Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 17
Case Report: Fecal Microbiota Transplantation for the Treatment of Generalized Eczema Occurring After COVID-19 Vaccination
Authors Huang T, Lv Y, Wang W, Chen Y, Fan L, Teng Z, Zhou X, Shen H, Fu G
Received 7 October 2023
Accepted for publication 9 January 2024
Published 26 January 2024 Volume 2024:17 Pages 229—235
DOI https://doi.org/10.2147/CCID.S443542
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Tao Huang,1,* Yongling Lv,2,* Wei Wang,1 Yunyao Chen,1 Lixin Fan,1 Zhaowei Teng,3 Xianfeng Zhou,2,4 Hexiao Shen,2 Guang Fu1
1Gastrointestinal Surgery, Wuhan Puren Hospital, Wuhan, People’s Republic of China; 2Maintainbiotech. Ltd. (Wuhan), Wuhan, People’s Republic of China; 3Central Laboratory, The Second Affiliated Hospital of Kunming Medical University, Kunming, People’s Republic of China; 4School of Life Sciences and Health Engineering, Hubei University of Technology, Wuhan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hexiao Shen; Guang Fu, Email [email protected]; [email protected]
Abstract: Adverse skin reactions caused by the COVID-19 vaccine have attracted considerable attention. As we all know, the development mechanism of some skin diseases is related to the gut and skin microbiome. A 78-year-old male patient who received the COVID-19 vaccine developed generalized eczema with multiple dense black patches over the body, a widespread rash, erosion, and scabs on his limbs, as well as facial edema. The patient experienced recurrent flare-ups after conventional treatment, but then recovered well without recurrence after undergoing three fecal microbial transplantation (FMT) treatments. This rare case is reported for the first time in this study. This report demonstrates the possible potential of FMT in targeting refractory skin diseases, such as eczema, as well as diseases associated with gut microbiota disturbance after vaccination.
Keywords: FMT, eczema, COVID-19 vaccine
Introduction
Eczema, which is not only found in young children’s bodies in common but can also appear in adults. The main clinical characteristics of dry, inflamed, and intensely itchy skin can occur in all parts of the body. Its development is usually accompanied by elevated levels of the immunomarker IgE, but the specific immunological mechanisms are not yet fully understood.1 The skin is the largest organ in the human body, harboring approximately 1 billion bacteria per 1 cm2 of surface area and the composition of skin microorganisms is closely related to dermatologic diseases.2 With the proposal of the concept “gut-microbe-skin axis”, more and more studies have shown that the gut microbiota may influence the development of skin diseases by mediating the immune system.3,4 However, evidence for gut microbiota-based therapies in the treatment of eczema is still lacking. Here, we describe a patient treated with fecal microbial transplantation (FMT) for recurrent flare-ups of generalized eczema after COVID-19 vaccination.
Case Report
In June 2022, a 78-year-old male patient was admitted to the department with generalized eczema three days after receiving the inactivated COVID-19 vaccine (0.5 mL). The patient’s medical history included hypertension, cardiac arrhythmia, adrenalectomy, and gastrointestinal dysfunction characterized by constipation, as well as sleep disorders. The patient denied having any previous drug or food allergies, as well as a prior COVID-19 infection. He presented with swelling of the face and lower limbs, as well as large erythematous, papular, and scaly patches that were densely distributed in multiple locations throughout the body. The immune marker IgE was 611.00 IU/mL (0–100 IU/mL), and the inflammatory markers PCT and IL-6 were 1.0865 ng/mL (0–0.051 ng/mL) and 0.2544 ng/mL (0–0.007 ng/mL), respectively. The diagnosis was “adverse reactions of COVID-19 vaccine - generalized eczema”. So he was treated with promethazine (25mg/day, intramuscular injection), diphenhydramine (20mg/night, intramuscular injection), ebastine (10mg/night, orally), loratadine (8.8 mg/day, orally), and traditional Chinese medicine. The patient was discharged from the hospital one week later with marked improvement in eczema symptoms. After discharge, the patient continued to take oral ebastine and topical fluticasone cream, moisturizing cream, and herbal lotion. However, as the symptoms subsided, the patient took intermittent medication at home. Because the patient did not adhere to the medication and due to the relapse-prone nature of the disease, the disease recurred repeatedly, and the symptoms worsened as time went on.
Five months later, the patient was admitted to the hospital presenting with a recurrence of eczema, facial swelling, widespread dark patches on the body, and a generalized rash with erosion and crusts on the extremities (Figure 1). Laboratory tests revealed elevated levels of IgE (2600 IU/mL), inflammatory markers (PCT) (0.110 ng/mL), and IL-6 (0.0174 ng/mL) beyond the normal range. As the patient had a history of gastrointestinal dysfunction, an intestinal functional barrier test was performed. The test showed that the concentration of diamine oxidase was within the normal range at 7.08 U/L (normal range: 0–10 U/L). However, the concentrations of D-lactate (32.91 mg/L) and serum endotoxin (25.11 U/L) were much higher than the normal range of 0–15 mg/L and 0–20 U/L, respectively. Considering the abnormalities in D-lactate and serum endotoxin levels, we suspected that the patient might have an imbalance in the intestinal flora. The subsequent results of the intestinal flora testing confirmed our suspicion. The concept of the gut-skin axis has advanced the study of the microbiome associated with the skin and gut.5,6 Numerous studies have shown that GM metabolites can be involved in the mechanisms of inflammatory responses. Short-chain fatty acids (SCFA), tryptophan, and pathogen-associated molecular patterns can activate different receptors to restore TH1/TH2 balance, thereby enhancing the epidermal barrier of the skin.7,8 This provides a theoretical basis for treating skin-related diseases by restoring the gut microbiota. Therefore, a customized comprehensive treatment plan, including FMT and anti-allergy medication, was eventually considered for this patient.
 |
Figure 1 Patient’s skin condition. |
The FMT donor and preparation of intestinal bacterial fluid were conducted according to established standards.9 The patient received a three-day course of orally administered vancomycin (500 mg, twice/day) up to two days before the FMT procedure. On the day before the FMT procedure, the patients were required to orally take polyethylene glycol to eliminate any remaining antibiotics and fecal material for bowel preparation. The bacterial solution was injected via colonic catheterization, using an injection tube to administer 50 mL of the solution into the terminal ileum and ileocecum once daily for three consecutive days (Figure 2). In addition, the patient received loratadine for anti-allergic medication and topical moisturizing treatment for the skin. Following the first FMT treatment, the patient reported a significant improvement in itchy skin symptoms and sleep quality. After the second day of FMT treatment, supplemented with probiotics, prebiotics, and other medications, the patient experienced a notable reduction in frontal and facial erythema, as well as alleviation of pimples and scales on the limbs (Figure 3A). By the fourth day, after the third FMT treatment, the papules on the patient’s entire body had significantly subsided, and there was a marked improvement in black spots. The patient also reported a significant relief in itchiness throughout their body (Figure 3B). After two days of observation, the patient was discharged from the hospital with a satisfactory recovery. The re-examination results before discharge showed a significant improvement in the levels of diamine oxidase (3.59 U/L), D-lactic acid (13.02 mg/L), and bacterial endotoxin (12.51 U/L) compared to the pre-FMT levels, returning to the levels observed in a healthy individual. The level of IL-6 (13.00 pg/mL) slightly decreased, while the levels of PCT (0.120 ng/mL) and IgE (3100 IU/mL) were slightly elevated, still indicating a higher risk of allergies. Treatment with oral anti-allergy medication (loratadine) and topical moisturizing medication for the skin was continued after discharge. Three months later, the patient reported no recurrence of eczema and a significant improvement in sleep quality and bowel movements.
 |
Figure 2 Colonoscopy administration. Bacterial fluid injection tube (A) and its positioning imaging (B). |
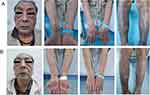 |
Figure 3 Patient’s skin condition after the second FMT (A); after the third FMT (B). |
Stool samples were collected from the patient before FMT treatment and one month after three FMT treatments. The composition of the gut microbiota was compared by utilizing high-throughput sequencing of the 16S V3+V4 region. After FMT, the Shannon index, which represents the alpha diversity of the sample, increased in the gut microbiota, indicating an increase in microbial diversity within the gut (Figure 4A). A higher diversity and richness of the intestinal microbial community are more conducive to the stability of the intestinal microecology. At the phylum level, the gut microbiota of patients was dominated by Firmicutes before FMT, while after FMT, there was a significant decrease in Firmicutes and a notable increase in Bacteroidota and Verrucomicrobiota (Figure 4B). A significant change in the microbiota was observed at the genus level after FMT (Figure 4C). The relative abundance of Bacteroides, Akkermansia, Faecalibacterium, and Bifidobacterium increased, while Subdoligranulum, Megamonas, Catenibacterium, Ruminococcus, and Holdemanella showed a decrease in relative abundance. It is worth mentioning that there was a significant increase in the abundance of Akkermansia in Verrucomicrobiota after FMT. However, it is important to note that the relative abundance of Akkermansia in the donors was relatively low.
 |
Figure 4 Shannon index (A); relative species abundance at phylum level (B) and genus level (C). |
Discussion
Vaccination is an effective measure for controlling epidemics. The new COVID-19 vaccine has played an important role in stopping the spread of the rapidly evolving SARS-CoV-2. However, there is also growing attention on the adverse reactions to the COVID-19 vaccine that has received accelerated approval. In addition to major discomforts such as fever, headache, and respiratory symptoms, some vaccinated individuals may experience dermatological conditions such as localized erythematous herpes zoster, pityriasis rosea, erythema multiforme, varicella, herpes simplex, psoriasis, and others.10 One study showed that eczema-like rashes accounted for 27.27% of all adverse skin reactions.11 Generalized eczema is an extremely rare condition with a complex mechanism of occurrence and development that is related to immune system dysfunction. The characteristics of a prolonged onset period, frequent recurrence, and difficulty in finding a cure seriously affect the quality of life for patients. As far as we all know, this is the first report of using FMT as a treatment for generalized eczema after vaccination.
Currently, the primary method of treating eczema is through the external application of glucocorticoids and emollients.12,13 In addition, immunosuppressive drugs (cyclosporine, methotrexate, etc.) are often clinically recommended for patients with severe generalized eczema. It is worth mentioning that the FDA’s approval of dupilumab and abrocitinib has benefited numerous patients with atopic dermatitis.14,15 However, the actual clinical situation is complex. Therefore, treatment strategies often require a comprehensive assessment of the patient’s age, medical history, and disease severity. In this case, the patient was initially treated with a combination of an anti-allergic drug (promethazine, diphenhydramine, ebastine, and loratadine), a glucocorticoid (fluticasone cream), and a moisturizing cream. This treatment approach resulted in a positive outcome. However, the eczema recurred, and the patient’s condition worsened even further after a few months. This result suggests that conventional treatment may only provide temporary relief for this patient. Therefore, safer and more effective treatments are needed for certain patients. Microbial therapies focused on the “gut-microbe-skin axis” are gaining attention from dermatologists as research in microbial therapies. Navarro-Lopez et al have demonstrated that novel probiotic agents can improve atopic dermatitis (AD) by regulating the composition of the microbiota.16,17 It was not until Huang18 and Mashiah et al19 began to attempt to use fecal fluid directly in the form of FMT to treat AD. However, there are currently relatively few clinical studies on FMT in AD, and there are no relevant reports on generalized eczema. Therefore, more research is needed. Considering the unique circumstances of this patient, we discovered that his intestinal microecology was disrupted after analyzing his gut microbiota. As a result, we have decided to implement the treatment plan of FMT.
We observed significant changes in the gut microbiome after FMT in this patient. Before FMT, the abundance of Subdoligranulum (associated with diseases such as food allergies),20 Megamonas (associated with systemic inflammatory cytokines),21 Catenibacterium (associated with metabolic syndrome),22 and Ruminococcus (associated with intestinal mucolysis)23 is relatively high. This finding is consistent with the results reported in other immune system-associated skin diseases (Symptomatic dermographism, psoriasis, and atopic dermatitis).21,24–26 However, another study by Zhang et al showed a reduction in the abundance of Megamonas and Dialister in patients with chronic spontaneous urticaria (CSU) compared to healthy individuals.27 In a case study by Huang et al on the treatment of patients with functional constipation and accompanying AD using FMT, it was found that Ruminococcus was enriched in the patient before FMT, while Bacteroides, Alistipes, Bilophila, and other bacteria related to bile metabolism increased after FMT.18 However, these changes may also be associated with constipation. In this case report, the patient had a lower abundance of Faecalibacterium and Bifidobacterium before FMT compared to healthy individuals. Although similar evidence has been found in the reported literature,28–31 there appear to be some inconsistencies regarding changes in Faecalibacterium. Faecalibacterium prausnitzii has been strongly associated with AD and infantile eczema.32,33 Interestingly, a macrogenomic analysis of psoriasis by Dei-Cas et al34 yielded similar results. However, the higher abundance of Bacteroides in non-psoriasis patients is consistent with the findings in this case. This contradiction may be related to a correlation between the specific mechanisms of different disease types. In addition, there are significant differences in Akkermansia before and after FMT, as well as between healthy donors. Although research results have shown that the abundance of Akkermansia is significantly reduced in patients with psoriasis,30,31,35 this phenomenon cannot be well explained from the perspective of fecal microbiota transplantation. Interestingly, the findings of Mashiah et al seem to help us understand this phenomenon.19 They demonstrated through continuous FMT capsule treatments that the gut microbiota of patients gradually became more similar to that of the donor over time, especially in terms of the transmission of specific bacterial strains rather than changes in relative abundance. The gut microbiota is a functionally complex microecosystem. Therefore, we speculate that this may be the result of the comprehensive effect of the gut microbiota. In the future, it is recommended to employ continuous sampling and other methods to dynamically monitor the gut microbiota. Additionally, as this study is a single case report, we need to consider limitations such as individual differences, donor differences, and lack of controls. Therefore, it is necessary to expand the study cohort to further elucidate the relationship between gut microbiota and immune system-related skin diseases, such as eczema.
The case we reported demonstrates the possible therapeutic effect of FMT in treating refractory eczema. This finding warrants further large-scale clinical research and the exploration of FMT as a potential treatment for other forms of eczema. While focusing on exploring specific microbial biomarkers and metabolic markers of particular microbial groups, it is also important to consider the dynamic changes of the entire microbial community over time. This will help further understand the microbial processes at different stages of the disease and provide scientific guidance for personalized treatment.
Data Sharing Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author (Guang Fu).
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Wuhan Puren Hospital. The patient provided their written informed consent to participate in this study. Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Acknowledgments
The authors thank the patient for providing consent and granting permission to draft and publish this case report. We acknowledge the medical staff of Wuhan Puren Hospital who were associated with this patient in the preparation of this manuscript.
Disclosure
Yongling Lv, Xianfeng Zhou and Hexiao Shen are employees of Maintainbiotech. Ltd. (Wuhan). The authors declare no other conflicts of interest in this work.
References
1. Suaini NH, Siah KT, Tham EH. Role of the gut–skin axis in IgE-mediated food allergy and atopic diseases. Curr Opin Gastroenterol. 2021;37(6):557–564. doi:10.1097/MOG.0000000000000780
2. Grice EA, Kong HH, Renaud G, et al. A diversity profile of the human skin microbiota. Genome Res. 2008;18(7):1043–1050. doi:10.1101/gr.075549.107
3. Su Y, Zhang F, Qin W, et al. ”Gut-skin” axis: understanding psoriasis from the gut. Int J Pharm Sci. 2021;76(11):523–527.
4. Salem I, Ramser A, Isham N, Ghannoum MA. The gut microbiome as a major regulator of the gut-skin axis. Front Microbiol. 2018;9:1459.
5. Thye AY, Bah YR, Law JW, et al. Gut–skin axis: unravelling the connection between the gut microbiome and psoriasis. Biomedicines. 2022;105:1037.
6. De Pessemier B. Gut–skin axis: current knowledge of the interrelationship between microbial dysbiosis and skin conditions. Microorganisms. 2021;2:353.
7. Alam M. Manipulating microbiota to treat atopic dermatitis: functions and therapies. Pathogens. 2022;6:642.
8. Stec A, Sikora M, Maciejewska M, et al. Bacterial metabolites: a link between gut microbiota and dermatological diseases. Int J Mol Sci. 2023;4:3494.
9. Alliance CM; Microecology Committee of Shanghai Preventive Medicine Association. Chinese experts consensus on standardized methodology and clinical application of fecal microbiota transplantation. Zhonghua Wei Chang Wai Ke Za Zhi. 2020;23(Z1):5–13.
10. Català A, Muñoz-Santos C, Galván-Casas C, et al. Cutaneous reactions after SARS-CoV-2 vaccination: a cross-sectional Spanish nationwide study of 405 cases. Br J Dermatol. 2022;186(1):142–152.
11. Li Y, Fan L, Mao Q, et al. Clinical observation and analysis of skin reactions caused by COVID‐19 vaccination. Dermatologic Therapy. 2022;35(10):e15746.
12. Wollenberg A, Barbarot S, Bieber T, et al. Consensus‐based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32(5):657–682.
13. Chan S. Biologics in atopic dermatitis. Curr Opin Allergy Clin Immunol. 1970;3:297–302.
14. FDA Approves Dupixent® (Dupilumab) For Moderate-To-Severe Atopic Dermatitis In AdolescENTS. Available from: https://investor.regeneron.com/news-releases/news-release-details/fda-approves-dupixentr-dupilumab-moderate-severe-atopic.
15. FDA Approves Pfizer’s Supplemental New Drug Application for CIBINQO® (abrocitinib). Available from: https://www.pfizer.com/news/press-release/press-release-detail/fda-approves-pfizers-supplemental-new-drug-application.
16. Navarro-López V, Martínez-Andrés A, Ramírez-Boscá A, et al. Efficacy and safety of oral administration of a mixture of probiotic strains in patients with psoriasis: a randomized controlled clinical trial. Acta Dermato Venereol. 2019;99(12):1078–1084.
17. Navarro-López V, Ramírez-Boscá A, Ramón-Vidal D, et al. Effect of oral administration of a mixture of probiotic strains on SCORAD index and use of topical steroids in young patients with moderate atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2018;154(1):37–43.
18. Huang HL, Xu HM, Liu YD, et al. Fecal microbiota transplantation as a novel approach for the treatment of atopic dermatitis. J Dermatol. 2021;48(12):e574–6.
19. Mashiah J, Karady T, Fliss‐Isakov N, et al. Clinical efficacy of fecal microbial transplantation treatment in adults with moderate‐to‐severe atopic dermatitis. Immun Inflamm Dis. 2022;10(3):e570.
20. Abdel-Gadir A, Stephen-Victor E, Gerber GK, et al. Microbiota therapy acts via a regulatory T cell MyD88/RORγt pathway to suppress food allergy. Nature Med. 2019;25(7):1164–1174.
21. Zhang X, Shi L, Sun T, Guo K, Geng S. Dysbiosis of gut microbiota and its correlation with dysregulation of cytokines in psoriasis patients. BMC Microbiol. 2021;21(1):1.
22. Phungviwatnikul T, Lee AH, Belchik SE, Suchodolski JS, Swanson KS. Weight loss and high-protein, high-fiber diet consumption impact blood metabolite profiles, body composition, voluntary physical activity, fecal microbiota, and fecal metabolites of adult dogs. J Anim Sci. 2022;100(2):skab379.
23. Vacca M, Celano G, Calabrese FM, Portincasa P, Gobbetti M, De Angelis M. The controversial role of human gut lachnospiraceae. Microorganisms. 2020;8(4):573.
24. Liu R, Peng C, Jing D, et al. Biomarkers of gut microbiota in chronic spontaneous urticaria and symptomatic dermographism. Front Cell Infect Microbiol. 2021;11:703126.
25. Liu R, Peng C, Jing D, et al. Identification of gut microbiota signatures in symptomatic dermographism. Exper Dermatol. 2021;30(12):1794–1799.
26. Rostaher A, Morsy Y, Favrot C, et al. Comparison of the gut microbiome between atopic and healthy dogs—preliminary data. Animals. 2022;12(18):2377.
27. Zhang X, Zhang J, Chu Z, Shi L, Geng S, Guo K. Gut microbiome alterations and functional prediction in chronic spontaneous urticaria patients. J Microbiol Biotechnol. 2021;31(5):747.
28. Yap GC, Loo EX, Aw M, Lu Q, Shek LP, Lee BW. Molecular analysis of infant fecal microbiota in an Asian at-risk cohort–correlates with infant and childhood eczema. BMC Res Notes. 2014;7:1–6.
29. Gore C, Munro K, Lay C, et al. Bifidobacterium pseudocatenulatum is associated with atopic eczema: a nested case-control study investigating the fecal microbiota of infants. J Allergy Clin Immunol. 2008;121(1):135–140.
30. Tan L, Zhao S, Zhu W, et al. The Akkermansia muciniphila is a gut microbiota signature in psoriasis. Exper Dermatol. 2018;27(2):144–149.
31. Koper M, Woźniacka A, Robak E. The intestinal microbiota in psoriasis. Adv Hygie Exper Med. 2020;74:236–246.
32. Song H, Yoo Y, Hwang J, Na YC, Kim HS. Faecalibacterium prausnitzii subspecies–level dysbiosis in the human gut microbiome underlying atopic dermatitis. J Allergy Clin Immunol. 2016;137(3):852–860.
33. Zheng H, Liang H, Wang Y, et al. Altered gut microbiota composition associated with eczema in infants. PLoS One. 2016;11(11):e0166026.
34. Dei-Cas I, Giliberto F, Luce L, Dopazo H, Penas-Steinhardt A. Metagenomic analysis of gut microbiota in non-treated plaque psoriasis patients stratified by disease severity: development of a new psoriasis-microbiome index. Sci Rep. 2020;10(1):12754.
35. Scher JU, Ubeda C, Artacho A, et al. Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis Rheumatol. 2015;67(1):128–139.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
