Back to Journals » International Medical Case Reports Journal » Volume 16
Case of Spontaneous Closure and Recurrence of Macular Hole in Vitrectomized Eye
Authors Komi Y, Katsumoto T, Yoshikawa Y, Shibuya M, Shoji T , Makita J, Shinoda K
Received 8 July 2023
Accepted for publication 14 September 2023
Published 2 October 2023 Volume 2023:16 Pages 641—645
DOI https://doi.org/10.2147/IMCRJ.S429577
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Yuki Komi,1 Takeshi Katsumoto,1 Yuji Yoshikawa,1 Masayuki Shibuya,1 Takuhei Shoji,1,2 Jun Makita,1 Kei Shinoda1
1Department of Ophthalmology, Saitama Medical University, Iruma, Saitama, Japan; 2Koedo Eye Institute, Kawagoe, Saitama, Japan
Correspondence: Kei Shinoda, Department of Ophthalmology, Saitama Medical University, 38 Morohongo, Moroyama, Saitama, 350-0495, Japan, Tel +81-4-9276-1250, Fax +81-4-9295-8002, Email [email protected]
Rationale: Development and spontaneous closure of a macular hole (MH) in a vitrectomized eye is relatively rare. We report our findings in a case in which vitrectomy was performed successfully to treat a vitreous hemorrhage (VH), but a MH developed eight months later. The MH spontaneously closed 2 weeks later, but then reopened. A second vitrectomy was performed with insertion of the internal limiting membrane flap into the MH which led to the successful closure of the MH. The purpose of this article is to present an explanation of how MH developed in this eye without vitreous traction.
Patient: A 64-year-old woman visited an eye clinic with vision reduction in her right eye of 3 days duration. A VH was detected in the right eye and pars plana vitrectomy (PPV) was performed. A retinal tear was detected which was the origin of the VH. The vision was restored to a decimal visual acuity of 1.2. Eight months later, the patient noticed that her vision was distorted and was referred to our hospital.
Diagnosis: Optical coherence tomographic (OCT) images showed a thin epiretinal membrane on the macula, cystoid changes in the macular area, and a full-thickness MH.
Interventions: The MH closed spontaneously in two weeks, however a lamellar MH with an epiretinal proliferation (EP) developed 11 months later. Two months later, OCT showed cyst-like changes in the retina and a full-thickness MH. A second PPV was performed with the insertion of the ILM flap and EP into the MH to close the MH. Her visual acuity improved, and distorted vision was not present.
Lessons: Clinicians should be aware that a MH can develop in a vitrectomized eye without vitreous traction but can close spontaneously. We conclude that careful follow-up examinations are necessary even in vitrectomized eyes.
Plain Language Summary: We report our findings in a case in which a macular hole (MH) developed 8 months after vitrectomy and spontaneously closed but then reopened. Finally, the MH was closed after a second vitrectomy. Optical coherence tomographic images showed changes in the macular microstructure, eg, the presence of a thin epiretinal membrane on the retina, cystoid changes in the retina, and a full-thickness MH. We discuss the mechanism of MH development and surgical treatment.
Keywords: epiretinal proliferation, full-thickness macular hole, lamellar macular hole, optical coherence tomography, spontaneous closure, vitrectomy
Introduction
Vitreous traction in the anteroposterior and tangential directions together with degeneration of the inner retinal layers at the central fovea, are believed to be important factors that can cause the formation and expansion of a full-thickness macular hole (FTMH).1–3 However, a macular hole (MH) can develop in eyes with a complete posterior vitreous detachment (PVD)4,5 and even after vitrectomy during which a PVD has been created.6–9 It has also been reported that a spontaneous closure and recurrence of an MH can occur in a previously vitrectomized eye.10–15 The associations of an epiretinal membrane (ERM) or an epiretinal proliferation (EP) with the development and closure of FTMH and lamellar macular hole (LMH) have been intensively studied by several researchers.16,17
We report our findings in a patient who underwent a successful vitrectomy for a vitreous hemorrhage (VH) associated with a retinal tear. A FTMH developed during the postoperative period but closed spontaneously. However, optical coherence tomography (OCT) 4 months later revealed a LMH that developed into a FTMH and a second vitrectomy had to be performed to close the MH.
Case Presentation
A 64-year-old woman was examined at a local clinic because of reduced vision in her right eye of three days duration. Her best-corrected visual acuity (BCVA) in the right eye was hand motion at 30 cm and she had no history of trauma. Her intraocular pressure (IOP) was 12 mmHg and the axial length was 23.52 mm in the right eye. Ophthalmoscopy revealed a dense vitreous hemorrhage (VH), and she underwent successful pars plana vitrectomy (PPV) combined with cataract surgery the following day. A retinal tear that caused the hemorrhage was detected during vitrectomy and was treated with photocoagulation and gas tamponade. The decimal BCVA in the right eye had improved to 1.2 one week later.
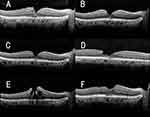
Eight months later, a MH developed in the vitrectomized right eye (Figure 1A). OCT showed cyst-like changes in the retina and a thin hyperreflective membrane on the retinal surface. The patient complained of distorted vision even though her decimal BCVA was 1.2. PPV was planned, but was canceled 2 weeks later due to the spontaneous closure of the MH.
Four months later, OCT showed a slight elevation of the ellipsoid zone and the interdigitation zone at the fovea but the patient reported that the distorted vision of her right eye was not present (Figure 1B). Eleven months after the spontaneous closure of the MH, a LMH with EP was detected in the vitrectomized right eye (Figure 1C and D). Nevertheless, her decimal BCVA was still 1.2. Two months later, the BCVA had decreased to 0.7 (Figure 1E), and OCT revealed a recurrence of the FTMH with cyst-like changes in the retina.
A second PPV combined with the insertion of the peeled internal limiting membrane (ILM) flap into the MH was performed. The yellowish tissue was not large and was able to be inserted into the MH without trimming. The retina was then tamponaded with 20% SF6 gas. The MH was closed, and the decimal BCVA was restored to 1.2 (Figure 1F). No recurrence was found at the last visit 5 months after the second vitrectomy.
Discussion
There have been several reports of a MH that developed in vitrectomized eyes, however only a limited number of cases had a recurrence of a MH after a spontaneous closure (Figure 1).10–15 The mechanism causing the MH formation after vitrectomy has not been definitively determined. Earlier studies have suggested two possible mechanisms: the formation of an epiretinal membrane (ERM) which exerts tangential traction on the foveal retina and causes the development of a MH. This type of MH is referred to as the tangential tractional type of MH6 and is related to the development of a MH after vitrectomy.7,8 The other type of MH develops in eyes with cystoid macular edema and is referred to as the cystoid degeneration type of MH.6 It is believed that the rupture or dehiscence of the roof of the cysts leads to the FTMH. There are also patients with repeated recurrences and spontaneous closures of a MH which one author suggested was caused by retinal inflammation.12
In our case, the first MH occurred 8 months after vitrectomy. Because an ERM was observed in the OCT images, we suggest that this MH is a tangential traction type of MH. Surgery was planned to release the traction but it was canceled due to a spontaneous closure of the MH. A spontaneous closure can be caused by a shift in the tractional forces caused by an ERM and the presence of bridging retinal tissues in the MH.9,10 An ERM was observed in our patient, and the spontaneous closure may have occurred because the MH opening was small and the direction of traction by the ERM had changed.
If the ILM peeling had been performed during the primary surgery, it may have led to another situation. Further analyses are needed to determine if ILM peeling is necessary during vitrectomy for eyes with a rhegmatogenous VH.
A LMH was detected immediately before the second vitrectomy for the MH and ERM. EPs were also observed in the retina around the MH in the OCT images. The cellular origin of the EPs was not determined, but Pang et al postulated that it is primarily caused by the proliferation of Müller cells in the inner retina.18 In addition, the connections between the foveolar Müller cells and EP were thought to stabilize the foveal structure by preventing the elevation of the inner layers of the foveal walls which could cause an enlargement of the degenerative cavitation.19
There are also reports that EPs can act on MH closure and reopening.20–25 Thus, Watanabe et al suggested that EPs exert a force while attempting to seal the hole, and the more widespread ERMs have sufficient force to create a reopening.21 It has been suggested that the main cellular components of EPs were glial and vitreal cells, such as fibroblasts and hyalocytes. However, a histological study showed that many of the cells of EPs were positive for glial fibrillary acidic protein, and the number of cells expressing α-smooth muscle actin was low.22 These findings suggested that EPs have less tractional force than ERMs. The suggestion that the mechanism that created the first MH also caused the second MH cannot be eliminated. On the other hand, it may be possible that the first MH was only partially closed as noted when the OCT images of the MH was examined more carefully. In our patient, the configuration of the macula appeared to be normal and micro and small MHs were not observed in the raster images taken serially at each visit (Supplemental Figure 1). The second MH developed 13 months later, and it is possible that cellular migration and phagocytosis occurred around the macula during this period. This may then exert tension on the retina through another mechanism, and due to the presence of EPs and cyst-like changes, we suggest that the development of the second MH was associated with a degenerative mechanism.
Although it has not been determined conclusively whether EPs should be completely removed or inserted into the MH during vitrectomy, we inserted the yellowish tissue and ILM flap into the MH during the surgery according to the findings of earlier cases.25–27 Further analyses of this procedure should lead to a better understanding of the pathology of MHs.
This study had several limitations. This was a single case, and the assumed mechanism for the development and spontaneous closure of the MH cannot be expanded to other cases. No histopathological examinations were performed on the surgically excised specimens. Nevertheless, we believe that these findings will contribute to a better understanding of the pathogenesis of MH opening and closing.
In conclusion, the findings made in our case illustrate the different OCT findings including macular configuration in eyes with a MH. Careful observations during its development is necessary to determine the possibility that repeated MH development is due to different mechanisms. Further analysis of microstructural changes is required to determine the exact pathogenesis of MHs.
Abbreviations
BCVA, best corrected visual acuity; EP, epiretinal proliferation; ERM, epiretinal membrane; FTMH, full thickness macular hole; ILM, internal limiting membrane; IOP, intraocular pressures; LMH, lamellar macular hole; MH, macular hole; OCT, Optical coherence tomography; PPV, pars plana vitrectomy; VH, vitreous hemorrhage.
Data Sharing Statement
The data supporting the conclusions of the article are included in the article and figure.
Ethics Statement
This study was conducted in accordance with the Declaration of Helsinki guidelines. The study protocol was reviewed and the need for approval was waived by the ethics committee of Saitama Medical University. Written informed consent was obtained from the patient for the publication of this case report and any accompanying images.
Consent for Publication
All authors agree with the content of the manuscript.
Acknowledgments
The authors thank Professor Duco Hamasaki for the English language review.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research received no specific grants from any public funding agency. commercial, or not-for-profit sectors.
Disclosure
The authors declare no conflict of interest.
References
1. Avila MP, Jalkh AE, Murakami K, et al. Biomicroscopic study of the vitreous in macular breaks. Ophthalmology. 1983;90(11):1277–1283. doi:10.1016/S0161-6420(83)34391-8
2. Gass JD. Idiopathic senile macular hole. Its early stages and pathogenesis. Arch Ophthalmol. 1988;106:629. doi:10.1001/archopht.1988.01060130683026
3. Smiddy WE, Flynn HW. Pathogenesis of macular holes and therapeutic implications. Am J Ophthalmol. 2004;137:525–537. doi:10.1016/j.ajo.2003.12.011
4. Gordon LW, Glaser BM, Le D, et al. Full thickness macular hole formation in eyes with a preexisting complete posterior vitreous detachment. Ophthalmology. 1995;102:1702–1705. doi:10.1016/S0161-6420(95)30806-8
5. Smiddy WE. Macular hole formation without vitreofoveal traction. Arch Ophthalmol. 2008;126(5):737–738. doi:10.1001/archopht.126.5.737
6. Lee SH, Park KH, Kim JH, et al. Secondary macular hole formation after vitrectomy. Retina. 2010;30:1072–1077. doi:10.1097/IAE.0b013e3181cd4819
7. Lipham WJ, Smiddy WE. Idiopathic macular hole following vitrectomy: implications for pathogenesis. Ophthalmic Surg Lasers. 1997;28:633–639. doi:10.3928/1542-8877-19970801-04
8. Kumagai K, Ogino N, Furukawa M, et al. Surgical outcomes for patients who develop macular holes after pars plana vitrectomy. Am J Ophthalmol. 2008;145:1077–1080. doi:10.1016/j.ajo.2008.01.030
9. Shaikh S, Garretson B. Spontaneous closure of a recurrent macular hole following vitrecomy corroborated by optical coherence tomography. Ophthalmic Surg Lasers Imaging. 2003;34:172–174. doi:10.3928/1542-8877-20030301-18
10. Lo WR, Hubbard GB. Macular hole formation, spontaneous closure, and recurrence in a previously vitrectomized eye. Am J Ophthalmol. 2006;141:962–964. doi:10.1016/j.ajo.2005.12.012
11. Sridhar J, Townsend JH, Rachitskaya AV. Rapid macular hole formation, spontaneous closure, and reopening after pars plana vitrectomy for macula-sparing retinal detachment. Retin Cases Brief Rep. 2017;11:163–165. doi:10.1097/ICB.0000000000000319
12. Hayashi I, Shinoda H, Nagai N, et al. Retinal inflammation diagnosed as an idiopathic macular hole with multiple recurrences and spontaneous closures: a case report. Medicine. 2019;98:e14230. doi:10.1097/MD.0000000000014230
13. Parikh PD, Day Ghafoori S. Multiple late recurrences of macular hole after vitrectomy and episode of spontaneous closure in a single eye. Retin Cases Brief Rep. 2021;15:635–639. doi:10.1097/ICB.0000000000000870
14. Ozdemir E, Ozdek S. Recurrent opening and closure of macular hole after pars plana vitrectomy for rhegmatogenous retinal detachment. Retin Cases Brief Rep. 2020;14:331–333. doi:10.1097/ICB.0000000000000699
15. Mori T, Kitamura S, Sakaguchi H, et al. Two cases of repeating recurrences and spontaneous closures of macular holes in vitrectomized eyes. Jpn J Ophthalmol. 2018;62:467–472. doi:10.1007/s10384-018-0596-3
16. Hubschman JP, Govetto A, Spaide RF, et al. Optical coherence tomography-based consensus definition for lamellar macular hole. Br J Ophthalmol. 2020;104:1741–1747. doi:10.1136/bjophthalmol-2019-315432
17. Duker JS, Kaiser PK, Binder S, et al. The international vitreomacular traction study group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology. 2013;120:2611–2619. doi:10.1016/j.ophtha.2013.07.042
18. Pang CE, Spaide RF, Freund KB. Epiretinal proliferation seen in association with lamellar macular holes: a distinct clinical entity. Retina. 2014;34:1513–1523. doi:10.1097/IAE.0000000000000163
19. Bringmann A, Unterlauft JD, Wiedemann R, et al. Morphology of partial-thickness macular defects: presumed roles of Müller cells and tissue layer interfaces of low mechanical stability. Int J Retina Vitreous. 2020;6:28. doi:10.1186/s40942-020-00232-1
20. Takahashi H, Kishi S. Optical coherence tomography images of spontaneous macular hole closure. Am J Ophthalmol. 1999;128:519–520. doi:10.1016/S0002-9394(99)00173-7
21. Watanabe M, Yokota H, Aso H, et al. Development of stage 4 macular hole after spontaneous closure in a patient with stage 2 macular hole and a lamellar macular hole-associated epiretinal proliferation. Case Rep Ophthalmol. 2021;12:481–484. doi:10.1159/000513132
22. Compera D, Entchev E, Haritoglou C, et al. Lamellar hole-associated epiretinal proliferation in comparison to epiretinal membranes of macular pseudoholes. Am J Ophthalmol. 2015;160:373–84.e1. doi:10.1016/j.ajo.2015.05.010
23. Asaad SZ. Full-thickness macular hole progressing from lamellar macular hole with epiretinal proliferation. Case Rep Ophthalmol. 2021;12:134–141. doi:10.1159/000514526
24. Cutler NE, Singh RP. Spontaneous closure of lamellar macular hole with epiretinal proliferation. Ophthalmol Retina. 2019;3:997. doi:10.1016/j.oret.2019.06.011
25. Fukushima M, Kato T, Hayashi A. Epiretinal proliferation embedding combined with internal limiting membrane flap inversion for secondary macular hole: two case reports. Am J Ophthalmol Case Rep. 2022;29:101774. doi:10.1016/j.ajoc.2022.101774
26. Shiode Y, Morizane Y, Takahashi K, et al. Embedding of lamellar hole-associated epiretinal proliferation combined with internal limiting membrane inversion for the treatment of lamellar macular hole: a case report. BMC Ophthalmol. 2018;18:257. doi:10.1186/s12886-018-0926-8
27. Kanai M, Sakimoto S, Takahashi S, et al. Embedding technique versus conventional internal limiting membrane peeling for lamellar macular holes with epiretinal proliferation. Ophthalmol Retina. 2023;7:44–51. doi:10.1016/j.oret.2022.07.009
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.