Back to Journals » Infection and Drug Resistance » Volume 15
Case Report: Vesicorectal Fistula Caused by Intestinal Tuberculosis Complicated with Systemic Lupus Erythematosus
Received 9 August 2022
Accepted for publication 19 October 2022
Published 27 October 2022 Volume 2022:15 Pages 6237—6243
DOI https://doi.org/10.2147/IDR.S383893
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Pengjia Wu, Jiashun Zeng, Lei Yang
Department of Rheumatology and Immunology, The Affiliated Hospital of Guizhou Medical University, Guiyang, 550000, People’s Republic of China
Correspondence: Jiashun Zeng, Email [email protected]
Abstract: Patients with systemic lupus erythematosus (SLE) are prone to various infections due to autoimmune defects and long-term use of immunosuppressive agents. Mycobacterium tuberculosis (TBC) infection is a common infection in patients with SLE, especially in developing countries such as China. SLE and TBC may overlap and confuse a clinical picture, bringing great difficulties for the diagnosis and treatment. This article reports a case of vesicorectal fistula caused by intestinal TBC complicated with SLE, where the manifestation was recurrent diarrhea, initially treated as lupus-associated intestinal vasculitis without notable response. This case suggests that we should pay attention to close monitoring of tuberculosis-related indicators during the follow-up period of SLE patients, especially in endemic areas, and early diagnosis and treatment of TBC can reduce tuberculosis-related complications and significantly improve the quality of life of patients.
Keywords: systemic lupus erythematosus, vesicorectal fistula, intestinal tuberculosis, infect, diarrhea
Introduction
The prevalence of TBC infection in patients with SLE is higher than that in the general population, especially in developing countries. According to the WHO, in 2019, about 10 million people were infected with TBC, in other words, on average, 130 per 100,000 people were suffering from TBC.1 During the treatment of SLE with glucocorticoids and immunosuppressive agents, the incidence of mycobacterium TBC infection increases significantly, mainly, in severe TBC and extrapulmonary TBC.2–5 A meta-analysis of data from 35 studies, including 46,327 SLE patients in 13 countries, showed that the prevalence of TBC in SLE patients was 3.59%.6 The clinical manifestations of TBC are diverse and non-specific, so the diagnosis is very difficult. In addition, because the clinical picture of intestinal TBC and lupus intestinal vasculitis are extremely similar, both of them are often manifested as abdominal pain, diarrhea, nausea, vomiting, etc, which often leads to misdiagnosis or untimely diagnosis, therefore, there are very few relevant reports. Intestinal TBC can lead to malabsorption syndrome, obstruction, perforation, and fistula. When patients have chronic perforation, the abscess can be formed in the abdominal cavity, and then rupture of the abscess can cause an intestinal fistula, resulting in a connection between bladder and intestine. This can significantly threaten the patient’s life without a clear diagnosis and timely treatment. Here we report a rare case of vesicorectal fistula caused by intestinal TBC complicated with SLE, and to our knowledge, no such case has been reported.
Case Report
A 24-year-old female patient experienced rash and oral ulcers with arthralgia at the age of 19 years, when antinuclear antibodies and double-stranded DNA antibodies were positive. The patient was diagnosed with SLE based on her clinical manifestations and relevant immune test results. She was initially treated with prednisone 50 mg/day and hydroxychloroquine orally. She returned regularly with reasonable disease control, followed by a gradual reduction to prednisone 10 mg for long-term maintenance therapy.
In August 2018, the patient began to experience repeated diarrhea with abdominal pain and occasional fever, with a body temperature up to 40°C, accompanied by nausea and anorexia. The stools varied from several to ten times a day and were yellow watery stools. No treatment was given. On October 17, 2018, the patient was admitted to the hospital. Blood routine: blood cell analysis (five-part differential): white blood cell count (WBC) 8.31G/L, neutrophil granulocyte% (NEUT%) 85%, red blood cell (RBC) 2.99T/L, hemoglobin (Hb) 74.00 g/L, hematocrit (HCT) 24.2%, platelet count (PLT) 295.00G/L. Urinalysis: RBC 1321.00 cells/ul, WBC 1636.00 cells/ul, urine protein + stool routine + occult blood test (OB): positive fecal occult blood. The stool smear revealed fungal spores and hyphae. High-sensitivity C-reactive protein 103.29 mg/L. The erythrocyte sedimentation rate was 94 mm/h. Coombs’ test was positive. ANA antibody spectrum (containing anti-dsDNA) antinuclear antibody 1:1000 positive, anti-double-stranded DNA antibody negative, anti-Smith antibody weakly positive, anti-ribosomal P protein antibody strongly positive. Complement3 0.61 g/l, Complement4 0.14 g/l. T-cells enzyme linked immunospot (T-SPOT) was positive. Chest CT: interstitial change in both sides. For the patient. Considering the initial treatment by a variety of antibiotics was ineffective, and the fundamental disease is SLE, for which lupus associated intestinal vasculitis is a common complication, when obvious gastrointestinal symptoms occurred, relevant physician of the hospital related the diarrhea and lupus-associated intestinal vasculitis to intestinal fungus infection, and gave methylprednisolone pulse therapy, 500 mg/d, for 3 days, twice in total; and gave fluconazol 200 mg/d, po (10.21–11.04), fluconazol 200 mg/d, ivgtt (11.04–11.06) for antifungal therapy. After the above treatment, the patient’s diarrhea was not significantly improved.
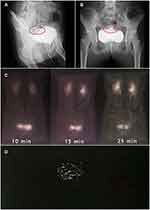
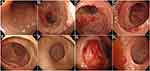
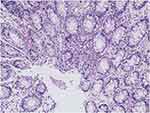
On November 6, 2018, the patient was transferred to our hospital for continuous treatment. Blood cell analysis (five-part differential) showed WBC 7.73G/L, RBC 2.68T/L, Hb 70.00 g/L, HCT21.9%, PLT 61.00G/L. Urine examination showed RBC 68.0 cells/ul, WBC 456.00 cells/ul, Blood 3 +. Stool routine + OB: positive fecal occult blood. Liver and kidney function and electrolytes showed no significant abnormalities. ANA antibody profile (containing anti-dsDNA) antinuclear antibody: 1:3200 positive, anti-Smith antibody negative, anti-double-stranded DNA antibody negative. Anti-ribosomal P protein antibody was strongly positive. Complement3 0.398 g/l, Complement4 0.124/l. High-sensitivity C-reactive protein 103.29 mg/L. The erythrocyte sedimentation rate was normal. QuantiFERON (R)-TB Gold In-Tube (QFT) was negative. Coombs’ test was positive. Thoracoabdominal CT showed interstitial changes in both sides (the main manifestations are left upper lung nodule and right lower lung calcification), edema and thickening of the intestinal wall of the ascending colon, and multiple surrounding exudates. During the course of the disease, the patient had no arthralgia, alopecia, oral ulcers, or new rash, and the SLEDAI score was 3. Methylprednisolone (40 mg/d) was given to inhibit immunity, micafungin (100 mg/d) for antifungal treatment, levofloxacin (0.3 g/d) and metronidazole (0.2 g Tid) for anti-infection, and nutritional support and other treatments. After the above treatment, the diarrhea was not significantly relieved. During hospitalization, we found that: the patient had repeated diarrhea, yellow watery stools, water content, floating fecal residue on the surface, and most of the time was regular, with an interval of 3–5 hours; in addition, the patient’s daily intake was about 2000mL, urine volume was significantly reduced (about 100mL/d), and there were many floccules in the urine, but the patient’s multiple examinations of renal function showed no abnormality (creatinine fluctuating between 28.00–41.01 umol/l), and there was no significant systemic edema. According to the character and regularity of urine and defecation, the presence of urinary fistula was considered. Therefore, to confirm the diagnosis, we performed the following verification: First, we obtained the stool of this patient, measured its urinary creatinine value after centrifugation of the stool (2504 umol/L), and obtained the urine of another patient with normal renal function to measure its urinary creatinine value (3442 umol/L); then cystography revealed that contrast agent was exuding from the bladder (see Figure 1A and B); thereafter, renal dynamic imaging was performed, and we found that over time, the contrast agent was gradually visualized in both kidneys and bladder (see Figure 1C), and the stool sample of the patient also shows this visualization (Figure 1D). In summary, the diagnosis of urinary fistula was confirmed in this patient. To understand the cause of urinary fistula, a colonoscopy was conducted and showed multiple ulcers in the large intestine: Crohn’s disease? (Figure 2), and colonic tissue biopsy revealed chronic inflammation and granulation tissue hyperplasia (Figure 3). Because Crohn’s disease and intestinal tuberculosis are similar in clinical manifestations and pathological findings, considering that this patient had fatigue, anorexia, weight loss, recent night sweats, and a history of long-term use of hormones and immunosuppressive agents, chest and abdomen CT revealed multiple thoracic, abdominal, and retroperitoneal lymphadenopathy with calcification, positive T-SPOT, and elevated high-sensitivity C-reactive protein, although the predilection site for intestinal tuberculosis was the ileocecum, it could also occur throughout the intestine, so intestinal TBC still could not be excluded. Therefore, we further conducted the relevant examinations: gastroscopy showed chronic non-atrophic gastritis, QFT was positive, the results of detecting mycobacterium TBC in colon biopsy tissues using PCR technique were positive twice, and acid-fast staining revealed positive bacilli. Combined with the patient’s medical history, clinical manifestations and relevant examinations, the diagnosis of vesicorectal fistula caused by intestinal TBC was confirmed. Quadruple anti-tuberculosis treatment (isoniazid + rifampicin + pyrazinamide + ethambutol) was given, and conservative treatment (indwelling catheter to observe whether the fistula could close spontaneously) was given based on the patient’s underlying disease and poor nutritional status; for SLE, prednisone (20 mg/d) and hydroxychloroquine (0.2 g bid) were given.
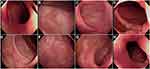
More than 1 month later, the patient was re-admitted to our hospital due to acute low intestinal obstruction, and was given gastrointestinal decompression and drainage with small intestinal drainage tube. The patient continued to receive anti-tuberculosis treatment and was discharged with improved condition. During this period, the patient returned to the outpatient department regularly, and enteroscopy showed ulcer healing in November 2020 (Figure 4). During follow-up until January 7, 2022, the patient had gradually reduced prednisone to 7.5 mg/d and hydroxychloroquine to 0.2 g/d, and discontinued anti-tuberculosis drugs for 1 year. At present, the patient’s condition is stable, urine and defecation are normal, and lupus is fairly controlled.
Discussion
SLE is an autoimmune disease with multiple organ damage. Infection has become one of the main causes of death in patients with SLE, and common pathogens also include mycobacterium TBC. TBC is an important global public health problem. In 2016, TBC was ranked among the top ten causes of death worldwide.7 It has been reported that patients with SLE in southern China are the population at high risk of TBC, especially extrapulmonary and disseminated TBC.8 Intestinal TBC is the sixth most common site of extrapulmonary TBC, and the site often involved in the intestine is the ileocecal region, which may be associated with increased lymphoid tissue and blood stasis. It usually presents with various complications such as intestinal obstruction, perforation, bleeding, and very rarely enterocutaneous fistula.9,10 The diagnosis of gastrointestinal manifestations in SLE is extremely challenging because its differential diagnosis is very wide and needs to be differentiated from Crohn’s disease, intestinal lymphoma, ulcerative colitis, eosinophilic enteritis, lupus cystitis, and infectious enteritis in addition to lupus-associated intestinal vasculitis. Lupus cystitis, as a rare complication of SLE mainly manifested as gastrointestinal symptoms and the urinary irritation, is often accompanied by lupus-associated intestinal vasculitis. For most of the patients, the glucocorticoid pulse therapy and immunosuppressive agent are effective. If relevant conditions are not treated, complications like intestinal pseudo-obstruction, mesenteric vasculitis, and hydronephrosis may occur. Theoretically, there may even be fistulous tract leading to vesical fistula, but there is still no relevant report at present. On the other hand, because fistula formation is more common in Crohn’s disease and colonoscopic features are equally misleading, it is likely to be misdiagnosed as Crohn’s disease. In this patient, we report recurrent diarrhea was the main clinical manifestation, which showed no notable response after steroid pulse therapy and anti-infective therapy, and urinary fistula was confirmed after stool creatinine detection, cystography, and renal dynamic imaging. In order to identify the cause of urinary fistula formation, colonoscopy and biopsy were performed, and PCR was performed on the biopsy tissue to detect mycobacterium tuberculosis and acid-fast staining, which finally confirmed the diagnosis of vesicorectal fistula caused by intestinal TBC. Anti-tuberculosis treatment and immunosuppressive therapy were given. After more than 3 years of follow-up, the patient’s condition was stable and urine and defecation were normal.
Patients with SLE are susceptible to infections due to immune system abnormalities, including immunoglobulin deficiency, complement deficiency, chemotaxis defects, phagocytosis, delayed allergy, and cellular immune abnormalities.11 Lao et al suggested that lymphopenia predominates in patients with lupus TBC, especially in disseminated patients with TBC.8 In addition, lupus nephritis, the average daily dose and cumulative dose of glucocorticoids are all important risk factors for the development of TBC.2,8,12–14 Therefore, patients with SLE, especially those from tuberculosis-endemic areas, need to be more closely monitored.
Enterovesical fistula can be secondary to Crohn’s disease, intestinal compartment, malignancy, ulcerative colitis, trauma, and TBC. Enterovesical fistula secondary to TBC is relatively rare, so there are few relevant reports. Pneumaturia and fecaluria are characteristic features in the later stages of enterovesical fistula.15 Although most enterovesical fistulas are the result of primary enteropathy, patients often experience symptoms of urinary tract infection, such as frequent urination, urgency, dysuria, and hematuria, and up to half of patients experience local intestinal symptoms such as abdominal pain, diarrhea, constipation, and intestinal obstruction.16 When the above symptoms occur, it is necessary to be highly vigilant for the possibility of TBC, especially in patients with SLE, otherwise, delayed treatment due to misdiagnosis may lead to poor prognosis. Conservative treatment with antituberculous drugs, as well as performing indwelling catheterization to divert the urethra, also has a good effect when the patient is at great risk for surgery.
In addition, for patients with SLE, lupus cystitis as a rare complication of systemic lupus erythematosus should also be noted if significant gastrointestinal and urinary symptoms occur. For this case, the main manifestations were diarrhea and urinary tract infection. Considering that lupus cystitis often coexists with lupus gastrointestinal vasculitis, lupus cystitis should be considered based on clinical manifestations. However, enterovesical fistula was confirmed by fecal creatinine control, cystography, and renal dynamic imaging. Of course, lupus cystitis may also lead to vesical fistula from a physiological view of point, but enterovesical fistula is not likely caused by a fistula formed between the intestine and the bladder, and also, there is still no relevant report. Besides, the poor response of the patient to glucocorticoid shock therapy provided in another hospital has suggested that other conditions should be considered. At last, intestinal tuberculosis was confirmed by colonoscopy, as well as mycobacterium tuberculosis detection by PCR and acid-fast staining of tissue for biopsy.
Conclusion
In summary, we report a case of SLE complicated with intestinal tuberculosis, and secondary enterovesical fistula. In the diagnosis and treatment of SLE, especially in tuberculosis-endemic areas such as China, attention should be paid to monitoring and regular screening for tuberculosis before and after treatment. The differential diagnosis needs to be rigorous, and relevant examinations or pathological biopsies can be repeated if necessary. Individualized treatment should pay attention to the balance between disease activity, infection and quality of life.
Date Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Compliance with Ethics Guidelines
Written informed consent to participate in the study was provided by the patient. The patient has signed and agreed to publish identifiable details, including: photos and/or videos and/or details in case history and/or text (“materials”) for publication in magazines and articles. According to the hospital protocol, no formal institutional approval was required to publish the case details in the manuscript.
Acknowledgments
We thank the patient and his family for their participation.
Funding
This work was supported by the Fund of Guizhou Provincial Health Commission [gzwkj2021-134]; the Fund of Guizhou Provincial Administration of Chinese Medicine [QZYY-2021-005]; and the Fund of Guiyang Science and Technology Project (grant no. ZHUKEHE [2019] 9-1-11).
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. Global tuberculosis report 2019 [EB/OL]. Available from: https://www.who.int/tb/publications/global.
2. Mok MY, Lo Y, Chan TM, et al. Tuberculosis in systemic lupus erythematosus in an endemic area and the role of isoniazid prophylaxis during corticosteroid therapy. J Rheumatol. 2005;32(4):609–615.
3. Alarcón GS. Infections in systemic connective tissue diseases: systemic lupus erythematosus, scleroderma, and polymyositis/dermatomyositis. Infect Dis Clin North Am. 2006;20(4):849–875. doi:10.1016/j.idc.2006.09.007
4. Zhang L, Wang DX, Ma L. A clinical study of tuberculosis infection in systemic lupus erythematosus. Zhonghua Nei Ke Za Zhi. 2008;47(10):808–810. Chinese.
5. Zandman-Goddard G, Shoenfeld Y. Infections and SLE. Autoimmunity. 2005;38(7):473–485. doi:10.1080/08916930500285352
6. Wu Q, Liu Y, Wang W, et al. and prevalence of tuberculosis in systemic lupus erythematosus patients: a systematic review and meta-analysis. Front Immunol. 2022;13:938406. doi:10.3389/fimmu.2022.938406
7. WorldHealthOrganization (WHO). Global tuberculosis report. Geneva, Switzerland: World Health Organization; 2016. Available from: http://www.who.int/tb/publications/global_report/gtbr2016.
8. Lao M, Chen D, Wu X, et al. Active tuberculosis in patients with systemic lupus erythematosus from Southern China: a retrospective study. Clin Rheumatol. 2019;38(2):535–543. doi:10.1007/s10067-018-4303-z
9. Akinoğlu A, Bilgin I. Tuberculous enteritis and peritonitis. Can J Surg. 1988;31(1):55–58.
10. Das HS, Panda CR, Singh SP, et al. Colovesical fistula: a rare presentation of colonic tuberculosis. World J Colorectal Surg. 2013;3(1):12.
11. Ginzler EM. Infections in systemic lupus erythematosus. In: Wallace DJ, Hahn BH, editors. Dubois Lupus Erythematosus.
12. Sayarlioglu M, Inanc M, Kamali S, et al. Tuberculosis in Turkish patients with systemic lupus erythematosus: increased frequency of extrapulmonary localization. Lupus. 2004;13(4):274–278. doi:10.1191/0961203303lu529xx
13. Tam LS, Li EK, Wong SM, et al. Risk factors and clinical features for tuberculosis among patients with systemic lupus erythematosus in Hong Kong. Scand J Rheumatol. 2002;31(5):296–300. doi:10.1080/030097402760375205
14. Yun JE, Lee SW, Kim TH, et al. incidence and clinical characteristics of Mycobacterium tuberculosis infection among systemic lupus erythematosus and rheumatoid arthritis patients in Korea. Clin Exp Rheumatol. 2002;20(2):127–132.
15. Larsen A, Bjerklund Johansen TE, Solheim BM, et al. Diagnosis and treatment of enterovesical fistula. Eur Urol. 1996;29(3):318–321. doi:10.1159/000473768
16. Ramchandra N, Mayank V, Sanjeev K, et al. Ileovesical fistula due to tuberculosis: a rare presentation. Int J Health Sci Res. 2015;5(2):414–417.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.