Back to Journals » International Medical Case Reports Journal » Volume 16
Case Report: Alternaria alternata keratitis
Authors Leite J , Romano J, Lopes V, Neves MM , Gomes M , Oliveira L
Received 9 October 2022
Accepted for publication 24 November 2022
Published 27 January 2023 Volume 2023:16 Pages 59—64
DOI https://doi.org/10.2147/IMCRJ.S392781
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
João Leite,1 João Romano,2 Virginia Lopes,3 Miguel Mesquita Neves,1 Miguel Gomes,1 Luis Oliveira1
1Department of Ophthalmology, Centro Hospitalar Universitário do Porto (CHUPorto), Porto, Portugal; 2Department of Ophthalmology, Centro Hospitalar de Leiria, Leiria, Portugal; 3Department of Microbiology, Centro Hospitalar Universitário do Porto (CHUPorto), Porto, Portugal
Correspondence: João Leite, Centro Hospitalar Universitário do Porto [CHUPorto], Largo do Prof. Abel Salazar, Porto, 4099-001, Portugal, Tel +351916523942, Email [email protected]
Background: Alternaria spp are ubiquitous pigmented filamentous fungi that can cause opportunistic human infections. These molds can be found in healthy individuals and the most frequently observed clinical manifestations are skin infections, ocular mycosis, rhinosinusitis, among others. Immunosuppression (both local and systemic) as well as contact or trauma with contaminated matter are important risk and predisposing factors for ocular mycosis. Accurate diagnosis with microscopy and culture is crucial since infections by filamentous fungi are sight-threatening, and clinically indistinguishable from bacterial disease. In general terms, conventional antifungal drugs are effective.
Case Presentation: We report a case of Alternaria alternata keratitis after ocular trauma with biological material in a 44-year-old man’s functional single eye, which had a good clinical and functional evolution after topical therapy with voriconazole.
Conclusion: This case accounts the importance of thinking about other etiological diagnoses in infectious keratitis refractory to the established therapy and with a history of trauma with biological material.
Keywords: keratitis, Alternaria alternata, eye infection
Background
Alternaria species is a filamentous fungus from the dematiaceous family1 that produces black pigmented molds due to melanin,2–4 and is frequently isolated from soil, plants and food.1,2
Some species are ubiquitous agents of decay and plant pathogens,4 and can be found in healthy individuals both on the skin5 and conjunctiva.6Alternaria infects mainly immunocompromised patients. The most important risk factors are solid organ transplantation, Cushing’s syndrome and bone marrow transplants. However, infections in immunocompetent patients have also been reported although rarely involving invasive disease.4 Infection usually occurs associated with a breaking of skin or corneal epithelial barrier mainly in patients with immunocompromised ocular surface – treated with topical corticosteroids – or in farmers and gardeners, exposed to soil and garbage, who, in the vast majority of cases, had with a history of contact lens wear, and accidental or surgical ocular trauma suffered 2–6 years before clinical presentation.1,4,7 Unlike bacteria, fungi can penetrate Descemet’s membrane and reach the anterior chamber, which can seriously compromise and threaten vision.2 Thus, in addition to keratitis, patients with Alternaria ocular infection may present themselves clinically with endophthalmitis.4,8
This fungus is also associated to hypersensitivity pneumonitis, bronchial asthma, and allergic sinusitis and rhinitis.2,4
The aforementioned case report describes a mycotic keratitis caused by Alternaria alternata after corneal trauma and with a good outcome after therapeutic with voriconazole.
Case Presentation
We report the case of a 44-year-old man who presents himself with an acute onset ocular pain and photophobia in his right eye. He had reported an eye trauma with a tree branch 3 days before. Patient had a previous history of type 1 diabetes mellitus, diagnosed 24 years before and with established microvascular disease – diabetic retinopathy and chronic kidney disease – having undergone a reno-pancreatic transplant 5 years before. Right eye best corrected visual acuity (BCVA) was 20/80; left eye BCVA was hand motion. Left eye low vision was secondary to a diabetic neovascular glaucoma. A slit-lamp examination revealed an extensive temporal corneal epithelium erosion and a peripheral anterior stromal infiltration in the inferotemporal quadrant, with 2×1.5mm, which was stained with fluorescein dye. A slight anterior chamber reaction was detected (Tyndall 1+, flare 1+).
Corneal samples were collected in this context, and although the pathogen was not found, a treatment was started with clotrimazole clotrimazole 10 mg/mL (1 drop 5 times a day), moxifloxacin hydrochloride 5 mg/mL (1 drop of 5/5 minutes during the 1st hour, then 1 drop of 1/1h during the first 24 hours, then 1 drop of 2/2h), tobramycin 3 mg/mL (1 drop of 5/5 minutes during the 1st hour, then 1 drop of 1/1h during the first 24 hours, then 1 drop of 2/2h), bromfenac 0.9 mg/mL (1 drop 2 times a day) and cyclopentolate hydrochloride 10 mg/mL (1 drop 3 times a day).
After four days of treatment (D4), the stromal infiltration had almost completely disappeared but epithelial damage kept similar to the presentation, so a therapeutic contact lens was placed and the treatment with cyclopentolate hydrochloride 10 mg/mL (1 drop 3 times a day), moxifloxacin hydrochloride 5 mg/mL (1 drop 5 times a day) and tobramycin 3 mg/mL (1 drop 5 times a day) was kept.
On D15, the patient reported a progressive deterioration of symptoms and the slit lamp examination revealed a paracentral corneal ulcer with an associated stromal infiltration occupying mainly the inferior temporal quadrant and measuring 4.7mmx2.7mm (shown in Figure 1A and B). Epithelial changes as well as stromal disorganization (shown in Figure 1C) were visible on corneal – Optical Coherence Tomography (Corneal-OCT) (Spectralis® Anterior Segment Module, version 1.10.2.0, Heidelberg Engineering, Heidelberg, Germany).
Given this clinical aggravation, corneal samples were collected again, and inoculated immediately in blood and chocolate agars, in liquid Thioglycolate and in Sabouraud selective fungal medium, and sent to the Microbiology Laboratory. Topical therapy was changed to fortified vancomycin 50 mg/mL (1 drop of 2/2h), fortified ceftazidime 50 mg/dL (1 drop of 2/2h) and fortified voriconazole 10 mg/mL (1 drop 5 times a day).
On D25, the biomicroscopy exam was similar to the D15 evaluation as well as the topical medication. The microbiological study revealed a considerable growth of pigmented filamentous fungus identified as Alternaria alternata (shown in Figure 2) both by microscopic morphology and mass spectrometry through the VITEK® MS system, which consists of an automated microbial identification system that uses Matrix Assisted Laser Desorption Ionization Time-of-Flight (MALDI-TOF).
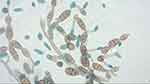 |
Figure 2 Microscopic examination of the colonies: pigmented septate hyphae with chains of ovate conidia with transverse and vertical septa. |
According to this diagnosis, topical therapy was administered. Therefore, therapy with fortified vancomycin 50 mg/mL and fortified ceftazidime 50 mg/dL was suspended. 5 mg/mL moxifloxacin hydrochloride (1 drop 4 times a day) was started and 10 mg/mL fortified voriconazole was maintained (1 drop 5 times a day).
On D32, the patient presented an improvement in pain symptoms, with an improvement in BCVA 20/50 and, at biomicroscopy, a corneal ulcer (less than 4.0 mm x 2.0 mm) with stromal infiltration and moderate ciliary injection.
These clinical changes persisted overlapping in subsequent evaluations, with a gradual decrease in corneal ulcer. At the beginning of the 3rd month, there was an improvement in BCVA to 20/25, with only a small ulcer less than 2 mm that was slightly coloured. Consequently, therapy with fortified voriconazole 10 mg/mL was suspended and only moxifloxacin hydrochloride 5 mg/mL (1 drop 4 times a day) was maintained. In the 4th month, the BCVA was 20/25 and, at the slit lamp observation, no ulcer was detected, there was only a paracentral leukoma no longer stained with fluorescein (shown in Figure 3A and B) and the Corneal-OCT demonstrated an improvement in corneal stromal and epithelial anatomy (shown in Figure 3C). Moxifloxacin hydrochloride 5 mg/mL was suspended. No adverse effects associated with the therapy process were observed.
 |
Figure 3 (A) – Slit-lamp image with visualization of a paracentral leukoma; (B) – Leukoma that no longer is stained with fluorescein; (C) - Corneal-OCT demonstrates an improvement in stromal organization – the green arrows under the caption on this figure (on the left) correspond to the cornea that is expressed in all its extension and by layers in Figure 1C (on the right). |
Discussion/Conclusion
Few cases of Alternaria keratitis were accounted for in literature. Hsiao et al reported in their article 32 cases documented up to 2014. Of those, 44% of the infections were secondary to trauma and in 31% there was a history of keratorefractive surgery or preexisting corneal disease; in 44% there was the use of corticosteroids. Most patients (72%) were cured with medical therapy: topical as monotherapy or in combination – natamycin, amphotericin B, miconazole, ketoconazole, voriconazole, flucytosine, fluconazole, and caspofungin; with oral therapy - itraconazole and voriconazole; and with intracameral or intrastromal voriconazole therapy. In 28% keratoplasty therapeutic was necessary.
Fungal keratitis has been increasingly reported in literature since the early 1960s. The first cases of Alternaria species were cultivated in 19759 and are an uncommon cause of corneal infection (3.3% to 8.7% of mycotic keratitis).1 Fungal keratitis accurate diagnosis and successful treatment is challenging and rely on microbiological study since bacterial and fungal eye infections may not be clinically distinguishable. Observation of hyphae in direct optical microscopy allows a fast diagnosis of fungal disease. The main limitations are the sample size, the suspicion of fungal disease and laboratory facilities. Corneal sample culture is essential for the genre and species identification and for adequate treatment selection (based on in vitro antifungal susceptibility studies).7 Culture sensitivity depends mainly on the sample size and previous use of antimicrobials. PCR is a developing and valuable tool for microbiological diagnosis but it is not available in most laboratories, namely for fungal infections diagnosis in general.
Taking the aforementioned reasons into consideration, Marta et al demonstrated in a retrospective study of all hospitalized patients for corneal abscess that in 25.6% of cases the microbiological analysis did not show a positive culture, the etiological agent being unknown. On the other hand, fungi usually grow in culture, usually in three days, but it is necessary to wait up to three weeks to discard the culture, given the inactivity intervals in the growth of some of these fungal agents.10
Regarding medical therapy, in vitro susceptibility studies have shown:1,4 amphotericin B revealed to be variable in vitro activity - 0.15% amphotericin B can be used (1 drop of 6/6 h), due to low intravitreal penetration. It is preferably administered intravenously or intravitreally (in cases of fungal endophthalmitis);4 triazoles: itraconazole, voriconazole (topical, 1 drop every 1h, and intravenously, 150 mg twice a day) and posaconazole (topical or oral) showed good activity with low minimum inhibitory concentrations (MIC);4,8 Alternaria was also susceptible to terbinafine and caspofungin, but resistant to micafungin, flucytosine and fluconazole. In recent years, and in following prospective studies, the efficacy and tolerability of voriconazole in the treatment of severe keratomycosis and the successful therapeutic results of Alternaria keratitis clinically resistant to other antifungals have been confirmed.1,4,8 Voriconazole has excellent bioavailability and achieves therapeutical aqueous and vitreous levels after topical and oral administration1 and voriconazole is currently established as the first-line treatment of keratomycosis.1,11 Some in vitro activity of Alternaria suggested that posaconazole could also be an alternative in the cases of keratitis and endophthalmitis.4
In this instance, and minding that this is the first report in which this outcome takes place, a good response was obtained with only topical voriconazole as the first therapeutic option. In other cases, topical therapy alone was undertaken with no clinical response; the efficacy of voriconazole was demonstrated as a first line but in two dosage forms (topical and oral); the efficacy of this drug was proved in topical form but as a second-line therapy; and lastly, other reports show the efficacy of voriconazole in association with other antifungal drugs.1
However, medical therapy with antifungal agents was not effective in up to 36% of cases of Alternaria keratitis resulting in either penetrating therapeutic or lamellar keratoplasty.8 In cases where it is not possible to administer the agent at the desired level, intracameral injection of voriconazole is possible.2
Hence, this case report describes a mycotic keratitis after corneal trauma with biological material that, as a result of the (initial) absence of a microbiological diagnosis, created difficulties both in the diagnosis and in the therapy to be instituted. With the microbiological result of Alternaria alternata, it was possible to direct the initiated therapy and the patient presented a good evolution after therapy with voriconazole.
Abbreviations
BCVA, best corrected visual acuity; MIC, minimum inhibitory concentrations.
Data Sharing Statement
The clinical data that support the findings of this clinical case are available in the electronic hospital register of CHUPorto. All data generated or analyzed during this study are included in this article. Further enquiries can be addressed to the author.
Ethics Approval and Consent to Participate
Ethical approval is not required for this study in accordance with local or national guidelines. The patient’s informed consent was obtained during the assessment visits.
Consent for Publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Author Contributions
All authors made a significant contribution to this report, may that be in the conception, design study, execution, acquisition, analysis or interpretation of data; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
Miguel Mesquita Neves is a consultant in Alcon Portugal – Produtos e Equipamentos, Lda.
The other authors have no conflicts of interest to declare for this work.
References
1. Hsiao CH, Yeh LK, Chen HC, et al. Clinical characteristics of Alternaria keratitis. J Ophthalmol. 2014;2014. doi:10.1155/2014/536985
2. Kang KM, Kim HK, Lee HS, et al. A case of Alternaria alternata keratitis isolated from corneal tissue. Korean J Med Mycol. 2015;20(2):27–33.
3. Naik M, Shahbaaz M, Sheth J, Sunderamoorthy SK. Alternaria keratitis after deep anterior lamellar keratoplasty. Middle East Afr J Ophthalmol. 2014;21(1):92–94. doi:10.4103/0974-9233.124121
4. Pastor FJ, Guarro J. Alternaria infections: laboratory diagnosis and relevant clinical features. Clin Microbiol Infect. 2008;14(8):734–746. doi:10.1111/j.1469-0691.2008.02024.x
5. Bloom PA, Laidlaw A, Warnock DW. A case of fungal contact lens contamination by Alternaria alternata: the first British case report. J Br Contact Lens Assoc. 1991;14(1):29–30. doi:10.1016/0141-7037(91)80059-U
6. Nema HV, Ahuja OP, Mohapatra LN, Mohapatra LN. Mycotic flora of the conjunctiva. Am J Ophthalmol. 1966;62(5):968–970. doi:10.1016/0002-9394(66)91928-3
7. Marta A, Silva N, Carneiro I, Neves MM, Gomes M, Oliveira L. Estudo epidemiológico das queratites infeciosas internadas num centro hospitalar terciário - revisão de 5 anos TT - Epidemiological study of infectious keratitis in patients of a tertiary hospital center - revision of 5 years. Rev Bras Oftalmol. 2019;78(6):370–374.
8. Konidaris V, Mersinoglou A, Vyzantiadis T-A, Papadopoulou D, Boboridis KG, Ekonomidis P. Corneal transplant infection due to Alternaria alternata: a case report. Case Rep Ophthalmol Med. 2013;2013:1–3.
9. Doughman DJ, Leavenworth NM, Campbell RC, Lindstrom RL. Fungal keratitis at the University of Minnesota: 1971–1981. Trans Am Ophthalmol Soc. 1982;80:235–247.
10. de Oliveira PR, Resende SM, de Oliveira FC, de Oliveira AC. Ceratite fúngica. Arq Bras Oftalmol. 2001;64(1):75–79. doi:10.1590/S0004-27492001000100015
11. Parchand S, Gupta A, Ram J, Gupta N, Chakrabarty A. Voriconazole for fungal corneal ulcers. Ophthalmology. 2012;119(5):1083–1083.e3. doi:10.1016/j.ophtha.2011.11.034
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.