminal route was also supported by an animal model.83 Further, an in vitro study showed the ascension of a Pseudomonas biofilm on a catheter, even in the presence of antibiotics.84 Together, these findings inform the catheter coating and materials approaches in development, and highlight the locations of interest for anti-fouling. Others are investigating modifications in catheter design. For example, Ionescu et al fabricated a catheter with an asymmetric balloon and additional drainage holes with the goal of reducing residual volume in the bladder and thus reducing CAUTI risk.85 The authors showed that the novel design was associated with less biofilm formation and a lower residual volume in vitro. The findings will need to be tested in clinical trials, but such engineering approaches to improve urine drainage hydrodynamics are promising to reduce CAUTI risk.
Nanoparticles
Nanoparticles serve as a drug delivery system and may enhance distribution and bioavailability.39,86 Nanoparticles have been used to deliver silver, gold, copper, and zinc.86 As discussed above, silver-impregnated catheters are associated with mixed clinical data. To improve their efficacy and minimize host tissue interaction, there has been interest in incorporating silver into nanoparticles. Silver nanoparticles are less than 100 nm in size and serve to deliver the silver ion where it can disrupt bacterial membranes, modifying permeability and leading to cell death.87 One study demonstrated that plastic catheters coated with silver nanoparticles reduced biofilm formation and growth of several uropathogens as compared to controls.88 In addition, the authors showed that, when implanted subcutaneously in mice, the particles did not induce inflammation or toxicity, and were mainly excreted in the feces or retained at the site of implant. Another study corroborated these results, showing that silver nanoparticles embedded in a hydrogel catheter coating reduced the growth of several uropathogens.89 A novel, green, fabrication wherein silver nanoparticles were synthesized using Spirulina extract, demonstrated inhibition of Pseudomonas aeruginosa biofilms on catheters.90 While these results are encouraging, a clinical trial in a central venous catheter impregnated with silver nanoparticles versus controls demonstrated no difference in colonization, infection, or mortality.91 Indeed, nanoparticle technology has progressed rapidly since that time, and future clinical trials will be needed to assess safety and efficacy of this technology in the context of the urinary catheter.
Gold nanoparticles have also been developed and investigated. Their mechanism of action is disruption of membrane potential and reduction of ATP, and inhibition of transfer ribonucleic acid (tRNA) binding to the ribosome.87,92 An in vitro study demonstrated efficacy of gold nanoparticles in bacterial growth inhibition of uropathogens at up to 48 hours.93 However, there appeared to be an efficacy reduction at the 48 hour time point relative to the 24 hour timepoint. Further investigation is needed regarding the safety and efficacy of gold nanoparticles.
Copper enters the bacterial cell and binds DNA-phosphate sites and degrades DNA.39 In turn, this deactivates enzymes, and disrupts membranes and cell walls. An in vitro study incorporated copper (both by itself and together with silver) nanoparticles onto catheter surfaces using a novel sputter coating technique, and demonstrated that within two minutes of interaction between Escherichia coli K12 strain and the copper/silver nanoparticle catheter surface, no viable bacteria were present.94 This rate was accelerated relative to either copper or silver nanoparticles alone. Zinc-doped copper oxide nanoparticles have also been investigated. The study showed a reduction of biofilm by over 90% on catheters in the context of this coating relative to uncoated controls at 24 h in an in vitro model.95 The coating was also effective in a catheterized rabbit model at 7 days of indwelling time, as confirmed by colony counts and scanning electron microscopy.
Antimicrobial Peptides
Antimicrobial peptides (AMP) are a group of host defense peptides with broad antimicrobial activity against Gram-negative and Gram-positive bacteria.39 They have a net positive charge, which interacts with and leads to destabilization and permeabilization of the bacterial membranes. The mechanism of action is polymodal and also includes autolysin activation, inhibition of nucleotide and protein synthesis, enzyme inhibition, and immunomodulation.44 One AMP molecule, CWR11 (CWFWKWWRRRRR-NH2), was studied as tethered to a polydopamine polymer on silicone surfaces and commercially available urinary catheters.96 The CWR11 coating had antimicrobial activity against both Gram-negative and Gram-positive bacteria and had decreased microbial adherence. Its antimicrobial activity was present for at least 21 days, and it had negligible cytotoxicity in urothelial or blood cells in vivo. Another AMP, RRWRIVVIRVRRC, exhibited broad and long-term antimicrobial activity.97 It prevented 99.9% of bacterial adhesion of both Gram-negative and Gram-positive bacteria in vitro. In a mouse model, it was highly effective at preventing infection. It also exhibited good biocompatibility with fibroblasts and uroepithelial cells in culture. Another group designed and studied AMP Chain201D (KWIVWRWRFKR).98 This AMP, immobilized on a model silicone-based surface, exhibited high anti-microbial efficacy against Escherichia coli and Staphylococcus aureus isolates. AMPs exhibit great potential, but challenges persist and include inadequate AMP surface density, suboptimal coating, altered AMP orientation, and pH sensitivity. Further, microbial resistance to AMPs is seen, albeit to a lesser extent than traditional antibiotics.44,99 AMPs require additional study in vivo and in clinical trials.
Bacteriophages
Bacteriophages are viruses that infect and replicate within bacteria. They have been used clinically for decades, and have demonstrated efficacy in treating a number of bacterial infections.100 Prior to antibiotics, bacteriophages, or phages, were used to halt outbreaks of dysentery, cholera, and the plague. In the era of antibiotics, enthusiasm for bacteriophages waned. Now, in the context of increasing antibiotic resistance, there is renewed interest in bacteriophage therapy. Phages have unique properties that confer advantages over traditional antibiotics. First, phages are extremely specific. Their specificity makes them less likely to exhibit off-target collateral damage to healthy gut or vaginal flora. Because of their specificity, phage prophylaxis would likely require a cocktail, or in the case of recurrent CAUTI, a targeted approach to the previously isolated organism(s) (or the most common causative organisms). Additionally, although phage resistance is possible, phages co-evolve with bacteria to counter phage resistance. Further, phages invade bacteria through interaction with bacterial receptors, which often also act as virulence factors. Thus, as bacteria evolve receptors (or loss of receptors) to resist phages, they often become less pathogenic.100
Bacteriophages reduced biofilm formation in a preclinical catheter mixed-species biofilm model.101 Hydrogel-coated catheters were pre-treated with phage cocktails designed to target Pseudomonas aeruginosa and Proteus mirabilis.101 The catheters were then challenged with these Pseudomonas and Proteus strains in a continuous flow reactor containing artificial urine media. The phage pretreatment was associated with a 4 log10 reduction of P. aeruginosa biofilm counts and a >2 log10 reduction in P. mirabilis biofilm count. Other studies reported phage efficacy in biofilm reduction of Escherichia coli and Staphylococcus epidermidis.102,103 Conversely, phages have shown to contribute to biofilms as structural elements, promote wound infection in mice, and be associated with chronic wound infection in humans.104–106 A recent study in men with urinary tract infection who underwent transurethral resection of the prostate demonstrated the safety of intravesical bacteriophage instillation, as well as its noninferiority to standard-of-care antibiotic treatment.107,108 About 38% of the subjects in this trial had an indwelling catheter prior to intervention. Further work is needed regarding the utility and safety of bacteriophages in CAUTI prevention.109 Given their specificity, and the multiple causative organisms of CAUTI, a bacteriophage cocktail may prove to be a promising approach. This may include a general approach, wherein the cocktail is designed to inhibit the most common causative organisms of CAUTI, or a personalized precision approach wherein the cocktail is designed for a specific patient based on clinical and microbiological history.
Antibiotics
There has been considerable effort in the development of catheters either coated or impregnated with various antibiotics. The nitrofurazone catheters are discussed above, as they remain commercially available, but there are multiple others in preclinical development. For example, catheters coated with gentamicin, a broad spectrum bactericidal aminoglycoside, reduced incidence and severity of infection in a rabbit model.39,110 Catheters coated with ciprofloxacin, a fluoroquinolone that blocks bacterial DNA gyrase and blocks DNA replication, delayed CAUTI onset from 3.5 days to 5.3 days in a rabbit model. Norfloxacin, sparfloxacin, triclosan, and chlorhexidine catheters have also been investigated.39 Most of such antibiotic coatings and impregnations have yet to be the subject of clinical trials. Given the known contributions of antibiotics to bacterial resistance,17 and the numerous other technological advances discussed in this section, the utility of catheters coated with traditional antibiotics in the future clinical landscape remains uncertain.
Bacterial Interference
Bacterial interference, or competition, is the use of native flora or bacteria of low virulence to outcompete pathogenic bacteria for colonization and infection (Figure 1). As it becomes more accepted that the urinary tract is not physiologically sterile, there is greater interest in utilizing bacterial interference in the urinary tract to prevent CAUTI. It is hypothesized that the mechanism of action includes competition for nutrients and attachment sites, regulation of gene expression, immunomodulation, and production of antibacterial virulence factors.111 In a clinical trial in men with spinal cord injury who managed their bladders with clean intermittent catheterization, catheters were pre-inoculated with the benign strain E. coli 83972 (which was initially isolated from a girl with three years of asymptomatic bacteriuria without alteration in renal function) for 48 hours. Then, 13 patients were catheterized with these catheters for an indwelling time of three days. Six weeks after catheter removal, 8 of 13 patients remained colonized E. coli 83972. The colonized patients had a lower rate of UTI than they did prior to enrollment (0.77 versus 2.27 per patient-year, respectively).112 The same group subsequently assessed the utility of bacterial interference in the context of long-term indwelling catheters.113 They used benign E. coli strain HU2117, a derivative of 83972 with a papG deletion (papG codes for the PapG protein, which adheres to the kidney uroepithelium) as a safety measure, in a population of older adults with chronic indwelling urinary catheters. A total of 8 of 10 participants became colonized with the E. coli strain, with a mean duration of colonization of 57.7 days. Rates of UTI did not differ before, during, or after the colonization period. Of note, 5 patients had febrile UTI or urosepsis following inoculation, two of which were caused by E. coli, and in one case E. coli HU2117 was detected at the time of urosepsis. This highlights the importance of identification and development of avirulent strains of bacteria that effectively outcompete uropathogens for the urinary tract.
A randomized controlled clinical trial of bacterial competition included adult patients with spinal cord injury and neurogenic bladder who managed bladder drainage with a urinary catheter and had recurrent symptomatic CAUTI.114 In patients with positive urine cultures, an antibiotic course was first administered and catheters were changed prior to inoculation. Then, bladders of participants were inoculated with either E. coli HU2117 or saline via a catheter, which was then clamped for an hour. This was repeated, and individuals were thus inoculated twice on three successive days. The average number of symptomatic UTIs was lower in the E. coli HU 2117 group than the control group, and E. coli HU2117 did not cause symptomatic UTI in this study.
One of the challenges in the use of avirulent E. coli strain 83972 in establishing adherence to withstand urinary tract turbulence, is that it lacks the fimH gene. fimH codes for the type 1 pilus adhesin FimH, the most distal pilus subunit that adheres to the uroepithelium.20 In an effort to circumvent this, E. coli 83972 was engineered to express type 1 pili.115 Type 1 pilus forming strains were shown to increase adherence to urinary catheters, increase efficacy in blocking colonization by virulent E. coli, and importantly did not adhere to shed uroepithelial cells, suggesting they remained non-pathogenic. It was subsequently shown that the type 1 pili on strain 83972 did not promote inflammation in the human urinary tract.116 P pili, expressed by the pap operon are associated with upper urinary tract colonization and infection. An additional genetic modification of E. coli 83972 was made to induce expression of a surface-located P pilus receptor mimic, which was demonstrated to bind P pilus expressing E. coli.117 The expression of the P pilus receptor mimic impaired P pilus-mediated adhesion to human erythrocytes and kidney epithelium.118 The strain impaired colonization by uropathogenic E. coli in a mouse UTI model.
Bacterial interference may also be accomplished through the engineering of pre-established biofilms on urinary catheters, which may serve as live protective catheter coatings. Establishing bacterial adherence to urinary catheters has been the subject of several studies. For example, a general method for the biofunctionalization of a silicone material (polydimethylsiloxane) with mannoside ligands has been developed to optimize adherence.119 Briefly, CO2 plasma was used to activate the silicone surface and then poly(amidoamine) (PAMAM) dendrimers were attached. This generated an amino-terminated surface. Carboxy-terminated mannose derivatives were then covalently attached to this surface. On this surface, dense and stable E. coli 83972 biofilms could be established within 48 hours, and these biofilms reduced uropathogenic Enterococcus faecalis adhesion by over 100-fold, whereas E. coli 83972 on the unmodified substrate only reduced E. faecalis adherence by 5.5-fold. In a subsequent report, the group modified their technique and synthesized a series of structurally heterogenous alkyl and aryl mannosides, and immobilized them on biofunctionalized silicone surface coated with poly(amidoamine) (PAMAM) dendrimers.120 They showed that fimH + E. coli 83972 adhered rapidly to biphenyl mannoside surfaces and prevented E. faecalis colonization, even when incubated at the high concentration of 108 CFU/mL, for 11 days. The technique also was associated with similar results in E. coli Nissle 1917, a strain initially isolated during World War I from a soldier who escaped a diarrheal outbreak, and commercialized for over 90 years as a probiotic for intestinal disorders.121 Subsequent modifications have been made to streamline and simplify the surface modification process, and biofilms grown on a preconjugated mannoside ligand tethered to PAMAM dendrimer have demonstrated robust and stable adherence and reduction of uropathogen colonization by more than 3.2 log10.122
Bacterial interference is a promising avenue for the prevention of CAUTI. As optimization of adherence and competition continue, and as clinical trials accrue, a microbial interference-based approach may become a common standalone or complementary strategy. Basic research continues to define the molecular underpinnings of bacterial competition and its clinical implications. For example, Ohlemacher et al recently identified a metallopore molecule – escherichelin – that is produced by both a uropathogenic and a commensal Escherichia coli strain, and has the ability to inhibit iron uptake by Pseudomonas aeruginosa. This work implicates eschericelin-producing bacterial strains as potential candidates to prevent P. aeruginosa colonization through niche exclusion.123 Studies such as this will further inform catheter and irrigant development designed to prevent CAUTI.
Vaccination Strategies and Candidates
Vaccination against UTI is a viable and exciting strategy. Although much of the research in this area has focused on recurrent uncomplicated UTI, many of the principles are common to CAUTI and vaccine candidates were developed against Escherichia coli and Enterococcus spp, which are also the most common causative organisms of CAUTI. Thus, the principles of vaccination against these organisms may be translatable to CAUTI and other complicated UTIs. Whether vaccination will ultimately play a role as a standalone versus complementary strategy for CAUTI management remains to be determined. Given the abiotic nature of indwelling catheters, vaccination may work in tandem with other methods described above that are designed to reduce pathogen colonization. In order for vaccines to be effective and specific, general ideal components of a vaccine target include: pathogen specificity, antigenicity, surface expression, and robust production during infection.124 A vaccine based on such a molecule should trigger a robust humoral and cellular immune response upon microbial breach of the uroepithelium.
OM-89 (Uro-Vaxom; OM Pharma, Myerlin, Switzerland) is a lyophilized 18 uropathogenic E. coli strain membrane protein cocktail125 It is administered in an oral formulation once daily for one month, and then again month later with a lower frequency, as a booster. A meta-analysis of 5 studies including a total of approximately 1000 patients found that the mean number of UTIs, as well as antibiotic usage, was significantly lower in OM-89 treated patients compared to placebo controls.126 Thus, it has been included in the European Association of Urology (EAU) Guidelines as immunoprophylaxis in women with recurrent UTIs.127 The guidelines note that its efficacy in other groups remains to be established. Other immunostimulant vaccines, generally multi-strain cell lysates, are under development. Their formulations include vaginal, parenteral, and oral, but most have not been tested in a Phase III clinical trial.124
MV-140 (Uromune, Syner-Med Ltd UK; Immunotek S.L. Spain), a sublingual spray, has been evaluated for safety and efficacy.128 The MV-140 vaccine is composed of inactivated cell lysates of common uropathogens. Specifically, it contains lysates of E. coli, Klebsiella pneumonia, Proteus vulgaris, and Enterococcus faecalis. In a prospective study that included 77 women with recurrent UTI (each had 3 or more UTI episodes in the preceding 12 months), the vaccine was administered for 3 months, and 78% of the population was UTI-free over the 12-month follow-up period.128 In this study, one patient experienced rash and thus had to stop treatment. The first phase III, randomized, placebo-controlled, double-blind clinical trial was recently reported.129 The trial included 240 women with recurrent UTI, randomized to either MV-140 (for 3 or 6 months) or placebo. The median number of UTIs in the 9 months post-vaccination was 3.0 in the placebo compared to 0.0 in the MV-140 treatment groups. There was a greater UTI-free rate in the MV-140 groups. Five subjects reported adverse reactions, all of which were non-serious (3 in the treatment groups, 2 in the placebo group). While these remarkable findings open new prevention avenues for this patient cohort, whether or not they are generalizable to a population with indwelling urethral catheters remains to be determined.
The above vaccines have generally been developed and tested in the context of uncomplicated UTI, and as discussed, will require further study in the context of CAUTI. Exciting basic science advances have led to a new understanding of the pathogenesis of CAUTI specifically, and have identified new potential treatment targets.130 One approach, wherein a molecule critical for Enterococcus spp colonization of catheters is targeted, holds considerable promise. EbpA, the minor subunit tip of the sortase-assembled endocarditis- and biofilm-associated (Ebp) pilus, has been shown to be critical for the colonization of urinary catheters.19 Flores-Mireles et al showed that EbpA is an adhesin, and mediates attachment to host fibrinogen, which is released from the bladder upon catheterization and deposited on catheters in their CAUTI mouse model. In the CAUTI model, active immunization with the N-terminal domain of EbpA protected against infection. Passive immunity using antibodies against EbpA N-terminal domain also were both preventive and therapeutic against CAUTI in the model.131 The group also showed that this strategy was effective against other enterococcal clinical isolates including E. faecalis, E. faecium, E. gallinarum, and vancomycin-resistant enterococci (VRE). Of note, fibrinogen binding may be a general phenomenon in Gram-positive and some fungal infections, and has been shown in the case of Staphylococcus aureus, Staphylococcus epidermidis, Group A streptococci, as well as Candida albicans.132,133
Type 1 pili have been shown to be required for biofilm formation and uropathogenic colonization on catheters.26 This highlights the importance of vaccines targeted against pili to prevent colonization and subsequent infection. The FimCH vaccine, which targets the adhesive type 1 pilus tip subunit FimH, has been shown to be protective in mice and cynomolgus monkey models, with the animals generating antigen-specific long-lasting IgG antibodies.134,135 Similarly, a FimH construct truncated to contain only its N-terminal adhesin domain, with CpG oligonucleotides used as an adjuvant, conferred protection against cystitis in mice vaccinated intramuscularly or intranasally.136 The FimH vaccine’s efficacy was largely due to the generation of antibodies that blocked FimH-mediated colonization of the bladder. The FimH vaccine (Sequoia Vaccines, St. Louis, Missouri, MO, USA) has completed a phase 1 clinical trial, in which it was shown to be safe and tolerable and display good immunogenicity.137 The drug product is now undergoing a phase 2 double-blind randomized, placebo-controlled clinical trial. Another candidate against uropathogenic E. coli is the PapDG vaccine, which targets the adhesive P pilus tip subunit PapG. This vaccine also elicited a specific IgG antibiody response in cynomolgus monkeys,138 and clinical trials are needed to assess its safety and efficacy. The vaccine development pipeline in UTIs is promising, and the possibility that the findings translate from the uncomplicated UTI cohort to those with indwelling catheters is an exciting prospect.
Diagnosis: Current Evidence and Considerations
Diagnosis of CAUTI generally includes two elements. First, the patient must demonstrate signs or symptoms compatible with UTI. Second, urine culture should grow a bacterial species.127,139 While these two elements are broadly agreed upon, specifics are debated and CAUTI poses unique diagnostic considerations.
Signs and Symptoms
Signs and symptoms of UTI may include increased bladder sensation, urgency, frequency, dysuria, pain in the urinary tract, suprapubic tenderness, fever, rigors, altered mental status, malaise, lethargy with no other identified cause, flank pain, and/or costovertebral angle tenderness.14,127,139,140 In the context of a catheter, these symptoms may be subtle, and the classic combination of urgency, frequency, and dysuria cannot be relied upon since there is no volitional voiding (outside the context of a recently removed catheter). Further, subpopulations of individuals with urinary catheters including those with neurogenic lower urinary tract dysfunction (neurogenic bladder) and those who are critically ill may exhibit a different symptom complex.141,142 For example, in patients who are spinal cord injured, increased spasticity, autonomic dysreflexia, and sense of unease are also compatible with CAUTI.14 The ability to detect symptoms in these populations is limited as sensorium may be impaired. Because of often subtle symptoms and overlap with those of other etiology, overdiagnosis of CAUTI is common in cases where asymptomatic bacteriuria is truly present.141 As discussed above in the Prevention subsection: asymptomatic bacteriuria, aside from rare exceptions, culture and/or treatment should not be performed if no symptoms are present.
Urine Culture
The urine culture as a diagnostic component of CAUTI is wrought with challenges. First, the diagnostic cutoff is controversial. The IDSA states that CAUTI is defined by symptoms (as above) in the context of a urine culture with growth of ≥103 colony-forming units (CFU)/mL of ≥1 bacterial species in a single catheter urine specimen, or a midstream voided specimen in an individual whose catheter was removed in the preceding 48 hours.14 The EAU also uses a 103 CFU/mL cutoff.127 The Centers for Disease Control (CDC)/National Healthcare Safety Network (NHSN) uses a cutoff of 105 CFU/mL.143 Of note, the IDSA states that the 103 CFU/mL cutoff represents a compromise between the sensitivity of detecting CAUTI and the feasibility of laboratory quantitation of organisms. Further, it notes that even counts below this threshold may indeed represent true bacteriuria and may be interpreted as such by the clinician in decision-making regarding treatment in the context of symptoms. For example, one study assessed 47 patients with acute spinal cord injury and intermittent catheterization, 70% of whom had symptoms that were clearly or possibly related to CAUTI. In this population, a 102 CFU/mL threshold represented optimal sensitivity and specificity as compared to the suprapubic aspirate.144 Additionally, the IDSA notes that in the absence of symptoms, >105 CFU/mL may also be compatible with asymptomatic bacteriuria, and outside the context of symptoms, generally does not warrant treatment.14
Whereas in uncomplicated UTI, pyuria is an important diagnostic criterion, it lacks utility in CAUTI diagnosis. Pyuria, or the presence of white blood cells in urine, is indicative of inflammation of the urinary tract. It is usually present in CAUTI as well as asymptomatic bacteriuria. In a study of over 700 recently catheterized patients, the presence of pyuria for bacteriuria (105 CFU/mL) was 47% and specificity was 90%, with a positive predictive value of 32%.145 In a study that assessed 177 urinalyses with urine cultures performed in sequence in a cohort of 14 long-term catheterized patients, both bacteriuria and pyuria were common in asymptomatic episodes, and levels did not change considerably in the context of symptoms.146 Similarly, testing for leukocyte esterase or nitrites has been demonstrated to lack utility in establishing CAUTI diagnosis in catheterized patients in the ICU setting.147
Based in part on the above studies, IDSA states that in those with urinary catheters, pyuria should not be diagnostic of either CAUTI or asymptomatic bacteriuria, should not differentiate the two, and should not be an indication for treatment.14 However, the absence of pyuria in those with urinary catheters suggests a diagnosis other than CAUTI. In addition, the presence of cloudy and/or malodorous urine should not be used to differentiate between CAUTI and asymptomatic bacteria, or as an indication for urine culture or treatment.
When obtaining urine specimens from catheterized individuals, some technical points should be considered.14 First, the specimen should be obtained prior to initiation of antibiotics. This is due to the wide variety of causative organisms and differential resistance profiles. Resistance information of the causative organism should be used to guide treatment, and this data will be obscured or altered following the initiation of antibiotics. In those with short-term indwelling catheters, it is recommended that specimens be obtained through the catheter port, if available, using aseptic technique. Another technique suggested by the IDSA is puncturing the catheter tubing with a needle and syringe. Though this technique is not commonly used in practice, these recommendations are in place to ensure the closed-drainage system remains intact, which is known to reduce the risk of CAUTI.148 In those with urinary catheters that have been indwelling for longer duration, the preferred collection method is to replace the catheter and obtain the specimen from the freshly placed catheter. This is due to the finding that culture results in the context of a catheter with biofilm may not accurately reflect the status of the infection in the bladder, as culture results from a present versus exchanged catheter may be discordant.149–151 In the case of those with symptoms suggestive of CAUTI, catheter removal or exchange should be done immediately prior to starting antibiotics. Culture specimens should not be obtained from the urine drainage bag due to high risk of contamination, although the CDC notes that large volume urine specimens may be obtained this way for special analyses other than culture.29
Ancillary Workup
In a typical case of CAUTI (such as in the postoperative setting), additional urologic diagnostic workup, including cystoscopy and imaging, may not be necessarily indicated. Decisions regarding these modalities should be made in the context of clinical symptoms, examination, and history, and in atypical cases (eg, wherein a foreign body or renal calculi are suspected), such workup should not be delayed. Details of such workup are beyond the scope of this review. Urological consultation should be considered based on the patient history, clinical examination, and all available data.
Diagnosis: Challenges and Future Prospects
Signs and Symptoms
Symptoms of CAUTI may be subtle and atypical, as detailed above. In fact, some question the reliability of the vague symptoms that often define CAUTI, and propose that reliance on the current definition of CAUTI may lead to a missed number of nonurinary etiologies of fever, and unnecessary antibiotic prescription.152 Thus, one potential opportunity for improvement in symptomatic assessment is the development of valid and reliable patient-reported outcome measures, or questionnaires. One group has developed a series of questionnaires termed the Urinary Symptom Questionnaires for People with Neurogenic Bladder (USQNB).153–155 These questionnaires have been validated in the neurogenic bladder population that uses an indwelling catheter, clean intermittent catheterization, or voids spontaneously. They were designed to determine whether one’s urinary symptoms are related to urinary tract infection. These questionnaires were developed recently, and their implementation in the clinic may prove useful in eliciting potential symptoms that may or may not be attributed to CAUTI.
Biomarkers
The subtlety of CAUTI symptoms and the imperfectness of the urine culture pose the common dilemma of distinguishing CAUTI from asymptomatic bacteriuria. Thus, there is an urgent need for novel diagnostic biomarkers. Further, since antibiotic susceptibility testing is based on urine culture, it generally takes at least two days to result. Thus, to improve antibiotic decision-making prior to the availability of final results, novel and rapid assays and algorithms are needed. As culture-independent sequencing techniques advance, specific bacteria or patterns of bacteria may serve as CAUTI biomarkers. Although such techniques have been demonstrated to detect bacteria and resistance genes with high sensitivity,20 the clinical relevance of this requires further investigation. There has been significantly more study in biomarkers for uncomplicated UTI.156 Potential candidates include procalcitonin, interleukins, lactoferrin, and IgA. In addition, urinary cell-free DNA shows promise in monitoring infections of the urinary tract.157 These biomarkers are associated with varying levels of evidence and predictive value, and require study in the catheterized population.
In an investigation of UTI biomarkers in catheterized patients, Olszyna et al assessed IL-6 and IL-8,158 both of which had previously been found to be present in those with asymptomatic bacteriuria and pyelonephritis.159,160 IL-8 is a prototypic member of the CXC chemokine family, primarily targeting neutrophils, which are commonly present in urine of individuals with UTI.161 The authors found that IL-8 was elevated on the day of UTI diagnosis, and IL-8 remained low in the non-UTI controls. They also found that IL-6 was released 2–4 days before the onset of UTI, but this increase was also seen in the group that did not develop UTI. Another study, which included a pediatric population with neurogenic bladder (regardless of bladder drainage management), found that the urinary antimicrobial peptide (AMP) neutrophil gelatinase-associated lipocalin (NGAL) differed significantly between the UTI group and the group with asymptomatic bacteriuria with an AUC of 0.82.162 Although NGAL shows promise based on this study, further work is needed to determine its utility in the adult population with indwelling urethral catheters.
Artificial intelligence (AI) is poised to impact the field of diagnostics in general, and CAUTI is no exception. For example, machine learning, a branch of AI, is being implemented in biomarker development processes.163 Machine learning may also assist with rapid antibiotic susceptibility testing. Bhattacharyya et al coupled machine learning with early antibiotic-induced transcriptional changes together with genetic determinants of resistance. Using this approach, the group classified resistance with 94–99% accuracy with <4 hours assay time.164 Machine learning has also demonstrated utility in guiding antibiotic choices prior to final susceptibility results. For example, Kanjilal et al used EMR data in a population with uncomplicated UTI to train machine learning algorithms to predict probability of antibiotic resistance of bacteria to first- and second-line antibiotic therapy.165 The algorithms resulted in a 67% reduction in second-line therapy use relative to clinicians, and reduced inappropriate antibiotic therapy by 18%. Whether such algorithms possess utility in the population with CAUTI and other complicated UTI requires further study and validation, but the prospect is promising.
As biomarker and antibiotic resistance assays and algorithm development continues, the medical community remains reliant on urine culture for the laboratory diagnosis and antibiotic susceptibility of CAUTI, which also may benefit from AI assistance.166 A common issue with urine culture is that it is frequently subject to contamination, and as discussed this may be particularly challenging in the context of those who are catheterized, wherein a culture may be obtained inappropriately (eg, from a drainage bag, or from a catheter that has been indwelling for a long period of time). Advances in automation and computing have led to the development and implementation of machine learning to assist in the laboratory diagnosis of positive versus negative versus contaminated urine cultures. In a recent study, a powerful deep learning model predicted culture result from blood agar plate images with 100% accuracy when compared to laboratory technologist consensus (the gold standard used in the study).167 A future extension of this work would be in the prediction of contamination versus infection in the subpopulation with urinary catheters, and, potentially, identifying patterns on urine cultures that may be reflective of CAUTI versus asymptomatic bacteriuria. This may be a one component of a multi-component algorithm that includes symptoms and biomarkers.
Treatment: Current Evidence and Considerations
Catheter Removal or Exchange
The CAUTI treatment paradigm includes catheter removal or exchange together with antibiotics. The catheter should be removed if no longer needed, or exchanged, prior to initiating antibiotic therapy (a urine culture must also be obtained prior to initiation of antibiotics, as discussed in the diagnostic section), particularly if it has been in place for longer than two weeks. A randomized, controlled clinical trial included 54 elderly participants living in nursing homes with long-term indwelling catheters and CAUTIs.168 The groups were randomized to either catheter replacement or no replacement prior to a course of fluoroquinolone antibiotics. The replacement group had significantly lower rates of polymicrobial bacteriuria at day 28, a shorter time to afebrile status and clinical improvement, and a lower rate of CAUTI at 28 days following treatment. Potential disadvantages of replacement include the risk of mucosal trauma and increased cost,168,169 which may be mitigated with adequate lubrication for insertion and atraumatic technique.
Antibiotics
Choice of antibiotics generally should be guided by severity of illness, the local antibiotic resistance data (antibiogram), host factors (including allergies), and sensitivity data of the isolated organisms.127 The EAU provides additional guidance regarding choice of antimicrobials.127 It should be noted that this guidance is based on regional antibiotic resistance patterns, and local patterns should be assessed prior to decision-making. The EAU guidelines state that treatment for CAUTI should follow the recommendations regarding treatment of other complicated UTIs. The guidelines recommend not using amoxicillin, TMP-SMX, or amoxicillin/clavulanic acid for empiric treatment of complicated UTI. This is based on current resistance profiles (and thus may vary based on local antibiograms).170 The EAU recommends that in those with systemic symptoms requiring hospitalization, amoxicillin plus an aminoglycoside, or a second-generation cephalosporin plus an aminoglycoside, or a third-generation cephalosporin intravenously may be used. The choice among these should be dictated by local resistance patterns, and the regimen should be tailored based on final culture resistance data. The guidelines recommend only using ciprofloxacin provided that the local resistance percentages are <10% when the patient is not seriously ill and it is considered safe to start initial oral treatment, or if the patient has had an anaphylactic reaction to a beta-lactam antimicrobial. Ciprofloxacin is especially not an optimal choice if the individual is a urology patient or has received ciprofloxacin in the past 6 months. The risk of tendonitis and tendon rupture, as is indicated in the FDA black box warning for ciprofloxacin, must also be considered.171 In those with a hypersensitivity to penicillin, a cephalosporin can still be prescribed according to the guidelines, unless the patient has had systemic anaphylaxis in the past.
Optimal duration of treatment for CAUTIs should be guided by illness severity, treatment response and host factors. The IDSA states that a seven-day course is the recommended duration for those who have prompt resolution of symptoms, and a 10- to 14-day regimen is recommended in cases of a delayed response.14 The IDSA states that a 5-day course of levofloxacin may be considered in those who are not severely ill. Finally, the IDSA states that in women ≤65 years of age with a CAUTI in whom the catheter has been removed, a three-day course of antibiotics may be considered. These recommendations are based on the treatment tenet of limiting unnecessary treatment duration to reduce the selective pressure for drug-resistant organisms. In the context of CAUTI diagnosis and treatment, urologic workup for any relevant pathology should be pursued and urological abnormalities and/or underlying complicating factors should be addressed.
Treatment: Challenges and Future Prospects
Antibiotics
Clinical Pipeline
Antibiotics remain the cornerstone of CAUTI treatment, but their use is associated with multiple challenges. First, less than a century into the antibiotic era, rates of antibiotic resistance have become alarmingly high, raising the possibility of a post-antibiotic era wherein even common infections could become life threatening.81,172 A major contributor to antibiotic resistance is clinical antibiotic use, a subset of which is unnecessary or unnecessarily prolonged. Further, the clinical pipeline of antibiotics remains insufficient to tackle these challenges. Large pharmaceutical companies continue to exit the development field, and both clinical and preclinical development is dominated by small and medium-sized companies.173,174 Despite the slow and insufficient antimicrobial pipeline, there are several examples of novel and effective drugs that have recently undergone FDA approval for complicated UTIs (including CAUTI) caused by multi-drug resistant organisms.
Cefiderocol (Fetroja, Shionogi, Florham Park, NJ, USA), a novel siderophore cephalosporin, was demonstrated to have broad activity against Enterobacteriaceae and non-fermenting bacteria, including Pseudomonas aeruginosa and Acinetobacter baumannii in vitro. Cefiderocol was shown to be safe and effective in phase 2 (versus imipenem-cilastatin) and Phase 3 clinical trials (versus best available therapy) in patients with complicated urinary tract infection at risk for multidrug-resistant Gram-negative infections.175,176 It had a significantly higher efficacy (clinical response and microbiological eradication at 7 days post-therapy) than high-dose imipenem/cilastatin. It was approved by the FDA in November 2019 for adults with complicated UTIs caused by susceptible Gram-negative organisms who have limited or no alternative treatment options.177 Another recently approved treatment for complicated UTI is meropenem-vaborbactam (Vabomere, Melinta Therapeutics, New Haven, CT, USA).178,179 It was shown to be noninferior to piperacillin-tazobactam in symptom improvement/resolution paired with microbiological eradication. It was approved by the FDA in August 2017 for adults with complicated UTI caused by susceptible Escherichia coli, Klebsiella pneumoniae, and Enterobacter cloacae.180 Plazomicin (Zemdri, Achaogen, San Francisco, CA, USA), an aminoglycoside antibiotic with bactericidal activity against multidrug resistant Enterobacteriaceae, recently was studied for safety and efficacy in a phase 3 clinical trial against meropenem, and was shown to be noninferior in the treatment of complicated UTI caused by Enterobacteriaceae, including multidrug resistant strains.181 It was approved by the FDA in June 2018.182 An additional drug product containing imipenem/cilastatin plus relebactam (Recarbrio, Merck, Kenilworth, NJ, USA) has been developed. Relebactam is a ß-lactamase inhibitor, and can restore imipenem activity against imipenem non-susceptible pathogens. In a phase 3 clinical trial that included individuals with complicated UTI, it was shown to be efficacious and well tolerated, and it was approved by the FDA in July 2019.183,184
One promising novel antibiotic, a synthetic penem termed sulopenem (Iterum Therapeutics, Dublin, Ireland), demonstrated promise in vitro against Enterobacteriaceae including ESBL-producing strains, and Gram-positive activity similar to other carbapenems, as well as safety in Phase II clinical trials. However, its phase III trial study in treatment of complicated UTI did not meet its primary endpoint.185 The randomized, double-blind trial included 1395 patients with complicated UTI was designed to measure sulopenem’s efficacy, tolerability and safety, with a primary endpoint of clinical and microbiological response on day 21. Patients were randomized to either IV followed by oral sulopenem, or IV ertapenem followed by oral ciprofloxacin (or amoxicillin-clavulanate for quinolone-resistant isolates). There was a lower response rate in the sulopenem group, driven almost entirely by higher rates of asymptomatic bacteriuria detected at the test of cure visit.
As the armamentarium of antibiotics improves, organisms will continue to develop resistance. Thus, the arms race between host and microbe continues. Each of the recently approved drugs has its own set of adverse effects and contraindications. The WHO notes that the newly approved products also generally have limited clinical benefit over existing treatments. The lack of differentiation from current treatments, together with lack of their inclusion in clinical guidelines and their higher costs, make their role in the future clinical landscape unclear.173 In addition, the majority of these new drug products are from existing drug classes wherein multiple resistance mechanisms are present, and thus there is the possibility of rapid evolution of resistance to these products. Furthermore, the WHO notes that based on anecdotal evidence and sales figures, it appears that clinicians are reluctant to use the novel antibiotic agents for the conditions (including complicated UTI) that were the initial targets for approval. Thus, novel antibiotics from new drug classes are critically needed.
Novel Methods of Antibiotic Identification and Development
Recent and exciting advances in basic science have led to new opportunities for the identification of novel classes of antibiotics. Natural products generated by cultured bacteria were a source of many antibiotics for decades, but their use has dwindled based on high re-discovery rates. Given that only a small fraction of microbes is cultureable, a team set out to develop a culture-independent discovery platform to identify natural product antibiotics produced by uncultureable bacteria obtained from soil.186 Using this platform, Hover et al discovered a novel drug class termed malacidins. The malacidins were active against multi-drug resistant bacteria, were demonstrated to sterilize methicillin-resistant Staphylcoccus aureus (MRSA) in an animal wound model, and did not contribute to antibiotic resistance under laboratory conditions. Specifically, even after exposure to sub-lethal levels of malicidin for 20 days, no malicidin-resistant Staphylococcus aureus were detected. Whether horizontal gene transfer from environmental bacterial strains could contribute to resistance warrants investigation. The authors note that the scaling and automation of their technique may allow for the systematic discovery of new natural product antibiotics, which have thus far remained hidden in the global metagenome, and represents a powerful approach to combat resistance.
A recent advance in antibiotic discovery was driven by the use of artificial intelligence.187 Stokes et al trained a deep neural network to predict molecules with antimicrobial activity from multiple chemical libraries. They used a training set of 2335 molecules to predict growth inhibition of Escherichia coli. Then, they applied their model to libraries comprising >107 million molecules. In doing so, they discovered a molecule termed halicin that had antimicrobial activity against a wide range of pathogens including Mycobacterium tuberculosis and carbapenem-resistant Enterobacteriaceae. Importantly, this molecule is structurally dissimilar to other known antibiotics. It demonstrated efficacy against Clostridioides difficile and pan-resistant Acinetobacter baumannii infections in murine models. Their model also identified 8 antibacterial compounds that were structurally dissimilar to known antibiotics. Such techniques open new avenues for novel classes of antibiotic discovery.
Small Molecule Alternatives to Traditional Antibiotics
Small molecule alternatives to traditional antibiotics represent a promising category of molecules to treat infection. They are less likely to contribute to resistance because they target microbial pathogenic mechanisms, specifically, without disrupting a central metabolic process. They do not attenuate growth or kill the microbe. Because of this, they are also less likely to have off-target effects on commensal flora, and thus may not significantly alter the healthy gut or vaginal flora.
Bacterial adhesion is a key early step for colonization and CAUTI. Adhesion is frequently mediated by pili, which function as virulence factors for many known uropathogens.20 Type 1 pili mediate adhesion to the bladder and have been shown to be important in biofilm formation, and P pili mediate adhesion to the kidneys. Both type 1 and P pili are assembled by the chaperone-usher pathway.188 Basic science has shed light on the chaperone-usher pilus structure and assembly mechanism.189–196 The detailed understanding of the pilus architecture, molecular interfaces, and structure-function relationships that these studies and others provide, makes pilus adherence and assembly attractive targets for novel small molecule alternatives to antibiotics.
The first pilus-targeting approach is to disrupt the pilus–receptor interaction between the microbe and the host. In the case of type 1 pili, the FimH pilus subunit is the adhesive subunit, and located at the most distal position, optimized for adherence to the host bladder.197 Mannosides, briefly discussed in the Bacterial Interference section, are soluble small molecule receptor analogs that contain a mannose group. They bind and occupy the FimH binding site, thus preventing adhesion and colonization of the urinary tract. They have been shown to be orally bioavailable and act against both established UTI and CAUTI (in a combinatorial fashion with TMP-SMX) in mouse models.26,198,199 Galabiose-based inhibitors are also the subject of investigation to target the P pilus adherence to host cells.200
Pilus assembly and secretion represents a second major target for small molecule inhibition. Pilicides are a class of small molecule inhibitors of chaperone-usher pilus assembly that bind to a periplasmic chaperone molecule and thus interfere with pilus subunit binding to the outer membrane usher, a bacterial membrane-spanning protein that assembles adhesive pili from the periplasmic pilus subunits. Pilicides reduce P pilus and type 1 pilus biogenesis, as well as pilus-mediated adhesion and biofilm formation in vitro201,202 Other pilicides have been developed to have activity against curli, and have been shown to reduce biofilm formation and colonization in a mouse UTI model.203 New pilicides are being investigated that attenuate pilus biogenesis through disruption of pilus subunit–subunit interactions, protein folding, and other aspects of the pilus assembly mechanism.204–206 Coilicides, another category of potential inhibitors, are designed to prevent uncoiling and recoiling of the pilus rod, and have been shown to lock the rod in a noncompliant form.207 Polyclonal antibodies were similarly shown to reduce the elastic properties of the P pilus rod.208
These novel pilus assembly- and adhesion-targeting therapeutics are moving forward in the development pipeline. Notably, the mannoside, GSK3882347, is now in phase 1 clinical trials (NCT04488770).209 Although a number of these small molecules have shown efficacy against biofilm formation in vitro, additional in vivo work and clinical trials will be needed to establish their safety and efficacy, and whether the findings are generalizable to the population with indwelling catheters.
Phytotherapy
Several herbal and other alternative therapies for UTIs have been studied. However, there are few studies on herbal therapies for CAUTI, specifically. For example, BNO-1045 (Canephron® N) is a herbal medicine product containing centaury powder (Centaurii herba), lovage root powder (Levistici radix) and rosemary leaf powder (Rosmarini folium). Studies have shown it has anti-inflammatory, spasmolytic, antiadhesive, antinociceptive, and diuretic properties.210 It has also been shown to preserve the gut microbiome.211 In a phase III clinical trial, BNO-1045 was noninferior to Fosfomycin in uncomplicated UTI treatment.210 Another study suggests that BNO-1045 could prevent postoperative UTI following surgery in which a catheter was placed.212 Additional trials are needed to determine whether BNO-1045 if effective in CAUTI and other complicated UTI treatment or prevention. Angocin® is a herbal medicinal product containing nasturtium and horseradish powder, and an observational study supported its potential efficacy in treatment and prevention of CAUTI.213 Similarly, trials are needed to further assess its safety and efficacy.
Conclusions
CAUTIs are the most common healthcare-associated infection and the most common cause of secondary bloodstream infections, and antibiotic resistance rates are alarmingly high. Diagnosis of CAUTI relies upon both urine culture and symptoms, both of which are problematic and controversial in the population with indwelling catheters. Catheter coatings, catheter materials, and vaccination are novel preventive strategies. Another interesting and promising prevention strategy is bacterial interference, wherein nonvirulent microbial strains are designed to outcompete pathogens for the urinary tract niche to reduce CAUTI risk. Novel diagnostic strategies and treatment decision aids are under development and include several classes of biomarkers often coupled with artificial intelligence algorithms, cell-free DNA, and others. Though the antibiotic drug development pipeline is currently insufficient to keep up with resistance rates, new drug products have recently undergone FDA approval for treatment of multi-drug resistant complicated UTI. Novel alternatives to traditional antibiotics are under study. They include inhibitors that specifically target bacterial pathogenesis mechanisms such as adhesive pilus assembly, to prevent catheter and bladder adherence. These molecules, termed mannosides and pilicides, target only a pathogenic mechanism rather than a central metabolic pathway. Thus, they are unique in that they do not select for antibiotic resistance, and are less likely to disrupt healthy gut or vaginal flora. These strategies are promising and welcome in the era of rampant and increasing antibiotic resistance. Clinical, translational, and basic sciences are continuing to provide new evidence and insight regarding the pathogenesis, prevention, and treatment of CAUTI. An understanding of the important challenges in the field coupled with the current evidence will inform the next generation of CAUTI management.
Funding
There is no funding to report.
Disclosure
The author reports no conflicts of interest in this work.
References
1. Stamm WE, Norrby SR. Urinary tract infections: disease panorama and challenges. J Infect Dis. 2001;183(Supplement_1):S1–S4. doi:10.1086/318850
2. Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13(5):269–284. doi:10.1038/nrmicro3432
3. Lichtenberger P, Hooton TM. Complicated urinary tract infections. Curr Infect Dis Rep. 2008;10(6):499–504. doi:10.1007/s11908-008-0081-0
4. Levison ME, Kaye D. Treatment of complicated urinary tract infections with an emphasis on drug-resistant gram-negative uropathogens. Curr Infect Dis Rep. 2013;15(2):109–115. doi:10.1007/s11908-013-0315-7
5. Lo E, Nicolle LE, Coffin SE, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Cont Hosp Epidemiol. 2014;35(5):464–479. doi:10.1086/675718
6. Foxman B. The epidemiology of urinary tract infection. Nat Rev Urol. 2010;7(12):653–660. doi:10.1038/nrurol.2010.190
7. Crnich CJ, Drinka P. Medical device–associated infections in the long-term care setting. Infect Dis Clin. 2012;26(1):143–164. doi:10.1016/j.idc.2011.09.007
8. Nicolle LE. Catheter associated urinary tract infections. Antimicrob Resist Infect Control. 2014;3(1):1–8. doi:10.1186/2047-2994-3-23
9. Umscheid CA, Mitchell MD, Doshi JA, Agarwal R, Williams K, Brennan PJ. Estimating the proportion of healthcare-associated infections that are reasonably preventable and the related mortality and costs. Infect Cont Hosp Epidemiol. 2011;32(2):101–114. doi:10.1086/657912
10. Chenoweth CE, Gould CV, Saint S. Diagnosis, management, and prevention of catheter-associated urinary tract infections. Infect Dis Clin. 2014;28(1):105–119. doi:10.1016/j.idc.2013.09.002
11. Stevenson KB, Moore J, Colwell H, Sleeper B. Standardized infection surveillance in long-term care interfacility comparisons from a regional cohort of facilities. Infect Cont Hosp Epidemiol. 2005;26(3):231–238. doi:10.1086/502532
12. Peng D, Li X, Liu P, et al. Epidemiology of pathogens and antimicrobial resistance of catheter-associated urinary tract infections in intensive care units: a systematic review and meta-analysis. Am J Infect Control. 2018;46(12):e81–e90. doi:10.1016/j.ajic.2018.07.012
13. Chant C, Smith OM, Marshall JC, Friedrich JO. Relationship of catheter-associated urinary tract infection to mortality and length of stay in critically ill patients: a systematic review and meta-analysis of observational studies. Crit Care Med. 2011;39(5):1167–1173. doi:10.1097/CCM.0b013e31820a8581
14. Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(5):625–663. doi:10.1086/650482
15. Avalos Vizcarra I, Hosseini V, Kollmannsberger P, et al. How type 1 fimbriae help Escherichia coli to evade extracellular antibiotics. Sci Rep. 2016;6(1):1–13. doi:10.1038/srep18109
16. Michael CA, Dominey-Howes D, Labbate M. The antimicrobial resistance crisis: causes, consequences, and management. Front Public Health. 2014;2:145. doi:10.3389/fpubh.2014.00145
17. Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. Pharm Therap. 2015;40(4):277.
18. Parsons CL. Pathogenesis of urinary tract infections: bacterial adherence, bladder defense mechanisms. Urol Clin North Am. 1986;13(4):563–568. doi:10.1016/S0094-0143(21)00262-7
19. Flores-Mireles AL, Pinkner JS, Caparon MG, Hultgren SJ. EbpA vaccine antibodies block binding of Enterococcus faecalis to fibrinogen to prevent catheter-associated bladder infection in mice. Sci Transl Med. 2014;6(254):254ra127–254ra127. doi:10.1126/scitranslmed.3009384
20. Werneburg GT, Nguyen A, Henderson NS, et al. The natural history and composition of urinary catheter biofilms: early uropathogen colonization with intraluminal and distal predominance. J Urol. 2020;203(2):357–364. doi:10.1097/JU.0000000000000492
21. Kostakioti M, Hadjifrangiskou M, Hultgren SJ. Bacterial biofilms: development, dispersal, and therapeutic strategies in the Dawn of the postantibiotic era. Cold Spring Harb Perspect Med. 2013;3(4):a010306. doi:10.1101/cshperspect.a010306
22. Trautner BW, Darouiche RO. Role of biofilm in catheter-associated urinary tract infection. Am J Infect Control. 2004;32(3):177–183. doi:10.1016/j.ajic.2003.08.005
23. Hadjifrangiskou M, Kostakioti M, Chen SL, Henderson JP, Greene SE, Hultgren SJ. A central metabolic circuit controlled by QseC in pathogenic Escherichia coli. Mol Microbiol. 2011;80(6):1516–1529. doi:10.1111/j.1365-2958.2011.07660.x
24. Foxman B. Urinary tract infection syndromes: occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect Dis Clin. 2014;28(1):1–13. doi:10.1016/j.idc.2013.09.003
25. Danese PN, Pratt LA, Dove SL, Kolter R. The outer membrane protein, antigen 43, mediates cell‐to‐cell interactions within Escherichia coli biofilms. Mol Microbiol. 2000;37(2):424–432. doi:10.1046/j.1365-2958.2000.02008.x
26. Guiton PS, Cusumano CK, Kline KA, et al. Combinatorial small-molecule therapy prevents uropathogenic Escherichia coli catheter-associated urinary tract infections in mice. Antimicrob Agents Chemother. 2012;56(9):4738–4745. doi:10.1128/AAC.00447-12
27. Mittal R, Aggarwal S, Sharma S, Chhibber S, Harjai K. Urinary tract infections caused by Pseudomonas aeruginosa: a minireview. J Infect Public Health. 2009;2(3):101–111. doi:10.1016/j.jiph.2009.08.003
28. Saint S, Greene MT, Krein SL, et al. A program to prevent catheter-associated urinary tract infection in acute care. N Engl J Med. 2016;374(22):2111–2119. doi:10.1056/NEJMoa1504906
29. Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA; Committee HICPA. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Cont Hosp Epidemiol. 2010;31(4):319–326. doi:10.1086/651091
30. Bagley K, Severud L. Preventing catheter-associated urinary tract infections with incontinence management alternatives: pureWick and condom catheter. Nurs Clin. 2021;56(3):413–425. doi:10.1016/j.cnur.2021.05.002
31. Fakih MG, Watson SR, Greene MT, et al. Reducing inappropriate urinary catheter use: a statewide effort. Arch Intern Med. 2012;172(3):255–260. doi:10.1001/archinternmed.2011.627
32. Rosenthal V, Todi S, Alvarez-Moreno C, et al. Impact of a multidimensional infection control strategy on catheter-associated urinary tract infection rates in the adult intensive care units of 15 developing countries: findings of the International Nosocomial Infection Control Consortium (INICC). Infection. 2012;40(5):517–526. doi:10.1007/s15010-012-0278-x
33. Saint S, Greene MT, Kowalski CP, Watson SR, Hofer TP, Krein SL. Preventing catheter-associated urinary tract infection in the United States: a national comparative study. JAMA Intern Med. 2013;173(10):874–879. doi:10.1001/jamainternmed.2013.101
34. Kunin CM, McCormack RC. Prevention of catheter-induced urinary-tract infections by sterile closed drainage. N Engl J Med. 1966;274(21):1155–1161. doi:10.1056/NEJM196605262742101
35. Tenke P, Kovacs B, Johansen TEB, Matsumoto T, Tambyah PA, Naber KG. European and Asian guidelines on management and prevention of catheter-associated urinary tract infections. Int J Antimicrob Agents. 2008;31:68–78. doi:10.1016/j.ijantimicag.2007.07.033
36. Maki DG, Tambyah PA. Engineering out the risk for infection with urinary catheters. Emerg Infect Dis. 2001;7(2):342. doi:10.3201/eid0702.010240
37. Darouiche RO, Goetz L, Kaldis T, Cerra-Stewart C, AlSharif A, Priebe M. Impact of StatLock securing device on symptomatic catheter-related urinary tract infection: a prospective, randomized, multicenter clinical trial. Am J Infect Control. 2006;34(9):555–560. doi:10.1016/j.ajic.2006.03.010
38. Al-Hazmi H. Role of duration of catheterization and length of hospital stay on the rate of catheter-related hospital-acquired urinary tract infections. Res Rep Urol. 2015;7:41. doi:10.2147/RRU.S75419
39. Andersen MJ, Flores-Mireles AL. Urinary catheter coating modifications: the race against catheter-associated infections. Coatings. 2020;10(1):23. doi:10.3390/coatings10010023
40. Talja M, Korpela A, Järvi K. Comparison of urethral reaction to full silicone, hydrogen‐coated and siliconised latex catheters. Br J Urol. 1990;66(6):652–657. doi:10.1111/j.1464-410X.1990.tb07203.x
41. Murakami S, Igarashi T, Tanaka M, Tobe T, Mikami K. Adherence of bacteria to various urethral catheters and occurrence of catheter-induced urethritis. Hinyokika Kiyo. 1993;39(1):107–111.
42. Schumm K, Lam TB. Types of urethral catheters for management of short‐term voiding problems in hospitalised adults. Cochrane Database Syst Rev. 2008;(2). doi:10.1002/14651858.CD004013.pub3
43. Kilonzo M, Vale L, Pickard R, Lam T, N’Dow J; Catheter Trial Group. Cost effectiveness of antimicrobial catheters for adults requiring short-term catheterisation in hospital. Eur Urol. 2014;66(4):615–618. doi:10.1016/j.eururo.2014.05.035
44. Zhu Z, Wang Z, Li S, Yuan X. Antimicrobial strategies for urinary catheters. J Biomed Mater Res A. 2019;107(2):445–467. doi:10.1002/jbm.a.36561
45. Johnson JR, Delavari P, Azar M. Activities of a nitrofurazone-containing urinary catheter and a silver hydrogel catheter against multidrug-resistant bacteria characteristic of catheter-associated urinary tract infection. Antimicrob Agents Chemother. 1999;43(12):2990–2995. doi:10.1128/AAC.43.12.2990
46. Menezes FG, Corrêa L, Medina‐Pestana JO, Aguiar WF, Camargo LFA. A randomized clinical trial comparing Nitrofurazone‐coated and uncoated urinary catheters in kidney transplant recipients: results from a pilot study. Transplant Infect Dis. 2019;21(2):e13031. doi:10.1111/tid.13031
47. Lee S-J, Kim SW, Cho Y-H, et al. A comparative multicentre study on the incidence of catheter-associated urinary tract infection between nitrofurazone-coated and silicone catheters. Int J Antimicrob Agents. 2004;24:65–69. doi:10.1016/j.ijantimicag.2004.02.013
48. Lam TB, Omar MI, Fisher E, Gillies K, MacLennan S. Types of indwelling urethral catheters for short‐term catheterisation in hospitalised adults. Cochrane Database Syst Rev. 2014;(9). doi:10.1002/14651858.CD004013.pub4
49. Niël‐Weise BS, van den Broek PJ. Antibiotic policies for short‐term catheter bladder drainage in adults. Cochrane Database Syst Rev. 2005;(3). doi:10.1016/j.jamcollsurg.2005.09.019
50. Lusardi G, Lipp A, Shaw C. Antibiotic prophylaxis for short‐term catheter bladder drainage in adults. Cochrane Database Syst Rev. 2013;(7). doi:10.1002/14651858.CD005428.pub2
51. Tenke P, Mezei T, Bőde I, Köves B. Catheter-associated urinary tract infections. Eur Urol Suppl. 2017;16(4):138–143. doi:10.1016/j.eursup.2016.10.001
52. Marschall J, Carpenter CR, Fowler S, Trautner BW. Antibiotic prophylaxis for urinary tract infections after removal of urinary catheter: meta-analysis. BMJ. 2013;346:f3147–f3147. doi:10.1136/bmj.f3147
53. Banks JA, McGuire BB, Loeb S, Shrestha S, Helfand BT, Catalona WJ. Bacteriuria and Antibiotic Resistance in Catheter Urine Specimens Following Radical Prostatectomy. Elsevier; 2013:1049–1053.
54. Berrondo C, Feng C, Kukreja JB, Messing EM, Joseph JV. Antibiotic prophylaxis at the time of catheter removal after radical prostatectomy: a prospective randomized clinical trial. Elsevier. 2019;181:e7–181. e14.
55. Wazait H, Van der Meullen J, Patel H, et al. Antibiotics on urethral catheter withdrawal: a hit and miss affair. J Hosp Infect. 2004;58(4):297–302. doi:10.1016/j.jhin.2004.06.012
56. Ehdaie B, Jibara G, Sjoberg DD, et al. The duration of antibiotics prophylaxis at the time of catheter removal after radical prostatectomy: clinically integrated, cluster, randomized trial. J Urol. 2021;206(3):662–668. doi:10.1097/JU.0000000000001845
57. Kaouk J, Beksac AT, Abou Zeinab M, Duncan A, Schwen ZR, Eltemamy M. Single port transvesical robotic radical prostatectomy: initial clinical experience and description of technique. Urology. 2021;155:130–137. doi:10.1016/j.urology.2021.05.022
58. Anger J, Lee U, Ackerman AL, et al. Recurrent uncomplicated urinary tract infections in women: AUA/CUA/SUFU guideline. J Urol. 2019;202(2):282–289. doi:10.1097/JU.0000000000000296
59. Sihra N, Goodman A, Zakri R, Sahai A, Malde S. Nonantibiotic prevention and management of recurrent urinary tract infection. Nat Rev Urol. 2018;15(12):750–776. doi:10.1038/s41585-018-0106-x
60. Zafriri D, Ofek I, Adar R, Pocino M, Sharon N. Inhibitory activity of cranberry juice on adherence of type 1 and type P fimbriated Escherichia coli to eucaryotic cells. Antimicrob Agents Chemother. 1989;33(1):92–98. doi:10.1128/AAC.33.1.92
61. Whiteside JL. Urine trouble without cranberries? Am J Obstet Gynecol. 2015;213(2):123–124. doi:10.1016/j.ajog.2015.04.030
62. Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012;(10). doi:10.1002/14651858.CD001321.pub5
63. Foxman B, Cronenwett AE, Spino C, Berger MB, Morgan DM. Cranberry juice capsules and urinary tract infection after surgery: results of a randomized trial. Am J Obstet Gynecol. 2015;213(2):
64. Gunnarsson A-K, Gunningberg L, Larsson S, Jonsson KB. Cranberry juice concentrate does not significantly decrease the incidence of acquired bacteriuria in female hip fracture patients receiving urine catheter: a double-blind randomized trial. Clin Interv Aging. 2017;12:137. doi:10.2147/CIA.S113597
65. Ji L, Badalato GM, Chung DE, Cooper KL, Rutman MP. Cranberry products for the prevention of catheter-associated urinary tract infections. Curr Bladder Dysfunct Rep. 2020;15:1–5.
66. Lo TS, Hammer KD, Zegarra M, Cho WC. Methenamine: a forgotten drug for preventing recurrent urinary tract infection in a multidrug resistance era. Expert Rev Anti Infect Ther. 2014;12(5):549–554. doi:10.1586/14787210.2014.904202
67. Chwa A, Kavanagh K, Linnebur SA, Fixen DR. Evaluation of methenamine for urinary tract infection prevention in older adults: a review of the evidence. Therap Adv Drug Saf. 2019;10:2042098619876749. doi:10.1177/2042098619876749
68. Kostiala AA, Nyrén P, Runeberg L. Effect of nitrofurantoin and methenamine hippurate prophylaxis on bacteria and yeasts in the urine of patients with an indwelling catheter. J Hosp Infect. 1982;3(4):357–364. doi:10.1016/0195-6701(82)90068-8
69. Norrman K, Wibell L. Treatment with methenamine hippurate† in the patient with a catheter. J Int Med Res. 1976;4(2):115–117. doi:10.1177/030006057600400206
70. Schiøtz HA, Guttu K. Value of urinary prophylaxis with methenamine in gynecologic surgery. Acta Obstet Gynecol Scand. 2002;81(8):743–746. doi:10.1080/j.1600-0412.2002.810810.x
71. Wellens A, Garofalo C, Nguyen H, et al. Intervening with urinary tract infections using anti-adhesives based on the crystal structure of the FimH–oligomannose-3 complex. PLoS One. 2008;3(4):e2040. doi:10.1371/journal.pone.0002040
72. Phé V, Pakzad M, Haslam C, et al. Open label feasibility study evaluating D‐mannose combined with home‐based monitoring of suspected urinary tract infections in patients with multiple sclerosis. Neurourol Urodyn. 2017;36(7):1770–1775. doi:10.1002/nau.23173
73. Falagas ME, Betsi GI, Tokas T, Athanasiou S. Probiotics for prevention of recurrent urinary tract infections in women. Drugs. 2006;66(9):1253–1261. doi:10.2165/00003495-200666090-00007
74. Toh S-L, Lee BB, Ryan S, et al. Probiotics [LGG-BB12 or RC14-GR1] versus placebo as prophylaxis for urinary tract infection in persons with spinal cord injury [ProSCIUTTU]: a randomised controlled trial. Spinal Cord. 2019;57(7):550–561. doi:10.1038/s41393-019-0251-y
75. Bossa L, Kline K, McDougald D, Lee BB, Rice SA. Urinary catheter-associated microbiota change in accordance with treatment and infection status. PLoS One. 2017;12(6):e0177633. doi:10.1371/journal.pone.0177633
76. Shepherd AJ, Mackay WG, Hagen S. Washout policies in long‐term indwelling urinary catheterisation in adults. Cochrane Database Syst Rev. 2017;(3). doi:10.1002/14651858.CD004012.pub5
77. Ramezani F, Khatiban M, Rahimbashar F, Soltanian AR. Efficacy of bladder irrigation in preventing urinary tract infections associated with short-term catheterization in comatose patients: a randomized controlled clinical trial. Am J Infect Control. 2018;46(10):e45–e50. doi:10.1016/j.ajic.2018.05.009
78. Cox L, He C, Bevins J, Clemens JQ, Stoffel JT, Cameron AP. Gentamicin bladder instillations decrease symptomatic urinary tract infections in neurogenic bladder patients on intermittent catheterization. Can Urol Assoc J. 2017;11(9):E350. doi:10.5489/cuaj.4434
79. Moussa M, Chakra MA, Papatsoris AG, Dellis A, Dabboucy B, Fares Y. Bladder irrigation with povidone‐iodine prevent recurrent urinary tract infections in neurogenic bladder patients on clean intermittent catheterization. Neurourol Urodyn. 2021;40(2):672–679. doi:10.1002/nau.24607
80. Nicolle LE, Gupta K, Bradley SF, et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis. 2019;68(10):e83–e110. doi:10.1093/cid/ciz021
81. World Health Organization. Antimicrobial Resistance: Global Report on Surveillance. World Health Organization; 2014.
82. Levy J. The effects of antibiotic use on gastrointestinal function. Am J Gastroenterol. 2000;95(1):S8–S10. doi:10.1016/S0002-9270(99)00808-4
83. Nickel J, Grant S, Costerton J. Catheter-associated bacterium: an experimental study. Urology. 1985;26(4):369–375. doi:10.1016/0090-4295(85)90185-2
84. Nickel JC, Downey J, Costerton J. Movement of pseudomonas aeruginosa along catheter surfaces A mechanism in pathogenesis of catheter-associated infection. Urology. 1992;39(1):93–98. doi:10.1016/0090-4295(92)90053-Y
85. Ionescu A, Brambilla E, Sighinolfi MC, Mattina R. A new urinary catheter design reduces in-vitro biofilm formation by influencing hydrodynamics. J Hosp Infect. 2021;114:153–162. doi:10.1016/j.jhin.2021.01.033
86. Mudshinge SR, Deore AB, Patil S, Bhalgat CM. Nanoparticles: emerging carriers for drug delivery. Saudi Pharma J. 2011;19(3):129–141. doi:10.1016/j.jsps.2011.04.001
87. Al-Qahtani M, Safan A, Jassim G, Abadla S. Efficacy of anti-microbial catheters in preventing catheter associated urinary tract infections in hospitalized patients: a review on recent updates. J Infect Public Health. 2019;12(6):760–766. doi:10.1016/j.jiph.2019.09.009
88. Roe D, Karandikar B, Bonn-Savage N, Gibbins B, Roullet J-B. Antimicrobial surface functionalization of plastic catheters by silver nanoparticles. J Antimicrob Chemother. 2008;61(4):869–876. doi:10.1093/jac/dkn034
89. Alshehri SM, Aldalbahi A, Al-Hajji AB, et al. Development of carboxymethyl cellulose-based hydrogel and nanosilver composite as antimicrobial agents for UTI pathogens. Carbohydr Polym. 2016;138:229–236. doi:10.1016/j.carbpol.2015.11.004
90. LewisOscar F, Nithya C, Vismaya S, et al. In vitro analysis of green fabricated silver nanoparticles (AgNPs) against Pseudomonas aeruginosa PA14 biofilm formation, their application on urinary catheter. Progress Organ Coatings. 2021;151:106058. doi:10.1016/j.porgcoat.2020.106058
91. Antonelli M, De Pascale G, Ranieri VM, et al. Comparison of triple-lumen central venous catheters impregnated with silver nanoparticles (AgTive®) vs conventional catheters in intensive care unit patients. J Hosp Infect. 2012;82(2):101–107. doi:10.1016/j.jhin.2012.07.010
92. Cui Y, Zhao Y, Tian Y, Zhang W, Lü X, Jiang X. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials. 2012;33(7):2327–2333. doi:10.1016/j.biomaterials.2011.11.057
93. Arunachalam K, Annamalai SK, Arunachalam AM, Raghavendra R, Kennedy S. One step green synthesis of phytochemicals mediated gold nanoparticles from Aegle marmales for the prevention of urinary catheter infection. Int J Pharm Pharm Sci. 2014;6(1):700–706.
94. Rtimi S, Sanjines R, Pulgarin C, Kiwi J. Quasi-instantaneous bacterial inactivation on Cu–ag nanoparticulate 3D catheters in the dark and under light: mechanism and dynamics. ACS Appl Mater Interfaces. 2016;8(1):47–55. doi:10.1021/acsami.5b09730
95. Shalom Y, Perelshtein I, Perkas N, Gedanken A, Banin E. Catheters coated with Zn-doped CuO nanoparticles delay the onset of catheter-associated urinary tract infections. Nano Res. 2017;10(2):520–533. doi:10.1007/s12274-016-1310-8
96. Lim K, Chua RRY, Ho B, Tambyah PA, Hadinoto K, Leong SSJ. Development of a catheter functionalized by a polydopamine peptide coating with antimicrobial and antibiofilm properties. Acta Biomater. 2015;15:127–138. doi:10.1016/j.actbio.2014.12.015
97. Yu K, Lo JC, Yan M, et al. Anti-adhesive antimicrobial peptide coating prevents catheter associated infection in a mouse urinary infection model. Biomaterials. 2017;116:69–81. doi:10.1016/j.biomaterials.2016.11.047
98. Monteiro C, Costa F, Pirttilä AM, Tejesvi MV, Martins MCL. Prevention of urinary catheter-associated infections by coating antimicrobial peptides from crowberry endophytes. Sci Rep. 2019;9(1):1–14. doi:10.1038/s41598-019-47108-5
99. Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3(3):238–250. doi:10.1038/nrmicro1098
100. Keen EC. Phage therapy: concept to cure. Front Microbiol. 2012;3:238. doi:10.3389/fmicb.2012.00238
101. Lehman SM, Donlan RM. Bacteriophage-mediated control of a two-species biofilm formed by microorganisms causing catheter-associated urinary tract infections in an in vitro urinary catheter model. Antimicrob Agents Chemother. 2015;59(2):1127–1137. doi:10.1128/AAC.03786-14
102. Curtin JJ, Donlan RM. Using bacteriophages to reduce formation of catheter-associated biofilms by Staphylococcus epidermidis. Antimicrob Agents Chemother. 2006;50(4):1268–1275. doi:10.1128/AAC.50.4.1268-1275.2006
103. Carson L, Gorman SP, Gilmore BF. The use of lytic bacteriophages in the prevention and eradication of biofilms of Proteus mirabilis and Escherichia coli. FEMS Immunol Med Microbiol. 2010;59(3):447–455. doi:10.1111/j.1574-695X.2010.00696.x
104. Sweere JM, Van Belleghem JD, Ishak H, et al. Bacteriophage trigger antiviral immunity and prevent clearance of bacterial infection. Science. 2019;363:6434. doi:10.1126/science.aat9691
105. Secor PR, Sweere JM, Michaels LA, et al. Filamentous bacteriophage promote biofilm assembly and function. Cell Host Microbe. 2015;18(5):549–559. doi:10.1016/j.chom.2015.10.013
106. Secor PR, Michaels LA, Smigiel KS, et al. Filamentous bacteriophage produced by Pseudomonas aeruginosa alters the inflammatory response and promotes noninvasive infection in vivo. Infect Immun. 2017;85(1):e00648–16. doi:10.1128/IAI.00648-16
107. Leitner L, Ujmajuridze A, Chanishvili N, et al. Intravesical bacteriophages for treating urinary tract infections in patients undergoing transurethral resection of the prostate: a randomised, placebo-controlled, double-blind clinical trial. Lancet Infect Dis. 2021;21(3):427–436. doi:10.1016/S1473-3099(20)30330-3
108. Leitner L, Sybesma W, Chanishvili N, et al. Bacteriophages for treating urinary tract infections in patients undergoing transurethral resection of the prostate: a randomized, placebo-controlled, double-blind clinical trial. BMC Urol. 2017;17(1):1–6. doi:10.1186/s12894-017-0283-6
109. Leitner L, Kessler TM, Klumpp J. Bacteriophages: a panacea in neuro-urology? Eur Urol Focus. 2020;6(3):518–521. doi:10.1016/j.euf.2019.10.018
110. Cho YH, Lee SJ, Lee J, et al. Prophylactic efficacy of a new gentamicin‐releasing urethral catheter in short‐term catheterized rabbits. BJU Int. 2001;87(1):104–109. doi:10.1046/j.1464-410x.2001.00978.x
111. Darouiche RO, Hull RA. Bacterial interference for prevention of urinary tract infection. Clin Infect Dis. 2012;55(10):1400–1407. doi:10.1093/cid/cis639
112. Prasad A, Cevallos ME, Riosa S, Darouiche RO, Trautner BW. A bacterial interference strategy for prevention of UTI in persons practicing intermittent catheterization. Spinal Cord. 2009;47(7):565–569. doi:10.1038/sc.2008.166
113. Horwitz D, McCue T, Mapes AC, et al. Decreased microbiota diversity associated with urinary tract infection in a trial of bacterial interference. J Infect. 2015;71(3):358–367. doi:10.1016/j.jinf.2015.05.014
114. Darouiche RO, Green BG, Donovan WH, et al. Multicenter randomized controlled trial of bacterial interference for prevention of urinary tract infection in patients with neurogenic bladder. Urology. 2011;78(2):341–346. doi:10.1016/j.urology.2011.03.062
115. Trautner BW, Cevallos ME, Li H, et al. Increased expression of type-1 fimbriae by nonpathogenic Escherichia coli 83972 results in an increased capacity for catheter adherence and bacterial interference. J Infect Dis. 2008;198(6):899–906. doi:10.1086/591093
116. Bergsten G, Wullt B, Schembri MA, Leijonhufvud I, Svanborg C. Do type 1 fimbriae promote inflammation in the human urinary tract? Cell Microbiol. 2007;9(7):1766–1781. doi:10.1111/j.1462-5822.2007.00912.x
117. Psonis JJ, Thanassi DG, Sandkvist M, Cascales E, Christie PJ. Therapeutic approaches targeting the assembly and function of chaperone-usher pili. EcoSal Plus. 2019;8(2). doi:10.1128/ecosalplus.ESP-0033-2018
118. Watts RE, Tan CK, Ulett GC, et al. Escherichia coli 83972 expressing a P fimbriae oligosaccharide receptor mimic impairs adhesion of uropathogenic E. coli. J Infect Dis. 2012;206(8):1242–1249. doi:10.1093/infdis/jis493
119. Lopez AI, Kumar A, Planas MR, Li Y, Nguyen TV, Cai C. Biofunctionalization of silicone polymers using poly (amidoamine) dendrimers and a mannose derivative for prolonged interference against pathogen colonization. Biomaterials. 2011;32(19):4336–4346. doi:10.1016/j.biomaterials.2011.02.056
120. Zhu Z, Wang J, Lopez AI, et al. Surfaces presenting α-phenyl mannoside derivatives enable formation of stable, high coverage, non-pathogenic Escherichia coli biofilms against pathogen colonization. Biomater Sci. 2015;3(6):842–851. doi:10.1039/C5BM00076A
121. Hancock V, Dahl M, Klemm P. Probiotic Escherichia coli strain Nissle 1917 outcompetes intestinal pathogens during biofilm formation. J Med Microbiol. 2010;59(4):392–399. doi:10.1099/jmm.0.008672-0
122. Zhu Z, Yu F, Chen H, et al. Coating of silicone with mannoside-PAMAM dendrimers to enhance formation of non-pathogenic Escherichia coli biofilms against colonization of uropathogens. Acta Biomater. 2017;64:200–210. doi:10.1016/j.actbio.2017.10.008
123. Ohlemacher SI, Giblin DE, d’Avignon DA, Stapleton AE, Trautner BW, Henderson JP. Enterobacteria secrete an inhibitor of Pseudomonas virulence during clinical bacteriuria. J Clin Invest. 2017;127(11):4018–4030. doi:10.1172/JCI92464
124. Magistro G, Stief CG. Vaccine development for urinary tract infections: where do we stand? Eur Urol Focus. 2019;5(1):39–41. doi:10.1016/j.euf.2018.07.034
125. Schmidhammer S, Ramoner R, Höltl L, Bartsch G, Thurnher M, Zelle-Rieser C. An Escherichia coli-based oral vaccine against urinary tract infections potently activates human dendritic cells. Urology. 2002;60(3):521–526. doi:10.1016/S0090-4295(02)01767-3
126. Naber KG, Cho Y-H, Matsumoto T, Schaeffer AJ. Immunoactive prophylaxis of recurrent urinary tract infections: a meta-analysis. Int J Antimicrob Agents. 2009;33(2):111–119. doi:10.1016/j.ijantimicag.2008.08.011
127. Bonkat G, Pickard R, Bartoletti R, et al. EAU guidelines on urological infections. Eur Assoc Urol. 2017;18:22–26.
128. Yang B, Foley S. First experience in the UK of treating women with recurrent urinary tract infections with the bacterial vaccine Uromune®. BJU Int. 2018;121(2):289–292. doi:10.1111/bju.14067
129. Nickel JC, Lorenzo-Gómez MF, Foley S, Saz-Leal P. PLLBA-02 A novel sublingual vaccine will dramatically alter the clinical management of recurrent urinary tract infections in women. J Urol. 2021;206(Supplement 3):e1181–e1181. doi:10.1097/JU.0000000000002150.02
130. Flores-Mireles A, Hreha TN, Hunstad DA. Pathophysiology, treatment, and prevention of catheter-associated urinary tract infection. Top Spinal Cord Inj Rehabil. 2019;25(3):228–240. doi:10.1310/sci2503-228
131. Flores-Mireles AL, Walker JN, Potretzke A, et al. Antibody-based therapy for enterococcal catheter-associated urinary tract infections. MBio. 2016;7(5):e01653–16. doi:10.1128/mBio.01653-16
132. Flores-Mireles AL, Walker JN, Bauman TM, et al. Fibrinogen release and deposition on urinary catheters placed during urological procedures. J Urol. 2016;196(2):416–421. doi:10.1016/j.juro.2016.01.100
133. Spaulding CN, Hultgren SJ. Adhesive pili in UTI pathogenesis and drug development. Pathogens. 2016;5(1):30. doi:10.3390/pathogens5010030
134. Langermann S, Palaszynski S, Barnhart M, et al. Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination. Science. 1997;276(5312):607–611. doi:10.1126/science.276.5312.607
135. Langermann S, Möllby R, Burlein JE, et al. Vaccination with fimh adhesin protects cynomolgus monkeys from colonization and infection by uropathogenic Escherichia coli. J Infect Dis. 2000;181(2):774–778. doi:10.1086/315258
136. Poggio TV, La Torre JL, Scodeller EA. Intranasal immunization with a recombinant truncated FimH adhesin adjuvanted with CpG oligodeoxynucleotides protects mice against uropathogenic Escherichia coli challenge. Can J Microbiol. 2006;52(11):1093–1102. doi:10.1139/w06-065
137. Eldridge GR, Hughey H, Rosenberger L, et al. Safety and immunogenicity of an adjuvanted Escherichia coli adhesin vaccine in healthy women with and without histories of recurrent urinary tract infections: results from a first-in-human phase 1 study. Hum Vaccin Immunother. 2021;17(5):1262–1270. doi:10.1080/21645515.2020.1834807
138. Roberts JA, Kaack MB, Baskin G, et al. Antibody responses and protection from pyelonephritis following vaccination with purified Escherichia coli PapDG protein. J Urol. 2004;171(4):1682–1685. doi:10.1097/01.ju.0000116123.05160.43
139. Averch TD, Stoffel J, Goldman HB, et al. AUA white paper on catheter associated urinary tract infections: definitions and significance in the urological patient. Urol Pract. 2015;2(6):321–328. doi:10.1016/j.urpr.2015.01.005
140. Haylen BT, De Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodynamics. 2010;29(1):4–20. doi:10.1002/nau.20798
141. Skelton-Dudley F, Doan J, Suda K, Holmes SA, Evans C, Trautner B. Spinal cord injury creates unique challenges in diagnosis and management of catheter-associated urinary tract infection. Top Spinal Cord Inj Rehabil. 2019;25(4):331–339. doi:10.1310/sci2504-331
142. Saran S, Rao NS, Azim A. Diagnosing catheter-associated urinary tract infection in critically ill patients: do the guidelines help? Indian J Crit Care Med. 2018;22(5):357. doi:10.4103/ijccm.IJCCM_434_17
143. Centers for Disease Control and Prevention. Urinary Tract Infection (Catheter-Associated Urinary Tract Infection [CAUTI] and Non-Catheter-Associated Urinary Tract Infection [UTI] and Other Urinary System Infection [USI]) Events. Centers for Disease Control and Prevention; 2015.
144. Gribble MJ, McCallum NM, Schechter MT. Evaluation of diagnostic criteria for bacteriuria in acutely spinal cord injured patients undergoing intermittent catheterization. Diagn Microbiol Infect Dis. 1988;9(4):197–206. doi:10.1016/0732-8893(88)90109-5
145. Tambyah PA, Maki DG. The relationship between pyuria and infection in patients with indwelling urinary catheters: a prospective study of 761 patients. Arch Intern Med. 2000;160(5):673–677. doi:10.1001/archinte.160.5.673
146. Steward DK, Wood GL, Cohen RL, Smith JW, Mackowiak PA. Failure of the urinalysis and quantitative urine culture in diagnosing symptomatic urinary tract infections in patients with long-term urinary catheters. Am J Infect Control. 1985;13(4):154–160. doi:10.1016/0196-6553(85)90102-6
147. Schwartz DS, Barone JE. Correlation of urinalysis and dipstick results with catheter-associated urinary tract infections in surgical ICU patients. Intensive Care Med. 2006;32(11):1797–1801. doi:10.1007/s00134-006-0365-5
148. Thornton GF, Andriole VT. Bacteriuria during indwelling catheter drainage: II. Effect of a closed sterile drainage system. JAMA. 1970;214(2):339–342. doi:10.1001/jama.1970.03180020059010
149. Tenney JH, Warren JW. Bacteriuria in women with long-term catheters: paired comparison of indwelling and replacement catheters. J Infect Dis. 1988;157(1):199–202. doi:10.1093/infdis/157.1.199
150. Grahn D, Norman DC, White ML, Cantrell M, Yoshikawa TT. Validity of urinary catheter specimen for diagnosis of urinary tract infection in the elderly. Arch Intern Med. 1985;145(10):1858–1860. doi:10.1001/archinte.1985.00360100120020
151. Bergqvist D, Brönnestam R, Hedelin H, Ståhl A. The relevance of urinary sampling methods in patients with indwelling Foley catheters. Br J Urol. 1980;52(2):92–95. doi:10.1111/j.1464-410X.1980.tb02936.x
152. Trautner BW, Morgan DJ. Imprecision medicine: challenges in diagnosis, treatment, and measuring quality for catheter-associated urinary tract infection. Clin Infect Dis. 2020;71(9):e520–e522. doi:10.1093/cid/ciaa467
153. Tractenberg RE, Frost JK, Yumoto F, Rounds AK, Ljungberg IH, Groah SL. Reliability of the Urinary Symptom Questionnaires for people with neurogenic bladder (USQNB) who void or use indwelling catheters. Spinal Cord. 2021;59(9):939–947. doi:10.1038/s41393-021-00665-x
154. Tractenberg RE, Frost JK, Yumoto F, Rounds AK, Ljungberg IH, Groah SL. Validity of the Urinary Symptom Questionnaires for people with neurogenic bladder (USQNB) who void or use indwelling catheters. Spinal Cord. 2021;59(9):948–958. doi:10.1038/s41393-021-00666-w
155. Tractenberg RE, Groah SL, Rounds AK, Ljungberg IH, Schladen MM. Preliminary validation of a Urinary Symptom Questionnaire for individuals with Neuropathic Bladder using Intermittent Catheterization (USQNB-IC): a patient-centered patient reported outcome. PLoS One. 2018;13(7):e0197568. doi:10.1371/journal.pone.0197568
156. Nanda N, Juthani-Mehta M. Novel biomarkers for the diagnosis of urinary tract infection–a systematic review. Biomark Insights. 2009;4:
157. Burnham P, Dadhania D, Heyang M, et al. Urinary cell-free DNA is a versatile analyte for monitoring infections of the urinary tract. Nat Commun. 2018;9(1):1–10. doi:10.1038/s41467-018-04745-0
158. Olszyna D, Vermeulen H, Baan A, et al. Urine interleukin-8 is a marker for urinary tract infection in postoperative patients. Infection. 2001;29(5):274–277. doi:10.1007/s15010-001-1157-z
159. Benson M, Jodal U, Agace W, et al. Interleukin (IL)-6 and IL-8 in children with febrile urinary tract infection and asymptomatic bacteriuria. J Infect Dis. 1996;174(5):1080–1084. doi:10.1093/infdis/174.5.1080
160. Ko Y-C, Mukaida N, Ishiyama S, et al. Elevated interleukin-8 levels in the urine of patients with urinary tract infections. Infect Immun. 1993;61(4):1307–1314. doi:10.1128/iai.61.4.1307-1314.1993
161. Baggiolini M, Dewald B, Moser B. interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv Immunol. 1993;55:97–179.
162. Gupta S, Preece J, Haynes A, Becknell B, Ching C. Differentiating asymptomatic bacteriuria from urinary tract infection in the pediatric neurogenic bladder population: NGAL as a promising biomarker. Top Spinal Cord Inj Rehabil. 2019;25(3):214–221. doi:10.1310/sci2503-214
163. Gadalla AA, Friberg IM, Kift-Morgan A, et al. Identification of clinical and urine biomarkers for uncomplicated urinary tract infection using machine learning algorithms. Sci Rep. 2019;9(1):1–11. doi:10.1038/s41598-019-55523-x
164. Bhattacharyya RP, Bandyopadhyay N, Ma P, et al. Simultaneous detection of genotype and phenotype enables rapid and accurate antibiotic susceptibility determination. Nat Med. 2019;25(12):1858–1864. doi:10.1038/s41591-019-0650-9
165. Kanjilal S, Oberst M, Boominathan S, Zhou H, Hooper DC, Sontag D. A decision algorithm to promote outpatient antimicrobial stewardship for uncomplicated urinary tract infection. Sci Transl Med. 2020;12:568. doi:10.1126/scitranslmed.aay5067
166. Herman DS, Rhoads DD, Schulz WL, Durant TJ. Artificial intelligence and mapping a new direction in laboratory medicine: a review. Clin Chem. 2021;67(11):1466–1482. doi:10.1093/clinchem/hvab165
167. Alouani DJ, Ransom EM, Jani M, Burnham C-A, Rhoads DD, Sadri N. Deep convolutional neural networks implementation for the analysis of urine culture. Clin Chem. 2022. doi:10.1093/clinchem/hvab270
168. Raz R, Schiller D, Nicolle LE. Chronic indwelling catheter replacement before antimicrobial therapy for symptomatic urinary tract infection. J Urol. 2000;164(4):1254–1258. doi:10.1016/S0022-5347(05)67150-9
169. Nicolle LE. Urine cultures and long-term indwelling catheters. Arch Intern Med. 1985;145(10):1794–1795. doi:10.1001/archinte.1985.00360100054006
170. Wagenlehner F, Tandogdu Z, Bartoletti R, et al. The global prevalence of infections in urology study: a long-term, worldwide surveillance study on urological infections. Pathogens. 2016;5(1):10. doi:10.3390/pathogens5010010
171. Tanne JH. FDA Adds “Black Box” Warning Label to Fluoroquinolone Antibiotics. British Medical Journal Publishing Group; 2008.
172. Fauci AS, Morens DM. The perpetual challenge of infectious diseases. N Engl J Med. 2012;366(5):454–461. doi:10.1056/NEJMra1108296
173. World Health Organization. 2019 Antibacterial Agents in Clinical Development: An Analysis of the Antibacterial Clinical Development Pipeline. World Health Organization; 2019.
174. World Health Organization. Antibacterial Agents in Preclinical Development: An Open Access Database. World Health Organization; 2019.
175. Portsmouth S, van Veenhuyzen D, Echols R, et al. Cefiderocol versus imipenem-cilastatin for the treatment of complicated urinary tract infections caused by Gram-negative uropathogens: a phase 2, randomised, double-blind, non-inferiority trial. Lancet Infect Dis. 2018;18(12):1319–1328. doi:10.1016/S1473-3099(18)30554-1
176. Bassetti M, Echols R, Matsunaga Y, et al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): a randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect Dis. 2021;21(2):226–240. doi:10.1016/S1473-3099(20)30796-9
177. U.S. Food and Drug Administration. FDA approves new antibacterial drug to treat complicated urinary tract infections as part of ongoing efforts to address antimicrobial resistance| FDA; 2019. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-new-antibacterial-drug-treat-complicated-urinary-tract-infections-part-ongoing-efforts.
178. Kaye KS, Bhowmick T, Metallidis S, et al. Effect of meropenem-vaborbactam vs piperacillin-tazobactam on clinical cure or improvement and microbial eradication in complicated urinary tract infection: the TANGO I randomized clinical trial. JAMA. 2018;319(8):788–799. doi:10.1001/jama.2018.0438
179. Patel TS, Pogue JM, Mills JP, Kaye KS. Meropenem–vaborbactam: a new weapon in the war against infections due to resistant Gram-negative bacteria. Future Microbiol. 2018;13(9):971–983. doi:10.2217/fmb-2018-0054
180. Elliott W, Chan J. Meropenem and vaborbactam injection (vabomere). Intern Med Alert. 2017;39(20). Available from: https://www.proquest.com/docview/1982784001/D10EDA0A57574F3FPQ/1. Accessed March 29, 2022.
181. Wagenlehner FM, Cloutier DJ, Komirenko AS, et al. Once-daily plazomicin for complicated urinary tract infections. N Engl J Med. 2019;380(8):729–740. doi:10.1056/NEJMoa1801467
182. Mui E. Plazomicin (Zemdri). Infect Dis Alert. 2018;38(2). Available from: https://www.proquest.com/docview/2129411491/F1716D96B294CBFPQ/2. Accessed March 29, 2022.
183. Motsch J, Murta de Oliveira C, Stus V, et al. RESTORE-IMI 1: a multicenter, randomized, double-blind trial comparing efficacy and safety of imipenem/relebactam vs colistin plus imipenem in patients with imipenem-nonsusceptible bacterial infections. Clin Infect Dis. 2020;70(9):1799–1808. doi:10.1093/cid/ciz530
184. Pourali S. Imipenem, Cilastatin, Relebactam (Recarbrio). Infect Dis Alert. 2020;39(7). Available from: https://www.proquest.com/docview/2506724594/5B4D5A3631884ED7PQ/1. Accessed March 29, 2022.
185. Iterum Therapeutics. Iterum therapeutics announces topline results for a phase 3 clinical trial of oral and IV sulopenem in complicated urinary tract infection. Available from: https://ir.iterumtx.com/press-releases/detail/37/iterum-therapeutics-announces-topline-results-for-a-phase-3.
186. Hover BM, Kim S-H, Katz M, et al. Culture-independent discovery of the malacidins as calcium-dependent antibiotics with activity against multidrug-resistant Gram-positive pathogens. Nat Microbiol. 2018;3(4):415–422. doi:10.1038/s41564-018-0110-1
187. Stokes JM, Yang K, Swanson K, et al. A deep learning approach to antibiotic discovery. Cell. 2020;180(4):688–702. e13. doi:10.1016/j.cell.2020.01.021
188. Thanassi DG, Saulino ET, Hultgren SJ. The chaperone/usher pathway: a major terminal branch of the general secretory pathway. Curr Opin Microbiol. 1998;1(2):223–231. doi:10.1016/S1369-5274(98)80015-5
189. Du M, Yuan Z, Werneburg GT, et al. Processive dynamics of the usher assembly platform during uropathogenic Escherichia coli P pilus biogenesis. Nat Commun. 2021;12(1):1–9. doi:10.1038/s41467-021-25522-6
190. Du M, Yuan Z, Yu H, et al. Handover mechanism of the growing pilus by the bacterial outer-membrane usher FimD. Nature. 2018;562(7727):444–447. doi:10.1038/s41586-018-0587-z
191. Werneburg GT, Henderson NS, Portnoy EB, et al. The pilus usher controls protein interactions via domain masking and is functional as an oligomer. Nat Struct Mol Biol. 2015;22(7):540–546. doi:10.1038/nsmb.3044
192. Phan G, Remaut H, Wang T, et al. Crystal structure of the FimD usher bound to its cognate FimC–FimH substrate. Nature. 2011;474(7349):49–53. doi:10.1038/nature10109
193. Remaut H, Tang C, Henderson NS, et al. Fiber formation across the bacterial outer membrane by the chaperone/usher pathway. Cell. 2008;133(4):640–652. doi:10.1016/j.cell.2008.03.033
194. Nishiyama M, Ishikawa T, Rechsteiner H, Glockshuber R. Reconstitution of pilus assembly reveals a bacterial outer membrane catalyst. Science. 2008;320(5874):376–379. doi:10.1126/science.1154994
195. Pham T, Henderson NS, Werneburg GT, Thanassi DG, Delcour AH. Electrostatic networks control plug stabilization in the PapC usher. Mol Membr Biol. 2015;32(5–8):198–207. doi:10.3109/09687688.2016.1160450
196. Volkan E, Ford BA, Pinkner JS, et al. Domain activities of PapC usher reveal the mechanism of action of an Escherichia coli molecular machine. Proc Natl Acad Sci. 2012;109(24):9563–9568. doi:10.1073/pnas.1207085109
197. Werneburg GT, Thanassi DG. Pili assembled by the chaperone/usher pathway in Escherichia coli and Salmonella. EcoSal Plus. 2018;8(1). doi:10.1128/ecosalplus.ESP-0007-2017
198. Cusumano CK, Pinkner JS, Han Z, et al. Treatment and prevention of urinary tract infection with orally active FimH inhibitors. Sci Transl Med. 2011;3(109):109ra115–109ra115. doi:10.1126/scitranslmed.3003021
199. Klein T, Abgottspon D, Wittwer M, et al. FimH antagonists for the oral treatment of urinary tract infections: from design and synthesis to in vitro and in vivo evaluation. J Med Chem. 2010;53(24):8627–8641. doi:10.1021/jm101011y
200. Salminen A, Loimaranta V, Joosten JA, et al. Inhibition of P-fimbriated Escherichia coli adhesion by multivalent galabiose derivatives studied by a live-bacteria application of surface plasmon resonance. J Antimicrob Chemother. 2007;60(3):495–501. doi:10.1093/jac/dkm251
201. Pinkner JS, Remaut H, Buelens F, et al. Rationally designed small compounds inhibit pilus biogenesis in uropathogenic bacteria. Proc Natl Acad Sci. 2006;103(47):17897–17902. doi:10.1073/pnas.0606795103
202. Chorell E, Pinkner JS, Bengtsson C, et al. Mapping pilicide anti-virulence effect in Escherichia coli, a comprehensive structure–activity study. Bioorg Med Chem. 2012;20(9):3128–3142. doi:10.1016/j.bmc.2012.01.048
203. Cegelski L, Pinkner JS, Hammer ND, et al. Small-molecule inhibitors target Escherichia coli amyloid biogenesis and biofilm formation. Nat Chem Biol. 2009;5(12):913–919. doi:10.1038/nchembio.242
204. Lo AW, Van de Water K, Gane PJ, et al. Suppression of type 1 pilus assembly in uropathogenic Escherichia coli by chemical inhibition of subunit polymerization. J Antimicrob Chemother. 2014;69(4):1017–1026. doi:10.1093/jac/dkt467
205. Chahales P, Hoffman PS, Thanassi DG. Nitazoxanide inhibits pilus biogenesis by interfering with folding of the usher protein in the outer membrane. Antimicrob Agents Chemother. 2016;60(4):2028–2038. doi:10.1128/AAC.02221-15
206. Psonis JJ, Chahales P, Henderson NS, Rigel NW, Hoffman PS, Thanassi DG. The small molecule nitazoxanide selectively disrupts BAM-mediated folding of the outer membrane usher protein. J Biol Chem. 2019;294(39):14357–14369. doi:10.1074/jbc.RA119.009616
207. Klinth JE, Pinkner JS, Hultgren SJ, Almqvist F, Uhlin BE, Axner O. Impairment of the biomechanical compliance of P pili: a novel means of inhibiting uropathogenic bacterial infections? Eur Biophys J. 2012;41(3):285–295. doi:10.1007/s00249-011-0784-2
208. Mortezaei N, Singh B, Bullitt E, Uhlin BE, Andersson M. P-fimbriae in the presence of anti-PapA antibodies: new insight of antibodies action against pathogens. Sci Rep. 2013;3(1):1–9. doi:10.1038/srep03393
209. GlaxoSmithKline. NCT04488770: safety, tolerability and pharmacokinetic investigation of GSK3882347 in healthy participants. 2021.
210. Wagenlehner FM, Abramov-Sommariva D, Höller M, Steindl H, Naber KG. Non-antibiotic herbal therapy (BNO 1045) versus antibiotic therapy (fosfomycin trometamol) for the treatment of acute lower uncomplicated urinary tract infections in women: a double-blind, parallel-group, randomized, multicentre, non-inferiority phase III trial. Urol Int. 2018;101(3):327–336. doi:10.1159/000493368
211. Naber KG, Kogan M, Wagenlehner FM, Siener R, Gessner A. How the microbiome is influenced by the therapy of urological diseases: standard versus alternative approaches. Clin Phytosci. 2017;3(1):1–4. doi:10.1186/s40816-017-0045-8
212. Rechberger E, Rechberger T, Wawrysiuk S, et al. A randomized clinical trial to evaluate the effect of canephron n in comparison to ciprofloxacin in the prevention of postoperative lower urinary tract infections after midurethral sling surgery. J Clin Med. 2020;9(11):3391. doi:10.3390/jcm9113391
213. Lau I, Albrecht U, Kirschner-Hermanns R. Phytotherapy in catheter-associated urinary tract infection: observational study recording the efficacy and safety of a fixed herbal combination containing Tropaeoli majoris herba and Armoraciae rusticanae radix. Urologe A. 2018;57(12):1472–1480. doi:10.1007/s00120-018-0740-1
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.