Back to Journals » International Medical Case Reports Journal » Volume 16
Case Report and Literature Review of an Atypical Polymyalgia Rheumatica and Its Management
Authors Ahmed SB, Ahmad S, Pan H
Received 15 September 2023
Accepted for publication 7 December 2023
Published 27 December 2023 Volume 2023:16 Pages 873—885
DOI https://doi.org/10.2147/IMCRJ.S440486
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Saad Bilal Ahmed,1 Saara Ahmad,2 Hanmei Pan1
1Monash Health Rehabilitation and Aged Care Services, Melbourne, Australia; 2Department of Biological and Biomedical Sciences, The Aga Khan University, Karachi, Pakistan
Correspondence: Saad Bilal Ahmed, Monash Health Rehabilitation and Aged Care Services, Melbourne, Australia, Email [email protected]
Abstract: Polymyalgia rheumatica (PMR) is a systemic inflammatory disease of the elderly population that increases in incidence as age advances. It is characterised by the sudden or sub-acute onset of symptoms affecting the shoulder and pelvic girdles, often accompanied by constitutional symptoms. Due to the lack of consensual diagnostic criteria and specific laboratory or radiological investigations for PMR, its diagnosis can be very challenging, particularly because it can be mimicked or masked by other geriatric syndromes. PMR responds well to glucocorticoid treatment, but if left untreated, can lead to morbidity and poor quality of life. We present the case of an 87-year-old male who presented with a one-week history of localised pain in the left hip joint, later involving the contralateral hip. Previously able to ambulate unaided, his mobility was now severely impaired. Due to his Alzheimer’s dementia and multiple comorbid geriatric conditions, extensive investigations were undertaken before a diagnosis of atypical PMR was reached. Treatment with a low dose of prednisolone led to a full recovery. This case highlights the inconsistency between an atypical presentation and the classic presentation of PMR and draws attention to the possibility of missed diagnosis in older, frail patients. Atypical symptomatology on top of cognitive impairment and language barriers can be easily overlooked and left untreated and could lead to severe adverse outcomes. Accurate diagnosis is crucial, as PMR is readily diagnosed, but the treatment with glucocorticoids, though generally straightforward, can pose challenges, particularly when dealing with polypharmacy and multiple coexisting health conditions.
Keywords: polymyalgia rheumatica, geriatrics, myositis, Alzheimer’s dementia
Case Summary
Mr JZ is an 87-year-old Cantonese-speaking man, who was referred to the Emergency Department (ED) with a one-week history of increasing pain and weakness in the left leg, impaired mobility, and one episode of urinary incontinence. He also had a history of anorexia with loss of approximately 12% of body weight over the preceding six months. He lived with family and received minimal assistance for personal activities of daily living. Prior to this episode, he had been able to ambulate independently and unaided around the house. A written consent was taken from the patient for presentation and publication of his data for research purposes, and here we have confirmed that approval was not required from Monash Health Rehabilitation and Aged Care Services, to publish this case report.
These symptoms occurred on a background of Alzheimer’s dementia (AD), hypertension, stroke, hyperlipidaemia, gout, peptic ulcer, and chronic kidney disease. His medications on admission included clopidogrel 75mg daily, irbesartan 300mg daily, atorvastatin 40mg daily, esomeprazole 40mg daily, lercanidipine 10mg daily, and rivastigmine 4.6mg patch daily. Rivastigmine was stopped on admission because of its documented side effect of anorexia.
On presentation, Mr JZ’s blood pressure was 88/45mmHg, but this improved after rehydration with 0.9% saline infusion. His heart rate was 96/min, temperature 36.4°C, and oxygen saturation 95% on room air with pulse oximetry. The anthropometric measurements of the patient were: weight 51kg, height 1.60m, and body mass index 19kg/m2. On clinical examination, he had dual heart sounds without any murmur; a chest examination revealed bilateral equal air entry, and his abdomen was soft with no evidence of visceromegaly. A neurological examination was essentially unremarkable except for reduced power of 4/5 in the left hip involving hip and knee flexors and extensors.
Initial investigations revealed raised inflammatory markers: C-reactive protein (CRP) 76mg/L, white cell count (WCC) 11.9 x109/L with neutrophil count of 8.5 x109/L, and evidence of pre-renal acute on chronic kidney disease (Table 1). An initial differential diagnosis of cauda equina syndrome or epidural abscess was considered in the ED based on the presenting features of left leg pain and weakness, urinary incontinence and raised inflammatory markers. An urgent magnetic resonance image (MRI) of the lumbar spine was performed, which showed mild foraminal narrowing at L5-S1 with no central spinal canal stenosis and no evidence of epidural abscess.
 |
Table 1 Mr JZ’s Test Results During Admission and Follow-Up |
The patient was admitted to the general medical unit for further investigation and management of possible underlying sepsis. It was difficult to obtain a proper history due to his AD and culturally and linguistically diverse (CALD) background. He could not communicate with healthcare staff, even with family members and a Cantonese interpreter present, and was only able to answer simple questions about pain and thirst. A chest X-ray, atypical pneumonia serology, urine microscopy and culture were all unremarkable. A plain X-ray film of the left hip showed no fracture. However, Mr JZ’s left hip symptoms continued to deteriorate. The pain was managed with paracetamol (1 gram every 8 hours) and oxycodone/naloxone (5/2.5mg orally twice daily) with oxycodone immediate release (5mg every 6 hours for breakthrough pain). Despite this, his pain continued to increase in intensity, and a repeat neurological examination revealed further reduction in power to 2/5 at the hip and 3/5 at the knee. Meanwhile, the acute on chronic kidney injury improved with intravenous hydration, but the inflammatory markers including the WCC and CRP continued to increase. The erythrocyte sedimentation rate (ESR) was also markedly high (Table 1).
This deterioration of symptoms with a lack of specific findings made the situation complex. Given the patient’s age and comorbidities, multiple other investigations were initiated to rule out alternative aetiologies. An ultrasound (US) of the left hip showed a small anterior hip joint effusion as shown in Figure 1. His weight loss pointed towards the possibility of a malignancy, but normal tumour markers with lack of specific findings on MRI hip and isotope bone scan ruled out this possibility as shown in Figures 2 and 3.
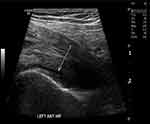 |
Figure 1 Left Hip ultrasound. Small left anterior Hip joint effusion of unknown aetiology. |
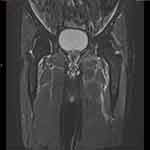 |
Figure 2 MRI with contrast showing diffuse T2 hyperintense swelling and enhancement involving bilateral iliacus muscles, distal psoas and iliopsoas tendons bilaterally. |
 |
Figure 3 Bone scan showing no osteoblastic abnormality throughout the pelvis. |
A differential diagnosis of septic arthritis and crystal arthropathy was considered, but an attempt to aspirate the left hip joint was unsuccessful. Based on patient’s previous history of gout, colchicine was commenced. Atorvastatin was ceased due to its potential to cause myositis and myalgia, although creatine kinase levels were within normal limits (Table 1). However, these attempts were all unsuccessful in improving his symptoms. An MRI of the hips and pelvis (Figure 2) was performed, which revealed bilateral iliacus and iliopsoas oedema with enhancement, indicative of myositis. Additionally, a left-sided insertional tendinopathy was reported with no evidence of trochanteric bursitis. These findings of muscular oedema with enhancement favoured inflammatory, as opposed to infective, aetiology. By then, the patient was complaining of pain in the contralateral hip.
Progress and Management
Based on the MRI findings and lack of alternative explanation such as sepsis, a trial of oral glucocorticoids (prednisolone, 15mg daily) was commenced. After 48 hours, Mr JZ’s pain levels significantly decreased to a level that allowed him to mobilise 30 meters using a 4-wheelframe with one person assisting. This helped to make the diagnosis of PMR. The oxycodone/naloxone was weaned off over the next six days and his inflammatory markers also reduced. A further plan was made to continue prednisolone 15mg daily for four weeks and then wean down to 10mg with continuation, with regular outpatient follow-up. The patient was then transferred for inpatient rehabilitation, where he was also diagnosed with depression by psychiatry team. He was commenced on citalopram 10mg with later up-titration to 20mg daily. He achieved significant gains in mobility during rehabilitation and was discharged home after two weeks.
Mr JZ attended rheumatology follow-up appointments at two and seven months after discharge. At follow-up, he was living at home with his family and was receiving home-based services. He could mobilise unaided for up to 100 meters. The maintenance dose of prednisolone was further reduced to 5mg daily. Inflammatory markers were within normal limits as listed in Table 1.
Due to the potential side effects of glucocorticoid treatment in elderly patients with dementia and risk of falls, Mr JZ was monitored for bone health with dual-energy X-ray absorptiometry (DEXA). Mr JZ was diagnosed with osteoporosis with a T-score of −3.8 on a DEXA bone density scan. Denosumab (60mg subcutaneous) six-monthly was therefore initiated.
Literature Review
Polymyalgia rheumatica (PMR) is an inflammatory rheumatic disorder that is most frequently seen in patients over 50 years of age and more common in females.1 Its most common symptoms are persistent inflammatory pain and morning stiffness of the shoulder2 and pelvic girdles, along with synovitis of proximal and distal joints and extra-articular synovial structures.3 Characteristically, the onset of the disease is sudden or sub-acute, and patients may also report systemic symptoms including weight loss, fever, malaise, and fatigue.4 The diagnosis of PMR is primarily made clinically,1 but is often supported by the presence of elevated inflammatory markers, particularly ESR and CRP.5
A multitude of clinical conditions may mimic PMR in the geriatric population. Frailty, depression, chronic diseases, and deconditioning share a lot of common constitutional symptoms with PMR such as weight loss, poor appetite, low energy levels and decreased mobility.6–8 The presence of cognitive impairment further complicates the acquisition of a comprehensive medical history and poses challenges in diagnosing cases with atypical presentations. A case report by Alisky in 2008 highlighted the potential oversight of PMR in individuals with dementia. In such patients, the ability to articulate symptoms may be compromised, and the expression of their feelings might be challenging, contributing to the difficulty in recognizing PMR in the context of dementia.9
Although several classification criteria have been developed to enable to study of PMR (see Table 2),10 discrepancies occur between them, meaning that careful clinical assessment remains the yardstick for PMR diagnosis.3 Recently, applications of US, MRI, and positron emission test (PET) imaging techniques have contributed to our understanding of the clinical pattern of PMR and our ability to distinguish it from rheumatoid arthritis (RA) and osteoarthritis (OA), inflammatory myopathies, occult malignancies, and infections.11–16 Sub-deltoid bursitis and shoulder and hip synovitis are not specific to PMR alone, so differentiating PMR from other disorders has treatment implications.17
 |
Table 2 Differences Between the 4 Classification Criteria of PMR.10,18–20 |
The gold standard for PMR treatment is the use of glucocorticoids.21,22 However, the roles of disease-modifying anti-rheumatic drugs (DMARDS) methotrexate (MTX) and leflunomide, and monoclonal antibodies like Tocilizumab (TCZ) and Etanercept23–25 will also be taken into consideration in this review.
Methods
A literature search was carried out using PubMed and Ovid Medline databases for observational studies, meta-analyses, randomised controlled trials, case reports, and series of PMR patients. A combination of the following keywords was used to accomplish the search: atypical PMR, PMR with giant cell arteritis, PMR and Alzheimer’s, myositis and PMR, PMR and arthritis, glucocorticoids and PMR, PMR and statins, dementia and PMR, fall and PMR, chronic pain with PMR, PMR in Asian, PMR and frailty. Manual searching of reference lists in retrieved articles was also performed to identify further relevant studies.
Results and Discussion
Signs and Symptoms
PMR is a chronic inflammatory disorder of the elderly population that increases in frequency with age.19,20 The distinguishing attributes of PMR include pain with enduring morning stiffness affecting the shoulders and pelvic girdles,26 raised inflammatory markers, and significant association with giant cell arteritis (GCA).27 The current patient presented with tiredness, depression, weight loss with decreased appetite, and one week of unilateral leg weakness. The current literature suggests that up to 95% of PMR cases present with shoulder pain and 50% with pelvic girdle pain and that unilateral shoulder or hip pain is uncommon, often becoming bilateral a few days later.1,28 This patient’s presentation of PMR was uncharacteristic, with no evidence of morning stiffness or involvement of the shoulders or bilateral hip until later in the presentation. The patient’s constitutional symptoms also had other potential origins—geriatric syndromes of dementia, frailty, and chronic pain may mimic PMR, where nonspecific musculoskeletal rheumatism makes the diagnosis complex.4,6–8,29 The atypical manifestation of symptoms, coupled with his concurrent geriatric syndromes, created complexity and prolonged diagnosis of PMR. This underscores the importance of additional research and documentation on atypical presentations of PMR for enhanced understanding and recognition in clinical settings.
Laboratory Investigations
Although markers of inflammation are frequently elevated in PMR,19 no distinct laboratory test exists for its diagnosis.19,20,30 Laboratory results in PMR are typically non-specific and may exhibit typical markers of generalized inflammation, including anemia, leukocytosis, elevated ESR and CRP, increased alkaline phosphatase, and mild transaminitis. Additionally, there may be an imbalance in thyroid-stimulating hormone, electrolytes, creatinine, and creatinine phosphokinase levels.20,30–35 ESR and CRP levels were found to be markedly elevated in this patient and were monitored during follow-ups (Table 1). Other laboratory tests that are supportive of suspected PMR and the exclusion of other disorders include assessment of the thyroid-stimulating hormone,36 electrolytes, alkaline phosphatase levels, creatinine, and creatine phosphokinases.37–42 All of these were normal in this patient (Table 1).
Imaging Investigations
A number of studies have investigated the utility of imaging techniques such as US, MRI and/or PET scans for the diagnosis, assessment, and treatment of PMR and its distinguishing from other ailments.42–45 A well-conducted case-control study involving PMR and RA patients revealed that MRI is a favourable and sensitive modality in PMR diagnosis.46 Meanwhile, a systematic review of 10 US studies and 6 MRI studies showed that the finding of sub-acromial bursitis in an US is the most helpful feature for diagnosis in PMR and that a peri-articular inflammatory process may be revealed in an MRI.47 In the present case, the US and MRI showed the presence of a thin sliver of fluid along with inflammation of the iliopsoas and iliacus muscles of the left lower limb (Figures 1 and 2). The effusion raised the possibility of an inflammatory or, less likely, an infectious aetiology,48,49 where potential causes could have been bacteria, fungi, parasites, or viral agents.50–53 However, the patient’s afebrile condition with mild leukocytosis made sepsis seem unlikely.
The utility of PET scans is limited in PMR and is mainly used to distinguish it from GCA,20 a condition with similar clinical features including headache, jaw and limb claudication, and visual disturbance.18,22,51,52,54–56 PET scans are expensive and seldom required in routine clinical evaluation of patients with PMR.57 Since there were no symptoms to suggest GCA in our patient, a PET scan was not performed.
Diagnostic Criteria and Difficulties in Diagnosis
A number of different criteria sets exist for PMR identification, with no international consensus, making the diagnosis of atypical PMR difficult (Table 2). The Chuang and Healey sets of criteria are rigid, requiring all points to be present, disallowing the possibility for atypical presentations.19 The ACR/EULAR classification criteria18 helpfully utilises positive as well as negative predictive values,3 ensuring consistency of diagnosis, which is especially important in comparing participants between research studies. However, failure to fulfil these criteria need not preclude a diagnosis of either PMR in an individual patient.27,58 Bird/Wood’s criteria, which reports a sensitivity rate of 92% and specificity of 80%,59 allows for some flexibility in the symptoms present (Table 2). Using any criteria but Bird/Wood’s would have missed PMR in the current case—there is clearly a need for a consensual criteria set with more flexibility for the recognition of potential atypical presentations.
Epidemiological studies have shown that PMR is more frequent in Caucasian compared to Asian populations, particularly in the northern European hemisphere.27,41,60,61 A comprehensive epidemiological study of PMR within the Asian population is lacking. From the available literature, it appears that the elderly Chinese population may exhibit a distinct PMR profile compared to Caucasians. This includes less stiffness, an extended duration of symptoms, reduced bilateral upper limb tenderness, and substantial overlap of PMR with RA and GCA.27,61–63 Interestingly, in one small study using a Chinese population, the mean age of presentation is a low 43.1 years with a range of 28–60 years,60 suggesting that GCA-type conditions including PMR may differ in key characteristics across ethnic groups. This also problematises the age limit used in the Chuang, Healey, and Bird/Wood criteria (Table 2).
Gender variations in PMR manifestations may exist. According to a case cohort study, female PMR patients tended to endure prolonged morning stiffness and intense pain, often necessitating a higher utilization of polypharmacy, which includes elevated doses of glucocorticoids, antidepressants, and opiate analgesics, in comparison to their male counterparts.61 With morning stiffness being a key point in many of the current classification criteria, the potential for missed diagnosis in men becomes heightened.
The current patient fulfilled the Bird/Wood criteria for diagnosis of PMR. However, the inappropriateness of the other 3 criteria, coupled with the emerging literature on the differing presentation patterns in different ethnic groups and sexes, highlights the need for PMR classification criteria to encompass a wider scope of possible signs and symptoms.
Mr JZ was previously diagnosed with mild to moderate AD by a geriatric multidisciplinary team. His AD may have rendered him unable to recall a true history of his symptoms and conditions, and previous research suggests that this can contribute to an easy misdiagnosis.9 Mr JZ’s AD may have therefore contributed to the delay in his diagnosis. To add to this, the patient’s CALD background became another barrier to diagnosis. Although the hospital team involved his family and a Cantonese interpreter, Mr JZ CALD background and AD combined to not only result in significant delays in obtaining his history but also difficulties in establishing rapport with medical staff and understanding the rationale for the investigations, the impacts of treatment, and the Australian health care system.64
In the present case where the PMR presentation was uncharacteristic, the major breakthrough in diagnosis was the prompt alleviation of pain after corticosteroid treatment. The classic speedy response to low-dose prednisolone (≤20 mg) becomes a substantiation of the PMR diagnosis.22,65,66 The use of a measure that does not require much self-report or history-taking is useful in patients with AD. However, as will be discussed under “Treatments”, administering corticosteroids to patients with AD may be dangerous. The patient was deemed frail on comprehensive geriatric assessment, displaying unintentional weight loss, slowness of walking, weakness, and low physical activity. It restrained the diagnosis, impacting on management decisions by requiring them to be conservative and non-invasive. For example, his muscle biopsy was not taken even though the MRI showed diffuse myositis. The need for non-invasive investigations is clearly important in a condition that becomes more common with age alongside dementia and frailness.
Establishing definitive diagnostic criteria for PMR remains a complex endeavor. The challenge lies in the multitude of variables that can influence diagnosis, including comorbidities and medication complexities. The nonspecific nature of PMR’s early presentation further complicates the development of true diagnostic criteria. While there is a need for precise and universally applicable criteria, achieving this goal proves challenging given the inherent complexities and variations in how PMR manifests. Efforts towards creating a diagnostic blood test are underway, but this, too, presents its own set of hurdles.
The present case highlights the difficulties of diagnosing PMR in the elderly population. It is worth noting that the geriatric population living in nursing homes are at risk of developing PMR and residential care nurses should be trained to recognise PMR symptoms that may be dismissed as normal for ageing. A UK-based qualitative study suggested that PMR has a significant disability and psychological impact on patients.67 Prompt recognition and early medical referral will improve patient outcomes at residential care facilities.
Differential Diagnoses
The differential diagnosis of PMR is a critical aspect of its clinical evaluation, given the overlapping symptoms with various rheumatic and non-rheumatic conditions. PMR shares clinical features, such as pain and stiffness, with conditions like rheumatoid arthritis, osteoarthritis, and fibromyalgia, necessitating a comprehensive approach to differentiation. One key factor is the age of onset, as PMR predominantly affects individuals over the age of 50. Distinguishing PMR from other inflammatory arthritides, particularly rheumatoid arthritis, is crucial. While both conditions exhibit joint pain and morning stiffness, the pattern of joint involvement, distribution, and response to glucocorticoids can aid in differentiation. Additionally, ruling out conditions like giant cell arteritis (GCA) is essential, as PMR and GCA often coexist. Investigations, including imaging studies such as ultrasound or MRI, can contribute to the diagnostic process. However, the absence of specific diagnostic markers makes the diagnosis challenging, emphasizing the need for a thorough clinical evaluation and careful consideration of differential diagnoses to ensure accurate and timely management of PMR.
Inflammatory effusion of joints, bursae, skeletal muscles and tendons is also seen in other idiopathic inflammatory myopathies. Myopathies may indeed share similar symptomatology with PMR, including constitutional symptoms of fever, myalgia, old age, muscle weakness with generalised fatigue, headaches, psycho-neurological deficits, and weight loss.14,34,68–71 However, no studies have compared the patterns of signs and symptoms between PMR and these conditions such as RA,17,46 crystalline arthritis, dermatomyositis, polymyositis, inclusion body myositis (IBM),45,57,72 infectious myositis,43 septic arthritis,50 and spondyloarthropathy.17,68 In inflammatory arthritis like spondyloarthritis and (chiefly seronegative) RA, patients may present with PMR-mimicking swelling and pitting oedema of hands and feet due to tenosynovitis,17,19,34 but these were not present in this case.
In the present scenario, the MRI revealed the inflammation of muscles and oedema of the iliacus and iliopsoas, but was unable to distinguish between its inflammatory or septic nature. To reveal inflammation of extra-articular synovial structures, other modalities including X-ray, US, CT scan, and fludeoxyglucose-PET are frequently used alongside MRI.22,47,49,51,52,56 These may show the expected findings of bursitis and synovitis of joints.
Dermatomyositis or polymyositis were unlikely diagnoses due to the absence of symmetrical proximal muscle weakness and cutaneous manifestations such as skin eruptions, erythema, and pruritus.57,72 IBM was a potential contender due to asymmetric proximal muscle weakness. This, however, was ruled out, as it is unresponsive to glucocorticoids therapy.72 Paraneoplastic presentations also need to be considered in older patients presenting with inflammatory musculoskeletal symptoms.54 Two single-institution observational studies in Western populations showed high incidences of malignancies within first few years of PMR diagnosis.15,73 In this case, reassuring results made this diagnosis less likely.
Statins are recognised to be associated with PMR and an established cause of polymyalgic symptoms, myositis, and rhabdomyolysis.39,74–76 They are associated with muscle pain, inflammation, and breakdown, along with muscle spasms, with or without elevated serum creatine kinase levels.39,75 Even after the discontinuation of statins in the current patient, the pain persisted and his condition continued to deteriorate, ruling out this reason for his symptoms.
Treatment
Glucocorticoids remain the mainstay for treating PMR, an acceptable dose being equal to 12.5–25mg prednisolone daily,21 which characteristically leads to a rapid and remarkable improvement in symptoms in a few days.16,31,65 However, a systematic review revealed that there are no high quality trials to delineate an initial optimal prednisolone dose and tapering regimen in patients with PMR.77 Despite excellent initial response, treatment failure and relapses are frequent and may happen in around 50% of the patients.49,65,78 Furthermore, it is well-established that steroid treatment can lead to osteoporosis, psychosis, mood disorders, proximal muscle weakness, increased risk of infection, and falls. Additionally, high doses of steroids can result in the heightened release of pro-inflammatory mediators, contributing to inflammation,16,79 causing neuronal damage that can worsen Alzheimer’s dementia.80 The current patient was at high falls risk due to multifactorial reasons including dementia, gout, proximal muscle weakness, and frailty. However, there was no history of fall in the preceding twelve months. Considering these, the patient was cautiously commenced on glucocorticoids with meticulous follow-ups to ensure improving clinical status.
There are limited studies looking into the role of disease-modifying anti-rheumatic drugs like methotrexate (MTX) and leflunomide as steroid-sparing agents or to decrease dose of prednisolone.23,24,77,81 A randomised double-blind placebo-controlled trial in Spain suggested that MTX plus prednisolone is more safe, effective, and efficient in maintaining disease remission than corticosteroids alone.81 While MTX is suggested to benefit individuals showing side effects of prolonged glucocorticoid use, further studies into the potential side effects of this treatment are still needed, and monitoring for gastrointestinal and haematological side effects is essential.24,77
Biological agents like Tocilizumab and Etanercept have also been trialled in patients with PMR. Tocilizumab, a humanised monoclonal antibody against the interleukin-6 receptor (IL-6R), has been used as an adjuvant therapy in small number of patients who are intolerant or resistant to glucocorticoids with good clinical response.78 It is still unclear when to use these agents, however, and safety in an elderly population due to the high risk of haematological and infectious complications is a possible concern.82 Etanercept, a tumour necrosis factor antagonist, has shown only a modest benefit in a Danish single-centre double-blind prospective randomised controlled trial—while it indicates a limited role of TNF-inhibitors in the treatment of PMR, more promising results are needed before they can be utilised in practice.83–85 Accurate diagnosis is crucial, as PMR is readily diagnosed, but the treatment with glucocorticoids, though generally straightforward, can pose challenges, particularly when dealing with polypharmacy and multiple coexisting health conditions.
Limitations
The best evidence for diagnosis of PMR is lacking due to the absence of clear diagnostic criteria, which could be better clarified first by more frequent reporting of the wide range of PMR presentations, including those from CALD backgrounds and those with complicating factors such as dementia and frailty. In elderly populations, these comorbid geriatric syndromes are not uncommon, and the risks of misdiagnosis or delays in diagnosis are high when PMR is not clearly defined. Current corticosteroid treatments are quick and effective, so swift diagnoses can help to reduce hospital stays and improve the quality of life of PMR patients. However, establishing precise diagnostic criteria for PMR remains a formidable challenge due to the multitude of variables that can influence diagnosis, such as comorbidities and medication complexities. Additionally, the diverse and nonspecific early presentation of PMR further complicates diagnostic efforts. The prospect of a diagnostic blood test seems promising, yet its development poses its own set of challenges.
Future Perspectives
It has become apparent that different ethnicities may present PMR differently. Further research into understanding these differences to facilitate timely diagnoses and education for migrant groups will be valuable in our diverse culture. Investigatory markers that do not rely on self-reported history or risky steroid treatments would also be valuable in understanding patients with CALD backgrounds or cognitive impairment.
Improved understanding of the disease pathogenesis may also allow for more precise, targeted therapy to be uncovered, with a more constructive safety profile. While steroid-sparing treatments are currently being trialled, the toxicity profile limits their use in geriatric patients. Safer treatments are needed for this condition that primarily affects the elderly as well as early onset.86–90
Conclusions
The present atypical case of PMR in an elderly patient was difficult to diagnose due to the presence of comorbid geriatric syndromes. Nonetheless, extensive analysis through physical examination and laboratory/radiological assessments helped not only in diagnosis but also timely treatment of the disease. It became apparent in this literature review that the documentation of atypical cases is lacking, especially those where common geriatric conditions of dementia and frailty may have made assessment and diagnosis difficult. As PMR becomes more common with age, reporting of these conditions’ effects on assessment and diagnosis are important for clinicians looking to make timely, effective diagnoses. The current patient regained much of his mobility in the weeks following treatment, restoring his former independence. The prompt diagnosis and treatment of atypical PMR cases is, as illustrated, important in improving the quality of life of sufferers.
Acknowledgments
I would like to thank Helena Ng (consultant geriatrician), Dr Stephanie Ward (Director of Training, Monash Health), and Dr Michael Gingold (consultant rheumatologist) for their guidance throughout this project and editorial feedback on the original and revised manuscripts.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Muratore F, Pazzola G, Pipitone N, Salvarani C. Recent advances in the diagnosis and treatment of polymyalgia rheumatica. Expert Rev Clin Immunol. 2016;12(10):1037–1045. doi:10.1080/1744666X.2016.1178572
2. Verhoeven F, Prati C, Guillot X, Boulahdour H, Wendling D. Sterno-clavicular involvement in polymyalgia rheumatica. Joint Bone Spine. 2016;83(3):363–364. doi:10.1016/j.jbspin.2015.04.015
3. Talke M, Schmidt WA. Polymyalgia rheumatica in der täglichen Praxis [Polymyalgia rheumatica in daily routine practice]. Z Rheumatol. 2014;73(5):408–414. German. doi:10.1007/s00393-013-1344-1
4. Mager DR. Polymyalgia rheumatica: common disease, elusive diagnosis. Home Healthcare Now. 2015;33(3):132–6; quiz 7–8. doi:10.1097/NHH.0000000000000199
5. Um YJ, Kim HA, Jung JH, Cho H, Kang JK. A case of amyloidosis presenting as chronic cholecystitis, misdiagnosed as polymyalgia rheumatica. Korean J Gastroenterol. 2016;68(1):49–53. doi:10.4166/kjg.2016.68.1.49
6. Klausen HH, Petersen J, Bandholm T, et al. Association between routine laboratory tests and long-term mortality among acutely admitted older medical patients: a cohort study. BMC Geriatr. 2017;17(1):62. doi:10.1186/s12877-017-0434-3
7. Lohman M, Dumenci L, Mezuk B. Depression and Frailty in Late Life: evidence for a Common Vulnerability. J Gerontol B Psychol Sci Soc Sci. 2016;71(4):630–640. doi:10.1093/geronb/gbu180
8. Vaughan L, Corbin AL, Goveas JS. Depression and frailty in later life: a systematic review. Clin Interv Aging. 2015;10:1947–1958. doi:10.2147/CIA.S69632
9. Alisky JM. Do not overlook polymyalgia rheumatica as a cause for immobility in poorly communicating dementia patients. South Med J. 2008;101(12):1277–1278. doi:10.1097/SMJ.0b013e3181819f48
10. Duarte C, Ferreira RJ, Mackie SL, Kirwan JR, Pereira da Silva JA; Group OPRSI. Outcome measures in polymyalgia rheumatica. A systematic review. J Rheumatol. 2015;42(12):2503–2511. doi:10.3899/jrheum.150515
11. Ozen G, Inanc N, Unal AU, et al. Assessment of the new 2012 EULAR/ACR clinical classification criteria for polymyalgia rheumatica: a prospective multicenter study. J Rheumatol. 2016;43(5):893–900. doi:10.3899/jrheum.151103
12. Ezeonyeji AN, Borg FA, Dasgupta B. Delays in recognition and management of giant cell arteritis: results from a retrospective audit. Clin Rheumatol. 2011;30(2):259–262. doi:10.1007/s10067-010-1616-y
13. Kermani TA, Warrington KJ. Advances and challenges in the diagnosis and treatment of polymyalgia rheumatica. Ther Adv Musculoskelet Dis. 2014;6(1):8–19. doi:10.1177/1759720X13512450
14. Manzo C, Natale M. Polymyalgia rheumatica and cancer risk: the importance of the diagnostic set. Open Access Rheumatol. 2016;8:93–95. doi:10.2147/OARRR.S116036
15. Muller S, Hider SL, Belcher J, Helliwell T, Mallen CD. Is cancer associated with polymyalgia rheumatica? A cohort study in the general practice research database. Ann Rheum Dis. 2014;73(10):1769–1773. doi:10.1136/annrheumdis-2013-203465
16. Yates M, Watts RA, Swords F, MacGregor AJ. Glucocorticoid withdrawal in polymyalgia rheumatica: the theory versus the practice. Clin Exp Rheumatol. 2017;35(1):1–2.
17. Plaza M, Nowakowska-Plaza A, Pracon G, Sudol-Szopinska I. Role of ultrasonography in the diagnosis of rheumatic diseases in light of ACR/EULAR guidelines. J Ultrason. 2016;16(64):55–64. doi:10.15557/JoU.2016.0006
18. Dasgupta B, Cimmino MA, Kremers HM, et al. 2012 Provisional classification criteria for polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Arthritis Rheum. 2012;64(4):943–954. doi:10.1002/art.34356
19. Kermani TA, Warrington KJ. Polymyalgia rheumatica. Lancet. 2013;381(9860):63–72. doi:10.1016/S0140-6736(12)60680-1
20. Salvarani C, Pipitone N, Versari A, Hunder GG. Clinical features of polymyalgia rheumatica and giant cell arteritis. Nat Rev Rheumatol. 2012;8(9):509–521. doi:10.1038/nrrheum.2012.97
21. Dejaco C, Singh YP, Perel P, et al. 2015 Recommendations for the management of polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Ann Rheum Dis. 2015;74(10):1799–1807. doi:10.1136/annrheumdis-2015-207492
22. Mackie SL, McGonagle DG. Response to: ‘A relationship between extracapsular involvement and response to steroid treatment in polymyalgia rheumatica: too soon to conclude?’ by Yang et al. Ann Rheum Dis. 2016;75(4):e17. doi:10.1136/annrheumdis-2015-208956
23. Diamantopoulos AP, Hetland H, Myklebust G. Leflunomide as a corticosteroid-sparing agent in giant cell arteritis and polymyalgia rheumatica: a case series. Biomed Res Int. 2013;2013:120638. doi:10.1155/2013/120638
24. Ferraccioli G, Salaffi F, De Vita S, Casatta L, Bartoli E. Methotrexate in polymyalgia rheumatica: preliminary results of an open, randomized study. J Rheumatol. 1996;23(4):624–628.
25. Watson P, Gaston H. Response to ‘Effect of etanercept in polymyalgia rheumatica: a randomized controlled trial’. Arthritis Res Therapy. 2011;13(2):403. doi:10.1186/ar3269
26. Schmidt WA. Polymyalgia rheumatica [Polymyalgia rheumatic--what’s new?]. Dtsch Med Wochenschr. 2016;141(7):490–492. German.. doi:10.1055/s-0042-100099
27. Li WL, Lo Y, Leung MH, Wong WS, Mok MY. The clinical course of polymyalgia rheumatica in Chinese. Clin Rheumatol. 2010;29(2):199–203. doi:10.1007/s10067-009-1315-8
28. Bordin G, Atzeni F, Bettazzi L, Beyene NB, Carrabba M, Sarzi-Puttini P. Unilateral polymyalgia rheumatica with controlateral sympathetic dystrophy syndrome. A case of asymmetrical involvement due to pre-existing peripheral palsy. Rheumatology (Oxford). 2006;45(12):1578–1580. doi:10.1093/rheumatology/kel334
29. van Dalen-Kok AH, Pieper MJ, de Waal MW, Lukas A, Husebo BS, Achterberg WP. Association between pain, neuropsychiatric symptoms, and physical function in dementia: a systematic review and meta-analysis. BMC Geriatr. 2015;15:49. doi:10.1186/s12877-015-0048-6
30. Kreiner FF, Galbo H. Activity of the neuroendocrine axes in patients with polymyalgia rheumatica before and after TNF-alpha blocking etanercept treatment. Arthritis Res Therapy. 2012;14(4):R186. doi:10.1186/ar4017
31. Visvanathan S, Rahman MU, Hoffman GS, et al. Tissue and serum markers of inflammation during the follow-up of patients with giant-cell arteritis--a prospective longitudinal study. Rheumatology (Oxford). 2011;50(11):2061–2070. doi:10.1093/rheumatology/ker163
32. Bas S, Perneger TV, Seitz M, Tiercy JM, Roux-Lombard P, Guerne PA. Diagnostic tests for rheumatoid arthritis: comparison of anti-cyclic citrullinated peptide antibodies, anti-keratin antibodies and IgM rheumatoid factors. Rheumatology (Oxford). 2002;41(7):809–814. doi:10.1093/rheumatology/41.7.809
33. Codreanu C, Enache L. Is ultrasound changing the way we understand rheumatology? Including ultrasound examination in the classification criteria of polymyalgia rheumatica and gout. Med Ultrason. 2015;17(1):97–103. doi:10.11152/mu.2013.2066.171.ccle
34. Emamifar A, Hess S, Gildberg-Mortensen R, Jensen Hansen IM. Association of remitting seronegative symmetrical synovitis with pitting edema, polymyalgia rheumatica, and adenocarcinoma of the prostate. Am J Case Rep. 2016;17:60–64. doi:10.12659/ajcr.895717
35. Nesher G, Breuer GS. Giant cell arteritis and polymyalgia rheumatica: 2016 update. Rambam Maimonides Med J. 2016;7(4):e0035. doi:10.5041/RMMJ.10262
36. Cherif Y, Zantour B, Alaya W, Berriche O, Younes S, Sfar MH. Primary hyperparathyroidism and hyperthyroidism in a patient with myotonic dystrophy: a case report and review of the literature. Case Rep Endocrinol. 2015;2015:735868. doi:10.1155/2015/735868
37. Dalkilic E, Tufan AN, Hafizoglu E, et al. The process from symptom onset to rheumatology clinic in polymyalgia rheumatica. Rheumatol Int. 2014;34(11):1589–1592. doi:10.1007/s00296-014-3034-y
38. Das P, Samanta A, Dasgupta B. Balancing on the edge: implications of a UK national audit of the use of BSR-BHPR guidelines for the diagnosis and management of polymyalgia rheumatica. RMD Open. 2015;1(1):e000095. doi:10.1136/rmdopen-2015-000095
39. de Jong HJ, Meyboom RH, Helle MJ, Klungel OH, Niskanen L, Cohen Tervaert JW. Giant cell arteritis and polymyalgia rheumatica after reexposure to a statin: a case report. Ann Intern Med. 2014;161(8):614–615. doi:10.7326/L14-5020-6
40. Dejaco C, Matteson EL, Buttgereit F. Diagnostik und Therapie der Polymyalgia rheumatica [Diagnostics and treatment of polymyalgia rheumatica]. Z Rheumatol. 2016;75(7):687–700. German. doi:10.1007/s00393-016-0105-3
41. Dejaco C, Singh YP, Perel P, et al. Current evidence for therapeutic interventions and prognostic factors in polymyalgia rheumatica: a systematic literature review informing the 2015 European League Against Rheumatism/American College of Rheumatology recommendations for the management of polymyalgia rheumatica. Ann Rheum Dis. 2015;74(10):1808–1817. doi:10.1136/annrheumdis-2015-207578
42. Wakura D, Kotani T, Takeuchi T, et al. Differentiation between polymyalgia rheumatica (PMR) and elderly-onset rheumatoid arthritis using 18F-fluorodeoxyglucose positron emission tomography/computed tomography: is enthesitis a new pathological lesion in PMR? PLoS One. 2016;11(7):e0158509. doi:10.1371/journal.pone.0158509
43. Crum-Cianflone NF. Bacterial, fungal, parasitic, and viral myositis. Clin Microbiol Rev. 2008;21(3):473–494. doi:10.1128/CMR.00001-08
44. Wysoki MG, Angeid-Backman E, Izes BA. Iliopsoas myositis mimicking appendicitis: MRI diagnosis. Skeletal Radiol. 1997;26(5):316–318. doi:10.1007/s002560050244
45. Beese MS, Winkler G, Maas R, Bucheler E. MRT der Muskulatur bei Myalgien. Indikationen und Bildbefunde [MRI of musculature in myalgia--indications and image findings]. Aktuelle Radiol. 1996;6(3):119–129. German.
46. Ochi J, Nozaki T, Okada M, et al. MRI findings of the shoulder and Hip joint in patients with polymyalgia rheumatica. Mod Rheumatol. 2015;25(5):761–767. doi:10.3109/14397595.2015.1008725
47. Mackie SL, Koduri G, Hill CL, et al. Accuracy of musculoskeletal imaging for the diagnosis of polymyalgia rheumatica: systematic review. RMD Open. 2015;1(1):e000100. doi:10.1136/rmdopen-2015-000100
48. Halls S, Sinnathurai P, Hewlett S, et al. Stiffness is the cardinal symptom of inflammatory musculoskeletal diseases, yet still variably measured: report from the OMERACT 2016 Stiffness Special Interest Group. J Rheumatol. 2016. doi:10.3899/jrheum.161073
49. Mackie SL, Pease CT, Fukuba E, et al. Whole-body MRI of patients with polymyalgia rheumatica identifies a distinct subset with complete patient-reported response to glucocorticoids. Ann Rheum Dis. 2015;74(12):2188–2192. doi:10.1136/annrheumdis-2015-207395
50. Jou IM, Chiu NT, Lai KA, Chuang YC. Synchronous pyomyositis and septic Hip arthritis. Clin Rheumatol. 2000;19(5):385–388. doi:10.1007/pl00011176
51. Kinoshita S, Aoki T, Takahashi H, et al. Thin-section chest CT findings in polymyalgia rheumatica: a comparison between with and without rheumatoid arthritis. Clin Imaging. 2016;40(3):382–385. doi:10.1016/j.clinimag.2015.11.013
52. Palard-Novello X, Querellou S, Gouillou M, et al. Value of (18)F-FDG PET/CT for therapeutic assessment of patients with polymyalgia rheumatica receiving tocilizumab as first-line treatment. Eur J Nucl Med Mol Imaging. 2016;43(4):773–779. doi:10.1007/s00259-015-3287-z
53. Te Riele MG, Schreuder TH, van Alfen N, et al. The yield of diagnostic work-up of patients presenting with myalgia, exercise intolerance, or fatigue: a prospective observational study. Neuromuscul Disord. 2017;27(3):243–250. doi:10.1016/j.nmd.2016.12.002
54. Mege D, Cammilleri S, Mundler O, et al. Circulating microparticles bearing Fibrin associated with whole-body 18FDG-PET: diagnostic tools to detect paraneoplastic polymyalgia rheumatica. Rheumatol Int. 2016;36(8):1099–1103. doi:10.1007/s00296-016-3510-7
55. Miceli MC, Zoli A, Peluso G, Bosello S, Gremese E, Ferraccioli G. Baseline shoulder ultrasonography is not a predictive marker of response to glucocorticoids in patients with polymyalgia rheumatica: a 12-month followup study. J Rheumatol. 2017;44(2):241–247. doi:10.3899/jrheum.160090
56. Yamashita H, Kubota K, Mimori A. Clinical value of whole-body PET/CT in patients with active rheumatic diseases. Arthritis Res Therapy. 2014;16(5):423. doi:10.1186/s13075-014-0423-2
57. Tateyama M, Fujihara K, Misu T, Arai A, Kaneta T, Aoki M. Clinical values of FDG PET in polymyositis and dermatomyositis syndromes: imaging of skeletal muscle inflammation. BMJ Open. 2015;5(1):e006763. doi:10.1136/bmjopen-2014-006763
58. Fitzcharles MA, Esdaile JM. Atypical presentations of polymyalgia rheumatica. Arthritis Rheum. 1990;33(3):403–406. doi:10.1002/art.1780330314
59. Bird HA, Esselinckx W, Dixon AS, Mowat AG, Wood PH. An evaluation of criteria for polymyalgia rheumatica. Ann Rheum Dis. 1979;38(5):434–439. doi:10.1136/ard.38.5.434
60. Hu Z, Yang Q, Zeng S, et al. Giant cell arteritis in China: a prospective investigation. Angiology. 2002;53(4):457–463. doi:10.1177/000331970205300413
61. Muller S, Hider SL, Helliwell T, et al. Characterising those with incident polymyalgia rheumatica in primary care: results from the PMR Cohort Study. Arthritis Res Therapy. 2016;18:200. doi:10.1186/s13075-016-1097-8
62. Cantini F, Niccoli L, Storri L, et al. Are polymyalgia rheumatica and giant cell arteritis the same disease? Semin Arthritis Rheum. 2004;33(5):294–301. doi:10.1016/j.semarthrit.2003.09.008
63. Chen C-H, Kung S-Y, Tsai -Y-Y, Liao H-T, Chou C-T, Huang D-F. Temporal arteritis. J Chin Med Assoc. 2005;68(7):333–335. doi:10.1016/S1726-4901(09)70170-4
64. Hyatt A, Lipson-Smith R, Schofield P, et al. Communication challenges experienced by migrants with cancer: a comparison of migrant and English-speaking Australian-born cancer patients. Health Expect. 2017;20(5):886–895. doi:10.1111/hex.12529
65. Benucci M, Olivito B, Manfredi M, et al. Polymyalgia rheumatica: inflammation suppression with low dose of methylprednisolone or modified-release prednisone. Eur Rev Med Pharmacol Sci. 2015;19(5):745–751.
66. Yang L, Zhou H, Tang H, Lee AM, Bai HX. A relationship between extracapsular involvement and response to steroid treatment in polymyalgia rheumatica: too soon to conclude? Ann Rheum Dis. 2016;75(4):e16. doi:10.1136/annrheumdis-2015-208956
67. Twohig H, Mitchell C, Mallen C, Adebajo A, Mathers N. ”I suddenly felt I’d aged”: a qualitative study of patient experiences of polymyalgia rheumatica (PMR). Patient Educ Couns. 2015;98(5):645–650. doi:10.1016/j.pec.2014.12.013
68. Lim T, Woo S, Mun YG, Yim E, Koh JH, Park KS. Polymyalgia rheumatica following paraspinal muscle inflammation and sacroiliitis. Korean J Intern Med. 2015;30(3):415–417. doi:10.3904/kjim.2015.30.3.415
69. Mansalis K. Myalgias and myopathies: polymyalgia rheumatica and giant cell arteritis. FP Essent. 2016;440:16–22.
70. Manzo C. Widespread headache as the first clinical manifestation of giant cell arteritis in patients affected by polymyalgia rheumatica. Reumatologia. 2016;54(5):236–238. doi:10.5114/reum.2016.63663
71. Nwadibia U, Larson E, Fanciullo J. Polymyalgia rheumatica and giant cell arteritis: a review article. S D Med. 2016;69(3):121–123.
72. Luo YB, Mastaglia FL. Dermatomyositis, polymyositis and immune-mediated necrotising myopathies. Biochim Biophys Acta. 2015;1852(4):622–632. doi:10.1016/j.bbadis.2014.05.034
73. Ji J, Liu X, Sundquist K, Sundquist J, Hemminki K. Cancer risk in patients hospitalized with polymyalgia rheumatica and giant cell arteritis: a follow-up study in Sweden. Rheumatology (Oxford). 2010;49(6):1158–1163. doi:10.1093/rheumatology/keq040
74. Barraclough K, Mallen CD, Helliwell T, Hider SL, Dasgupta B. Diagnosis and management of giant cell arteritis. Br J Gen Pract. 2012;62(599):329–330. doi:10.3399/bjgp12X649313
75. de Jong HJ, Saldi SR, Klungel OH, et al. Statin-associated polymyalgia rheumatica. An analysis using WHO global individual case safety database: a case/non-case approach. PLoS One. 2012;7(7):e41289. doi:10.1371/journal.pone.0041289
76. Schmidt WA. Myalgien bei Polymyalgia rheumatica, Arteriitis temporalis und anderen Vaskulitiden [Myalgia in polymyalgia rheumatica, temporal arteritis and other vasculitides]. Z Rheumatol. 2009;68(6):446–450. German. doi:10.1007/s00393-009-0453-3
77. Buttgereit F, Dejaco C, Matteson EL, Dasgupta B. Polymyalgia rheumatica and giant cell arteritis: a systematic review. JAMA. 2016;315(22):2442–2458. doi:10.1001/jama.2016.5444
78. Toussirot E, Martin A, Soubrier M, Redeker S, Regent A. Rapid and sustained response to tocilizumab in patients with polymyalgia rheumatica resistant or intolerant to glucocorticoids: a multicenter open-label study. J Rheumatol. 2016;43(1):249–250. doi:10.3899/jrheum.150599
79. Fry CS, Nayeem SZ, Dillon EL, et al. Glucocorticoids increase skeletal muscle NF-kappaB inducing kinase (NIK): links to muscle atrophy. Physiol Rep. 2016;4(21). doi:10.14814/phy2.13014
80. Ennis GE, An Y, Resnick SM, Ferrucci L, O’Brien RJ, Moffat SD. Long-term cortisol measures predict Alzheimer disease risk. Neurology. 2017;88(4):371–378. doi:10.1212/WNL.0000000000003537
81. Jover JA, Hernandez-Garcia C, Morado IC, Vargas E, Banares A, Fernandez-Gutierrez B. Combined treatment of giant-cell arteritis with methotrexate and prednisone. a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;134(2):106–114. doi:10.7326/0003-4819-134-2-200101160-00010
82. Toussirot E, Regent A, Devauchelle-Pensec V, Saraux A, Puechal X. Interleukin-6: a promising target for the treatment of polymyalgia rheumatica or giant cell arteritis? RMD Open. 2016;2(2):e000305. doi:10.1136/rmdopen-2016-000305
83. Kreiner F, Galbo H. Effect of etanercept in polymyalgia rheumatica: a randomized controlled trial. Arthritis Res Ther. 2010;12(5):R176. doi:10.1186/ar3140
84. Manzo C, Hysa E, Castagna A, Isetta M. The role of tumor necrosis factor alpha antagonists (Anti TNF-α) in personalized treatment of patients with isolated polymyalgia rheumatica (PMR): past and possible future scenarios. J Pers Med. 2022;12(3):329. doi:10.3390/jpm12030329
85. Hysa E, Bond M, Ehlers L, et al. Andreas Kerschbaumer, Evidence on treat to target strategies in polymyalgia rheumatica and giant cell arteritis: a systematic literature review. Rheumatology. 2023;kead471. doi:10.1093/rheumatology/kead471
86. Wu J, Yang F, Ma X, Lin J, Chen W. Elderly-onset rheumatoid arthritis vs. polymyalgia rheumatica: differences in pathogenesis. Front Med. 2023;9:1083879. doi:10.3389/fmed.2022.1083879
87. Ohta R, Sano C. Differentiating between seronegative elderly-onset rheumatoid arthritis and polymyalgia rheumatica: a qualitative synthesis of narrative reviews. Int J Environ Res Public Health. 2023;20:1789. doi:10.3390/ijerph20031789
88. Lange U, Piegsa M, Teichmann J, et al. Ultrasonography of the glenohumeral joints – a helpful instrument in differentiation in elderly onset rheumatoid arthritis and polymyalgia rheumatica. Rheumatol Int. 2000;19:185–189. doi:10.1007/s002960000051
89. Coskun Benlidayi I. Why is polymyalgia rheumatica a disease of older adults? Explanations through etiology and pathogenesis: a narrative review. Clin Rheumatol. 2023. doi:10.1007/s10067-023-06708-3
90. Carvajal Alegria G, Boukhlal S, Cornec D, Devauchelle-Pensec V. The pathophysiology of polymyalgia rheumatica, small pieces of a big puzzle. Autoimmun Rev. 2020;19(11):102670. doi:10.1016/j.autrev.2020.102670
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
