Back to Journals » Drug Design, Development and Therapy » Volume 12
Care pathways and treatment patterns for patients with heart failure in China: results from a cross-sectional survey
Authors Jackson JDS , Cotton SE , Bruce Wirta S, Proenca CC, Zhang M, Lahoz R , Balas B , Calado FJ
Received 22 February 2018
Accepted for publication 3 May 2018
Published 26 July 2018 Volume 2018:12 Pages 2311—2321
DOI https://doi.org/10.2147/DDDT.S166277
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Tuo Deng
James DS Jackson,1 Sarah E Cotton,1 Sara Bruce Wirta,2 Catia C Proenca,3 Milun Zhang,4 Raquel Lahoz,5 Bogdan Balas,5 Frederico J Calado5
1Real World Research, Adelphi Real World, Bollington, UK; 2Real World Evidence, Cardio-Metabolic Franchise, Novartis Sweden AB, Stockholm, Sweden; 3Wellmera AG, Basel, Switzerland; 4Health Economics and Outcomes Research, Novartis Pharma China, Beijing, China; 5Medical Affairs, Cardio-Metabolic Franchise, Novartis Pharma AG, Basel, Switzerland
Purpose: The objective of this study was to describe the clinical care pathways, management and treatment patterns, and hospitalizations for patients with heart failure (HF) in China.
Subjects and methods: A cross-sectional survey of cardiologists and their patients with HF was conducted. Patient record forms were completed by 150 cardiologists for 10 consecutive patients. Patients for whom a patient record form was completed were invited to complete a patient self-completion questionnaire.
Results: Most of the 1,500 patients (mean [SD] age 66 [10] years; 55% male) included in the study received care in tier-2 and -3 hospitals in large cities. Cardiologists were responsible for initial consultation, diagnosis, and treatment of patients with HF. The use of guideline-recommended diagnostics was high. However, guideline-recommended double- and triple-combination therapy was received by only 51% and 18% of patients, respectively. In total, 20% of patients with HF reported that they were not consulted on the choice of therapy. Concordance was high (≥80%) between matched cardiologist and patient pairs for the occurrence of side effects, while cardiologists more often under- than overreported the occurrence of side effects of treatment reported by patients.
Conclusion: The management of HF was predominantly overseen by cardiologists. The use of diagnostic tests was high, but the use of guideline-recommended treatment was low in this population. Improved communication between patients and cardiologists is essential to optimize treatment decision making and to increase awareness of treatment side effects.
Keywords: heart failure, disease management, patient preference, treatment satisfaction,
real-world evidence
Introduction
Heart failure (HF) represents a major clinical and public health problem worldwide, affecting 62 million people.1 In China, there are ~4.5 million individuals with HF, equating to a prevalence of 0.9%.2 HF can be classified as HF with reduced ejection fraction (HFrEF) or HF with preserved ejection fraction (HFpEF). HFrEF and HFpEF each comprise 50% of all cases of HF in China.3–5
China has one of the world’s largest health care systems, with ~28,000 hospitals, a third of which are tier-2 hospitals (medium-sized regional hospitals serving medium-sized cities) or tier-3 hospitals (large municipal hospitals providing care at a national level).6 In Western countries, care for patients with HF is generally provided by hospital- or office-based cardiologists and/or family physicians.7 Patients usually have a family physician who refers them to a more specialized physician when further input is required.8 Information on the typical care pathway of patients with HF in China has not been published in the literature. Treatment is based on recommendations made by the Chinese Society of Cardiology of the Chinese Medical Association,9 which recommend that routine examination for the diagnosis of patients with HF should comprise an echocardiogram, an electrocardiogram, laboratory tests (complete blood count, urinalysis, blood chemistry, fasting glucose level, glycated hemoglobin level, and thyroid function), levels of biomarkers (B-type natriuretic peptide or N-terminal pro-B-type natriuretic peptide), and an X-ray.
Most clinical trials published after 1990 report efficacy data for patients with HFrEF, based predominantly on a left ventricular ejection fraction threshold of 40% or less. For this reason, the Chinese Society of Cardiology guidelines mainly provide recommendations for the pharmacological treatment of this patient population (although left ventricular ejection fraction thresholds of <50% and ≥50% are suggested for HFrEF and HFpEF, respectively, and these definitions are not unified).9 The guidelines recommend angiotensin-converting enzyme inhibitors (ACEis), angiotensin receptor blockers (ARBs), β-blockers (BBs), mineralocorticoid receptor antagonists (MRAs), digoxin, diuretics, and nitrates for patients with HFrEF.
These drugs are available in most countries, including China; however, treatment gaps in the management of patients with HF still exist.10 Several large, real-world studies of treatment patterns for HF in China conducted in the early 2000s3–5,11,12 showed that nitrates, digitalis, and diuretics were the main drugs used to treat HF; the proportions of patients using these agents were 30%–91%, 40%–72%, and 49%–77%, respectively. ACEis and BBs were less widely used, although their use increased between the 1980s and the 2000s (from 9% to 19% of patients for BBs and from 14% to 40% for ACEis).11,13 A more recent survey conducted in 2009 of physicians from primary care hospitals across 17 areas of China observed trends similar to those of the earlier studies in terms of high use of diuretics and digitalis, although BBs and ACEis were more widely used in this study (40% and 80% of patients, respectively), likely reflecting increased use of these drug classes over time.14
The aim of the current cross-sectional study of patients with HF treated by cardiologists in a real-world setting in China was threefold: 1) to describe the patient pathway in terms of how patients with HF interact with and are treated by health care professionals; 2) to evaluate the use of treatment classes and how treatment decisions are being made; and 3) to understand the burden of patients with HF in terms of adverse events and hospitalizations.
Subjects and methods
Study design
Data were drawn from the Adelphi HF Disease Specific Programme (DSP), a cross-sectional survey of cardiologists, their patients with HF, and those patients’ informal caregivers, conducted in a real-world setting in China in 2016. Full details of the methodology are provided elsewhere.15,16 Briefly, a DSP comprises three key phases: preparatory, data collection, and data processing/analysis. No formal validation procedure was undertaken; however, all numerical data were double entered.
Preparatory phase
Development of fieldwork materials
Four questionnaires were developed to inform the DSP – a physician survey, a patient record form (PRF), a patient self-completion questionnaire (PSC), and a caregiver self-completion questionnaire. The physician survey was used in face-to-face interviews with cardiologists. PRFs were completed by the cardiologists for their patients presenting with HF using data from medical records. PSCs were completed by the same patients, and caregiver self-completion questionnaires were completed by the informal caregivers of the patients (results gathered from the caregiver self-completion questionnaires have been reported by Jackson et al).16 The questionnaires were developed empirically with input from experts in HF care; the concepts deemed most relevant to patients were selected empirically, and their pharmacometric properties were not systematically assessed. The questionnaires were developed in English and then translated into Chinese by a local DSP fieldwork agency. A second independent UK-based translation agency verified the translated materials. Samples of the questionnaires are available on request.
Participant recruitment
Cardiologists were identified from the public lists of health care professionals and were invited to participate in the DSP based on predefined eligibility criteria defined elsewhere.16 The first 150 cardiologists who met these criteria and agreed to participate in the study were enrolled and asked to complete PRFs for the next 10 patients they saw who presented with HF, immediately after each consultation. The same patients were invited to complete PSCs at the practice, independently of their cardiologist and immediately after their consultation. Included patients gave informed consent to participate by ticking a box on the front page of the questionnaire to indicate that they have read the information provided and that they agree to take part in the study.
Data collection phase
For this analysis, the physician survey with the cardiologists was used to capture information on practice type and location. Information collected from the PRFs covered patient demographics; clinical characteristics; consultation history; scans/investigations, physical examinations, and blood/urine tests during diagnosis and monitoring of HF; HF treatment; and all-cause and HF-related inpatient visits, outpatient visits, and emergency room visits (PRF questions pertaining to results presented herein are included in Supplementary materials, Appendix 1). Information gathered from the PSCs covered the duration of time between symptom onset and first visit to a physician (PSC questions pertaining to results presented herein are included in Supplementary materials, Appendix 1).
All responses were anonymized to preserve participant confidentiality and to avoid potential bias. The questionnaire applied in this study followed the European Pharmaceutical Market Research Association guidelines.17 The Code of Conduct states that within this context, ethical approval is not necessary, considering that the goal of research rather than being to test a hypothesis is to improve understanding rather than testing the hypotheses. The research was conducted in accordance with the US Health Insurance Portability and Accountability Act 1996 and European equivalents.17,18
Data processing/analysis phase
For the purposes of analysis and interpretation, HFrEF was defined as left ventricular ejection fraction <50% and HFpEF as ≥50%, in accordance with the Chinese Society of Cardiology guidelines.9 Descriptive statistics were used for all baseline characteristics and outcomes of interest with the analyses conducted using the software package QPSMR Reflect, version 2007.1g (QPSMR Ltd, Wallingford, UK). Continuous variables were presented as mean ± SD, or median (in the case of health care visit frequency), while categorical variables were presented as number (n) and percentage (%) of patients. For the patient–physician paired analyses, concordance was calculated as: n (concordant absence of symptom/adverse event + concordant presence of symptom/adverse event)/N (total numbers of matched pairs). Furthermore, the patient perspective was taken to evaluate physician underreporting (side effect reported by patient but not physician/total number of patients reporting side effect) and physician overreporting (side effect reported by physician but not patient/total number of patients not reporting side effect).
Results
Study population
The study comprised 150 cardiologists and 1,500 patients. Almost two-thirds (62%, n=933) of the patients for whom a PRF was completed also completed a PSC.16
Cardiologist demographics
Of the 150 cardiologists, the majority (60%) were based in Beijing, Shanghai, or Guangdong (Figure S1). Most (91%) of the cardiologists worked only in a hospital; of these, 69% worked in tier-3 hospitals.
Patient demographics and baseline clinical characteristics
Overall, 55% of the patients were male, and their mean (SD) age was 66 (10) years in both the PRF and PSC subsamples. The baseline clinical characteristics for patients are shown in Table 1, separately for the PRF and PCS patient samples.
Patient pathway
Reasons for consultation and physician specialties
Based on data from the PRFs, patients mainly consulted physicians for a routine follow-up or a repeat prescription (46%), or for a diagnosis of HF or testing of HF symptoms (44%). Few patients (1%) scheduled a consultation to discuss HF treatment (ie, because their treatment was not working or because of the occurrence of side effects), as shown in Figure S2.
Use of scans/investigations, physical examinations, and blood/urine tests during diagnosis and monitoring of HF
The use of scans/investigations, physical examinations, and blood/urine tests during diagnosis and monitoring of patients with HF (n=1,219–1,474) is shown in Figure 1. In general, scans/investigations were more commonly used during diagnosis of HF than when monitoring it, with the exception of electrocardiograms, which were more commonly used when monitoring HF than for diagnosing it (78% vs 63% of patients, respectively). Almost all patients (92%) had undergone an echocardiogram during their diagnosis of HF. Similarly, examination of physical signs was generally more commonly used during diagnosis than monitoring; assessment of heart rate was the most common physical sign examined during HF diagnosis (82%), while assessment of blood pressure was the most common physical sign used when monitoring HF (94%). B-type natriuretic peptide and N-terminal pro-B-type natriuretic peptide were the most common blood/urine markers used to diagnose HF (69% and 66%, respectively). Total cholesterol (68%) and triglyceride (62%) tests were most frequently performed during patient monitoring, followed by B-type natriuretic peptide (56%), fasting glucose (55%), glycated hemoglobin (47%), and serum creatinine (45%).
HF treatment patterns
Information on HF treatment patterns was available for 1,498 patients. The most common treatment class used at the time the survey was conducted was ACEi/ARB (94%), followed by BB (74%) and loop diuretic (60%), as shown in Figure 2. MRAs were received by 28% of patients. With regard to combination therapy, the largest proportion of patients (51%) received ACEi/ARB+BB, followed by 18% of patients who received ACEi/ARB+BB+MRA. More patients with HFrEF (21%) than HFpEF (18%), as well as those with more severe symptoms as determined by the New York Heart Association class (class I 10%; class II–IV 24%) received this combination, as shown in Table 2. The most common monotherapy received was ACEi or ARB (17%), with higher proportions of patients with HFrEF (22%) than HFpEF (10%) receiving these classes. BB monotherapy was uncommon (4%).
Of 1,489 patients, cardiologists reported that the ability of the drug to lower blood pressure (reported for 75% of patients) was the most common reason for treatment choice, which might be explained by the fact that 79% of patients had hypertension. Cardiologists’ own familiarity/experience with the drug was the second most common reason for treatment choice (reported for 73% of patients; Figure 3). Whether the choice of the drug was in accordance with the guidelines influenced cardiologists’ choice for 57% of patients.
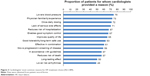  | Figure 3 Cardiologists’ most common reasons for HF treatment choice (N=1,489). |
Treatment decision making
A high level of concordance (84%) between matched cardiologist and patient pairs (n=842) was observed for the degree of patient input into treatment decisions. In total, 20% of patients with HF reported that they were not consulted on the choice of therapy, and 71% of matched cardiologist and patient pairs reported that treatment choice was based on a discussion, while the cardiologist had the final call (Table 3).
Treatment satisfaction
Concordance was high between matched cardiologist and patient pairs (n=842) for the occurrence of side effects, ranging from 80% (fatigue/tiredness and dizziness) to 100% (numbness/tingling on skin and sexual dysfunction), as shown in Figure 4A. Individual side effects were reported by 0%–22% of patients (Figure 4B). Cardiologists more often under- than overreported the occurrence of side effects of treatment reported by patients (Figure 4B).
Hospitalizations
In China, doctors do not have access to patients’ medical records outside of the hospital in which they see the patient. Therefore, the number of hospitalizations recorded by cardiologists in this study covered only hospitalizations that occurred at their particular hospital. Information on all-cause and HF-related inpatient, outpatient, and emergency room visits was available for 1,278–1,490 patients. In the 12 months before the survey, 55% and 49% of patients had ≥1 inpatient visit to the hospital for any reason (median=1 visit) or due to HF (median=0 visit), respectively (Figure 5). The corresponding proportions of patients experiencing ≥1 all-cause and HF-related outpatient and emergency room visit were 99% (median=11 visits) and 97% (median=7), and 31% (median=0) and 28% (median=0), respectively. Information on hospital readmission was available for 590 patients; of the patients hospitalized due to HF, 16% were readmitted to hospital within 30 days.
Discussion
This survey assessed care pathways of patients with HF in a real-world setting in China and provides evidence of HF treatment patterns in clinical practice. According to the population sampled in this study, cardiologists are the main physician speciality involved in first consultations of patients with a suspicion of HF, as well as in confirming HF diagnoses and initiating treatment; there is little involvement of family physicians, with only 10% being responsible for a first consultation and even fewer involved in diagnosis and treatment. These findings reflect the fact that involvement of primary care in HF management programs in China is extremely limited19 and differ from studies of HF care pathways in Western countries,7,8 where the family physician acts as a primary contact for patients with HF and will refer patients to specialists for further input when needed. Shared decision making is important for patient-centered care, whereby the physician educates the patient about treatment options, possible outcomes, and side effects, in order to reach an informed treatment decision. This study shows that even when a discussion occurs, the physician is most often the final decision maker for treatment decisions. Another important finding of this study relates to underreporting of adverse events by cardiologists. Improved communication between patients and cardiologists is essential to optimize treatment decision making and to increase awareness of the occurrence of treatment side effects.
The Chinese Society of Cardiology guidelines, similar to those published by the European Society of Cardiology, recommend that diagnosis of patients with HF should involve an echocardiogram, electrocardiogram, laboratory tests, and a chest X-ray. In the current study, the use of these diagnostic tests and tools was relatively high. Higher utilization rates of these diagnostic examinations by cardiologists in a hospital setting compared with family physicians in an office-based setting are not unexpected.20 Furthermore, the use of guideline-recommended HF drug classes, such as ACEis, ARBs, and BBs, was high and in line with the rates observed in specialist settings in Western countries.21–23 However, less than a third of patients with HFrEF received an MRA; this is notably lower than the rates reported for patients with HFrEF receiving care in specialist settings in Western countries (~40% in the USA and ~60% in European counties),21–23 highlighting an important gap exists in the utilization of a foundational disease-modifying therapy in HFrEF. In addition, less than half of patients with HFrEF were receiving the guideline-recommended combination of ACEi/ARB+BB and just one in five received a combination of ACEi/ARB+BB+MRA (which is recommended for patients with HFrEF who remain symptomatic on ACEi/ARB+BB).24 These findings are in line with a recent study conducted in China by Huang et al which used national claims data and reported that 48% and 23% of patients with HF were receiving these treatment combinations, respectively.25 The HF phenotype of patients included in the study by Huang et al was unspecified, so it might be expected that the proportion of patients with HFrEF who were receiving these combinations would be higher than for the overall study population. Nonetheless, the findings of the current study demonstrate that adherence to guidelines in China is suboptimal and should be improved.
The current study surveyed cardiologists, who may adhere more closely to clinical guidelines than family physicians. For example, in a review conducted by Jiang and Ge, it was reported that physicians in small hospitals and community hospitals may not follow guidelines regarding the use of BBs and ACEis.10 In addition, the cardiologists in the current study were practising within tier-2 and -3 hospitals; therefore, the findings may not be generalizable to care in rural settings or in smaller hospitals as assessed by Jiang and Ge.10 Indeed, previous studies have shown that there is great disparity in the use of HF treatments between developed and developing areas in China.14 A large survey conducted by Cao et al found that BBs and ACEis were used in 50% and 90% of patients in the Hunan province, respectively; however, in the Qinghai and Guizhou provinces, these drugs were used in 30%–33% and 33%–80% of patients, respectively.14 This imbalance in China with regard to access and utilization of health care between wealthier (usually urban) and poorer (usually rural) areas has long been recognized.26
The disease burden due to hospitalizations in the current study was overall high compared to those reported in Western studies.27,28 However, the total resource utilization of the patients from the study remains elusive, since in China, patients often receive care from multiple doctors and/or hospitals and doctors do not have access to patients’ medical records of their visits to other hospitals. Therefore, the cardiologists in the current study might only have known the number of visits to the hospital in which they were treating the patient. In contrast, Huang et al analyzed data from a national claims database (covering all of a patient’s hospitalizations regardless of at which hospital they took place) and reported higher hospitalization rates, as expected.25 In this study, 16% of patients who had been hospitalized for HF were readmitted within 30 days of discharge. This value is in line with the 15% reported by Huang et al25 and is within the range of 30-day readmission rates reported in the studies of Medicare patients with HF aged ≥65 years in the USA (11%–25%),29–32 suggesting that patients in China with HF experience similar rates of hospital readmission to patients in Western countries.
The inevitable limitations associated with data collected from surveys are relevant for the current study, including recall bias, missing data, and overreporting of surveyed events. Nevertheless, the Adelphi DSP is an established method for investigating real-world treatment practices across a wide range of disease areas.33
The patient population included in the study represents a sample of consulting patients with HF who are treated in a cardiology setting, and not those managed in primary care or by other specialities such as internal medicine or geriatricians. Only patients receiving care in tier-2 or -3 hospitals were included in the current study; these are more commonly found in large cities, and therefore, may not reflect patients receiving care in tier-1 hospitals, which are usually found in rural areas. Furthermore, in the current study, the split of tier-2 and -3 hospitals was 31% and 69%, respectively, which does not reflect the split in the Chinese health care system (78% vs 21%), and may therefore impact the generalizability of the data.6
Although cardiologists were requested to collect PRF data on a series of consecutive patients to avoid selection bias, formal source data verification (ie, checks against patient medical records) was not performed. Moreover, diagnosis in the target patient group is based primarily on the judgment and diagnostic skills of the respondent cardiologist rather than on a diagnostic checklist, although patients are managed in accordance with the same routine diagnostic procedures representative of that clinical practice setting. More complex diagnostic procedures or methods for assessing functional status (eg, cardiopulmonary exercise testing) were not captured in our research; more research is needed to understand the impact of holistic integrative medicine on physician’s choice of treatments. Choice of treatments given for HF may have been influenced by the presence of different comorbid conditions; yet, our questionnaire was unable to detect such potential interactions. In this respect, the cardiologists’ responses are likely to reflect real-world data.
The Chinese guidelines recommend a 50% ejection fraction cut-off to differentiate HFrEF from HFpEF phenotypes, which likely results in an overestimation of the HFrEF prevalence as compared to other geographies where 40% is used as the cut-off.
The selection of patients favors inclusion of those who consult more frequently, so patients with more severe disease or complications may be overrepresented in this sample. In addition, the inclusion criteria of this study required each cardiologist to recruit 10 patients, half of whom had HFrEF and the other half had HFpEF. Because there is an equal distribution of HF phenotype in the general HF population in China,3–5 this would be unlikely to introduce bias.
Conclusion
The results of this study allow for a better understanding of how patients with HF are diagnosed and treated, the extent to which patients influence treatment decisions, and how adverse events perceived by patients are recognized by the treating physicians in the sample of patients treated by cardiologists from China primarily practicing at tier-2 and -3 hospitals in large cities. Although use of guideline-recommended diagnostic procedures was high, guideline-recommended double-combination therapy was prescribed to only half of the patients and triple combination to only one-fifth of the patients in the survey. A high burden of disease was observed with around half of the patients experiencing a hospitalization for HF in the 12 months before the survey. Finally, a high level of concordance was observed between matched cardiologist and patient pairs. One-fifth of the patients reported that they were not consulted regarding the choice of therapy, and that cardiologists tended to underestimate patient-reported side effects. In conclusion, this study reinforces an unmet need in the care for patients with HF and suggests that improved communication is needed to optimize treatment decision making and to increase awareness of treatment side effects. Further studies with larger and more representative samples are needed to extrapolate these findings to the overall population of patients with HF in China.
Acknowledgments
This study was funded by Novartis Pharma AG, Basel, Switzerland. The manuscript was developed with important intellectual input from Heike Schwende and Daniel Viriato of Novartis Pharma AG, Basel, Switzerland. Medical writing support was provided by Carly L Sellick, BSc, of PharmaGenesis London, London, UK, and was funded by Novartis Pharma AG, Basel, Switzerland.
Disclosure
James DS Jackson and Sarah E Cotton are employees of Adelphi Real World, and were contracted by Novartis to conduct this study. Sara Bruce Wirta is an employee of Novartis Sweden AB; Raquel Lahoz, Bogdan Balas, and Frederico J Calado are employees of Novartis Pharma AG; and Milun Zhang is an employee of Novartis Pharma, China. Catia C Proenca is an employee of Wellmera AG contracted by Novartis Pharma AG. The authors report no other conflicts of interest in this work.
References
Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800. | ||
Gu DF, Huang GY, Jiang HE, et al. Investigation of prevalence and distributing feature of chronic heart failure in Chinese adult population. Chin J Cardiol. 2003;31(1):3–6. | ||
Ma JP, Wang L, Dang Q, et al. Retrospective analysis of drug treatment on inpatients with chronic heart failure. Chin J Epidemiol. 2007;28(1):78–82. | ||
Shanghai Investigation Group of Heart Failure. The evolving trends in the epidemiologic factors and treatment of hospitalized patients with congestive heart failure in Shanghai during the years of 1980, 1990 and 2000. Chin J Cardiol. 2002;30:24–26. | ||
Collaborative Group on Survey of Heart Failure in Shanghai. Cross-sectional survey on the current status of drug therapy in patients with stable heart failure in Shanghai. Chin J Cardiol. 2001;29:644–648. | ||
National Health and Family Planning Commission (NHFPC). The number of national medical and health institutions as of the end of June 2016. Available from: http://www.nhfpc.gov.cn/mohwsbwstjxxzx/s7967/201608/87343c7d63ce41ca8d8fddf7a5db66b7.shtml. Accessed August 4, 2017. | ||
Mehrhof F, Loffler M, Gelbrich G, et al; Competence Network Heart Failure. A network against failing hearts – introducing the German “Competence Network Heart Failure”. Int J Cardiol. 2010;145(1):135–138. | ||
Tebbe U, Tschope C, Wirtz JH, et al. Registry in Germany focusing on level-specific and evidence-based decision finding in the treatment of heart failure: REFLECT-HF. Clin Res Cardiol. 2014;103(8):665–673. | ||
Chinese Society of Cardiology of the Chinese Medical Association, Editorial Board of the Chinese Journal of Cardiology. Chinese heart failure diagnosis and treatment guidelines; 2014. Available from: http://www.cjcv.org.cn/xinxueguan20144202/33844.htm?locale=zh_CN. Accessed August 4, 2017. | ||
Jiang H, Ge J. Epidemiology and clinical management of cardiomyopathies and heart failure in China. Heart. 2009;95(21):1727–1731. | ||
Society of Cardiology, Chinese Medical Association. Retrospective investigation of hospitalized patients with heart failure in some parts of China in 1980, 1990 and 2000. Chin J Cardiol. 2002;30:450–454. | ||
Weihu F. [Cross-sectional survey on the current status of drug therapy in patients with stable heart failure in Shanghai]. Zhonghua Xinxueguanbing. 2001;29(11):644–648. Chinese. | ||
Ma X, Xiang YT, Li SR, et al. Prevalence and sociodemographic correlates of depression in an elderly population living with family members in Beijing, China. Psychol Med. 2008;38(12):1723–1730. | ||
Cao YM, Hu DY, Wang HY, Wu Y. [A survey of medical therapies for chronic heart failure in primary hospitals in China]. Zhonghua Nei Ke Za Zhi. 2006;45(11):907–909. Chinese. | ||
Anderson P, Benford M, Harris N, Karavali M, Piercy J. Real-world physician and patient behaviour across countries: disease-specific programmes – a means to understand. Curr Med Res Opin. 2008;24(11):3063–3072. | ||
Jackson J, Cotton S, Bruce Wirta S, et al. Burden of heart failure on patients in China: results from a cross-sectional survey. Drug Des Devel Ther. 2018;12:1659–1668. | ||
European Society for Opinion and Marketing Research (ESOMAR). International Code of Marketing and Social Research Practice 2007. Available from: http://www.icc.se/reklam/english/engresearch. Accessed August 22, 2013. | ||
U.S. Department of Health & Human Services. Summary of the HIPAA Privacy Rule, July 2013. Availble from: https://www.hhs.gov/hipaa/for-professionals/privacy/laws-regulations/index.html. Accessed January 24, 2018. | ||
Gu M, Ma Y, Zhou T, Xia Y. Evaluation of a community health service center-based intervention program for managing chronic heart failure. Balkan Med J. 2016;33(1):45–51. | ||
Bohlken J, Kostev K. [Diagnostic and prescription behavior of general practitioners and specialist physicians in patients with dementia in 2005 and 2015 in Germany]. Psychiatr Prax. 2018;45(3):154–159. German. | ||
Maggioni AP, Anker SD, Dahlstrom U, et al; Heart Failure Association of the ESC. Are hospitalized or ambulatory patients with heart failure treated in accordance with European Society of Cardiology guidelines? Evidence from 12,440 patients of the ESC Heart Failure Long-Term Registry. Eur J Heart Fail. 2013;15(10):1173–1184. | ||
Crespo-Leiro MG, Segovia-Cubero J, Gonzalez-Costello J, et al; Project research team. Adherence to the ESC heart failure treatment guidelines in Spain: ESC Heart Failure Long-term Registry. Rev Esp Cardiol (Engl Ed). 2015;68(9):785–793. | ||
von Scheidt W, Zugck C, Pauschinger M, et al. Characteristics, management modalities and outcome in chronic systolic heart failure patients treated in tertiary care centers: results from the EVIdence based TreAtment in Heart Failure (EVITA-HF) registry. Clin Res Cardiol. 2014;103(12):1006–1014. | ||
Ponikowski P, Voors AA, Anker SD, et al; ESC Scientific Document Group. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200. | ||
Huang J, Yin H, Zhang M, Ni Q, Xuan J. Understanding the economic burden of heart failure in China: impact on disease management and resource utilization. J Med Econ. 2017;20(5):549–553. | ||
Meng Q, Xu L, Zhang Y, et al. Trends in access to health services and financial protection in China between 2003 and 2011: a cross-sectional study. Lancet. 2012;379(9818):805–814. | ||
Parrondo J, Sarria A. Heart failure in a health area of Madrid, Spain. Description and management from electronic medical records. Value Health. 2015;18(7):A381–A382. | ||
Aydogdu AD, Bozkurt E, Cavusoglu Y, et al. The direct cost components in heart failure with reduced ejection fraction (HF-REF) in Turkey: the results of a Delphi panel. Abstract presented at: ISPOR 19th Annual European Congress; November, 2016; Vienna, Austria. | ||
Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309(4):355–363. | ||
Chen J, Ross JS, Carlson MD, et al. Skilled nursing facility referral and hospital readmission rates after heart failure or myocardial infarction. Am J Med. 2012;125(1):100.e1–e9. | ||
Krumholz HM, Merrill AR, Schone EM, et al. Patterns of hospital performance in acute myocardial infarction and heart failure 30-day mortality and readmission. Circ Cardiovasc Qual Outcomes. 2009;2(5):407–413. | ||
Ross JS, Chen J, Lin Z, et al. Recent national trends in readmission rates after heart failure hospitalization. Circ Heart Fail. 2010;3(1):97–103. | ||
Anderson P, Benford M, Harris N, Karavali M, Piercy J. Real-world physician and patient behaviour across countries: Disease-Specific Programmes – a means to understand. Curr Med Res Opin. 2008;24(11):3063–3072. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.







