Back to Journals » Clinical Ophthalmology » Volume 8
Cataract surgery during active methicillin-resistant Staphylococcus aureus infection
Received 20 January 2014
Accepted for publication 22 February 2014
Published 15 April 2014 Volume 2014:8 Pages 739—742
DOI https://doi.org/10.2147/OPTH.S61037
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Ahmad M Mansour,1,2 Haytham I Salti1
1Department of Ophthalmology, American University of Beirut, 2Rafic Hariri University Hospital, Beirut, Lebanon
Abstract: We present two patients with active, foul-smelling, methicillin-resistant Staphylococcus aureus (MRSA) wounds of the forehead and sternum following craniotomy or open heart surgery. Both had debilitating cataracts and were told by the infectious diseases team that cataract surgery is very risky. Both underwent sequential bilateral phacoemulsification with no sign of infection. Patients with active MRSA wound infections may safely undergo cataract surgery with additional precautions observed intraoperatively (good wound construction) and postoperatively (topical antibiotics and close observation). Banning such surgeries can unnecessarily jeopardize the lifestyles of such patients.
Keywords: cataract, infection, methicillin-resistant Staphylococcus aureus, phacoemulsification
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) is a multidrug-resistant organism that has infiltrated the hospital and the community. MRSA can be symptomatic (infection) or asymptomatic (carrier). An MRSA carrier is a person having bacterial colonization of the skin or nose usually without symptoms. Although most MRSA infections are not serious, some can be life-threatening. Most often, it causes a mild skin infection in the form of sores, but also more serious skin infections, or infection of surgical wounds, the bloodstream, or lungs. According to the Centers for Disease Control and Prevention’s National Nosocomial Infections Surveillance System, the percentage of MRSA nosocomial infections has escalated from 2% in 1974, to 22% in 1995, to 63% in 2003.1,2 However, from 2005 to 2011, the adjusted estimated national incidence rates for community-acquired infections have decreased by 27.7% and hospital-acquired infections by 54.2%.2 According to a recent analysis, postsurgical mediastinitis occurs in 1%–4% of patients after coronary artery bypass graft surgery, of which up to 65% of cases are caused by MRSA.1 Can patients with active MRSA wounds undergo phacoemulsification?
Case reports
Case 1
A 69-year-old diabetic, hypertensive, dyslipidemic, morbidly obese (body mass index 40 kg/m2) housewife underwent bifrontal craniotomy in 1996 that was complicated by osteomyelitis. Several courses of combination antibiotics failed to eradicate the infection. Repeated attempts at closing the craniotomy site with flaps failed. The wound (Figure 1) continued to be foul-smelling. The infectious diseases team was reluctant to allow any ocular surgery. Examination revealed counting finger vision with mature cataract bilaterally. Funduscopy was negative for diabetic retinopathy. The patient was consented for surgery with the risks of endophthalmitis explained. Sequential sutureless phacoemulsification was done, with final uncorrected vision of 6/7.5 in each eye and a 5-month interval between surgeries. Surgery was carried out under topical anesthesia with xylocaine gel and without the application of topical povidone iodine that could decrease corneal clarity (due to severe dry eye povidone iodine drops were omitted to avoid corneal epithelial toxicity that would affect visualization in this very dense cataract).3,4 The eyelid and eyebrow region was scrubbed with povidone iodine. Special attention was paid to creating elongated, three-plane, self-sealing limbal tracts (using a crescent blade), avoiding multiple entry and exit from the eye, and keeping the eye at a pressure of 25 mmHg throughout the procedure. No intracameral antibiotics were given at the end of surgery. The eye was patched with tobramycin and dexamethasone ointment for 1 hour. One hour after surgery, drops were applied every hour during waking time for 1 day with gradual taper (first week every 2 hours, second week every 4 hours, and third week every 6 hours). The postoperative regimen consisted of topical moxifloxacin along with a combination of netilmicin sulfate 0.3% with dexamethasone phosphate 0.1% drops for 3 weeks together with 5 days of oral moxifloxacin. Close observation (examination every 2 days) failed to reveal any signs of anterior chamber inflammation in the postoperative period of 2 months. The patient had no cultures (wound, nose, blood, conjunctival cul de sac), and no preoperative antibiotics.
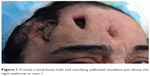  | Figure 1 Frontal cranial bone melt and overlying yellowish exudates just above the right eyebrow in case 1. |
Case 2
A 71-year-old retired lawyer who was a known diabetic, hypertensive, heavy smoker with coronary artery disease, renal failure, and benign prostate hypertrophy (on tamsulosin) had a chronically infected sternal wound with an indwelling catheter after coronary artery bypass surgery 6 months before presentation to the eye clinic. Repeated medical attempts at sterilizing the sternal wound failed, with accompanying irreversible antibiotic nephropathy. He presented with severe visual loss to counting fingers with dense nuclear sclerosis and posterior subcapsular cataract bilaterally. The patient accepted the risks of postoperative infection as previously detailed by the infectious diseases team. The patient underwent phacoemulsification of the right eye under topical anesthesia with xylocaine gel and without instillation of topical povidone iodine (because of dry eye, risk of epithelial toxicity, and loss of clarity during surgery).3,4 Special attention was paid to creating elongated, three-plane, self-sealing limbal tracts. The patient did not receive intracameral antibiotics or oral antibiotics because of recent antibiotic nephrotoxicity.5 The eye patch was placed for 1 hour and removed to allow topical therapy. He was put on topical moxifloxacin and a combination of netilmicin sulfate 0.3% with dexamethasone phosphate 0.1% drops. The drops were administered hourly on the day of surgery with taper thereafter, as in the first case. Vision in the right eye recovered to 6/6. One month later, the patient underwent phacoemulsification of the left eye with 4.5 mm anterior capsulorhexis. The foldable implant was placed in the bag under balanced salt irrigation. Excess pressure was exerted at 6 o’clock (in order to engage the superior haptic) in a thinned out capsular bag, leading to localized capsular rupture with no vitreous loss. The implant was centered intraoperatively and slowly started decentering inferiorly. He received the same topical regimen as for the right eye. Two months later, a secondary anterior chamber intraocular lens was implanted with the help of viscoelastic material (under topical anesthesia with xylocaine gel and without topical povidone iodine). Upon aspiration of the viscoelastic material, no vitreous was noted in the anterior chamber. Suturing of the three-plane, temporal 6 mm limbal incision was done with 10-0 nylon, ensuring a tight wound. The same regimen of topical drops allowed good visual recovery with no signs of inflammation on close observation (examination every 2 days) in the early postoperative period. Six weeks after the last surgery, uncorrected visual acuity was 6/12 in the left eye.
Discussion
Among a total of 3,640 patients with a culture positive for MRSA in Dallas county,6 30% were considered to have acquired MRSA nosocomially and 70% to have community-acquired MRSA. Of these 3,640 patients, 49 (1.3%) had ophthalmic involvement. The most common manifestations of ophthalmic MRSA infection include preseptal cellulitis, lid abscess, and conjunctivitis, and rarely sight-threatening infections such as corneal ulcers, orbital cellulitis, and endogenous endophthalmitis.6 Unlike in adults, periocular infections accounted for all of 137 ocular MRSA infections in a large northern pediatric health care system in California.7 The average rate of MRSA in ocular S. aureus infections was reported to be around 52.8%.8 Further, the rate of endophthalmitis after cataract surgery was reported to be in the range of 0.06%–0.1%, with MRSA accounting for very few cases.9 Another way to look at this subject is to consider what percentage of MRSA infections occur after cataract surgery. In a study at the University of California, San Francisco, 2.4% of all ocular MRSA infections manifested as postoperative endophthalmitis.10 Diabetes, asthma, chronic blepharitis, active conjunctivitis, ocular discharge, and immunosuppressive and autoimmune disorders increase the risk of bacterial resistance and colonization by MRSA.11 Proximity to swine and livestock is also associated with MRSA.12
Active surveillance consists of screening for MRSA, isolation, contact precautions, and decolonization. The anterior nares are the most important site to screen for colonization with S. aureus. Mertz et al13 screened 2,966 individuals for S. aureus carriage with swabs of both the nares and throat, and found that 37.1% were nasal carriers and 12.8% were solely throat carriers. Screening of throat swabs significantly increases the sensitivity of detection of carriers by 25.7%. Numerous studies support the use of intranasal mupirocin for decolonization as well as use of chlorhexidine for skin cleansing, decolonization, and impregnated dressings and catheters.
Various measures have been suggested to reduce the risk of postoperative bacterial endophthalmitis. Both the Royal College of Ophthalmologists and the American Academy of Ophthalmology recommend preoperative conjunctival irrigation with 5% povidone-iodine as prophylaxis against infection. The European Society of Cataract & Refractive Surgeons Endophthalmitis Study Group investigators reported reductions in the incidence of postoperative endophthalmitis following cataract surgery in patients receiving intracameral cefuroxime injection in addition to povidone-iodine irrigation. However, cefuroxime does not cover MRSA. Wound integrity also seems to be an important feature influencing the risk for developing endophthalmitis. Long tunnels made through the limbus seem to offer better wound apposition and less risk for wound leakage intraoperatively and postoperatively than corneal incisions.14–16 We did not place povidone-iodine (because of the potential interaction between xylocaine gel and iodine and subsequent corneal haze),3,4 but we did create long-tract, self-sealing limbal wounds and applied hourly antibiotics with close monitoring of the patient. Infectious diseases specialists often warn patients with MRSA against cataract surgery, especially if the disease is still active. This is understandable, given that failure to treat the infected wound would be associated with difficulties in controlling potential MRSA endophthalmitis.
Further studies are needed to delineate whether or not MRSA colonization is directly correlated with MRSA infection at the surgical site. Moreover, cataract surgery in patients with active MRSA infection at sites near to or distant from the eye (as in the present cases) has not received much attention in the literature. The eye is amenable to intensive regimen of topical therapy yielding very high concentrations of antibiotics in ocular tissues surpassing the minimal inhibitory concentration and overcoming so-called antibiotic resistance inherent with the systemic route of antibiotics. Close follow-up and careful surgery to avoid complications (vitreous loss) and wound leak in the immediate postoperative period are of paramount importance. Addition of oral moxifloxacin may offer additional protection in selected patients if tolerated and help to protect the surgeon from possible medicolegal issues, given that surgical antibiotic prophylaxis is one major mainstay of infection prevention (systemic treatment to clear MRSA infection is indicated in many such cases; however, this kind of therapy was unsuccessful in our two patients who did not respond).17 A single dose of vancomycin 15 mg/kg administered within one hour of skin incision17 has been proposed as prophylaxis in MRSA cases. In a critical review of the literature, Gordon18 advised against routine use of vancomycin after cataract surgery; however, its use is warranted in cases with active MRSA infection. Other antibiotics have been used, such as rifampin, doxycycline, sulfamethoxazole-trimethoprim,19 clindamycin, and minocycline.1 Lemaire et al17 found moxifloxacin to be highly effective against MRSA. In our cases, repeated courses of systemic antibiotics had failed to control the infection, and no attempt was made to start oral antibiotics preoperatively, particularly in view of the fact that these antibiotics had caused nephrotoxicity (case 2).5
The literature is scarce regarding appropriate management of cataract in patients with active MRSA wounds. Porter et al9 described three patients with MRSA colonization (only one case had active infection) and significant cataract with advanced vision loss. The first patient was repeatedly turned down for ophthalmic surgery over several years at two nearby ophthalmic institutions solely because of her MRSA colonization and the failure of MRSA eradication regimens. This patient was housebound with severe visual impairment (1/60 in the right eye and 6/60 in the left eye). The first two patients were colonized with MRSA and the third patient had active chronic MRSA-infected leg ulcers. In the current case reports, active MRSA infection was present as a foul-smelling wound of the chest or above the eyebrow. Patients colonized with MRSA infection may safely undergo cataract surgery with appropriate intraoperative technique, postoperative antibiotic infection control measures, and close postoperative follow-up. If appropriate, decolonization regimens,1,2 povidone-iodine irrigation of the cul de sac, and intracameral antibiotics remain the standard therapies in such cases.
Disclosure
The authors report no financial interest in any product mentioned in this paper and no conflicts of interest in this work.
References
Tom TSM, Kruse MW, Reichman RT. Update: methicillin resistant Staphylococcus aureus screening and decolonisation in cardiac surgery. Ann Thorac Surg. 2009;88:695–702. | ||
Dantes R, Mu Y, Belflower R, et al; Emerging Infections Program-Active Bacterial Core Surveillance MRSA Surveillance Investigators. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. 2013;173:1970–1978. | ||
Chalam KV, Murthy RK, Agarwal S, Gupta SK, Grover S. Comparative efficacy of topical tetraVisc versus lidocaine gel in cataract surgery. BMC Ophthalmol. 2009;9:7. | ||
MacRae SM, Brown B, Edelhauser HF. The corneal toxicity of presurgical skin antiseptics. Am J Ophthalmol. 1984;97:221–232. | ||
Cappelletty D, Jablonski A, Jung R. Risk factors for acute kidney injury in adult patients receiving vancomycin. Clin Drug Investig. 2014;34:189–193. | ||
Blomquist PH. Methicillin-resistant Staphylococcus aureus infections of the eye and orbit (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc. 2006;104:322–345. | ||
Amato M, Pershing S, Walvick M, Tanaka S. Trends in ophthalmic manifestations of methicillin-resistant Staphylococcus aureus (MRSA) in a northern California pediatric population. J AAPOS. 2013;17:243–247. | ||
Chuang CC, Hsiao CH, Tan HY, et al. Staphylococcus aureus ocular infection: methicillin-resistance, clinical features, and antibiotic susceptibilities. PLoS One. 2012;8:e42437. | ||
Porter LF, Khan RU, Hannan A, Kelly SP. MRSA and cataract surgery – reflections for practice. Clin Ophthalmol. 2010:4:1223–1227. | ||
Freidlin J, Acharya N, Lietman TM, et al. Spectrum of eye disease caused by methicillin-resistant Staphylococcus aureus. Am J Ophthalmol. 2007;144:313–315. | ||
Miño de Kaspar H, Shriver EM, Nguyen EV, et al. Risk factors for antibiotic-resistant conjunctival bacterial flora in patients undergoing intraocular surgery. Graefes Arch Clin Exp Ophthalmol. 2003;241:730–733. | ||
Casey JA, Curriero FC, Cosgrove SE, Nachman KE, Schwartz BS. High-density livestock operations, crop field application of manure, and risk of community-associated methicillin-resistant Staphylococcus aureus infection in Pennsylvania. JAMA Intern Med. 2013;173:1980–1990. | ||
Mertz D, Frei R, Jaussi B, et al. Throat swabs are necessary to reliably detect carriers of Staphylococcus aureus. Clin Infect Dis. 2007;45:475–477. | ||
Cooper BA, Holekamp NM, Bohigian G, Thompson PA. Case-control study of endophthalmitis after cataract surgery comparing scleral tunnel and clear corneal wounds. Am J Ophthalmol. 2003;136:300–305. | ||
Cao H, Zhang L, Li L, Lo SK. Risk factors for acute endophthalmitis following cataract surgery: a systematic review and meta-analysis. PLoS One. 2013;8:e71731. | ||
Taban M, Rao B, Reznik J, Zhang J, Chen Z, McDonnell PJ. Dynamic morphology of sutureless cataract wounds – effect of incision angle and location. Surv Ophthalmol. 2004;49 Suppl 2:S62–72. | ||
Lemaire S, Kosowska-Shick K, Appelbaum PC, Glupczynski Y, Van Bambeke F, Tulkens PM. Activity of moxifloxacin against intracellular community-acquired methicillin-resistant Staphylococcus aureus: comparison with clindamycin, linezolid and co-trimoxazole and attempt at defining an intracellular susceptibility breakpoint. J Antimicrob Chemother. 2011;66:596–607. | ||
Gordon YJ. Vancomycin prophylaxis and emerging resistance: are ophthalmologists the villains? The heroes? Am J Ophthalmol. 2001;131:371–376. | ||
Feiz V, Nijm L, Glickman RD, et al. Vitreous and aqueous penetration of orally administered trimethoprim-sulfamethoxazole combination in humans. Cornea. 2013;32:1315–1320. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
