Back to Journals » Clinical Epidemiology » Volume 9
Cardiovascular risks in smokers treated with nicotine replacement therapy: a historical cohort study
Authors Dollerup J, Vestbo J, Murray-Thomas T, Kaplan A , Martin RJ , Pizzichini E, Pizzichini MMM , Burden A, Martin J, Price DB
Received 15 November 2016
Accepted for publication 23 January 2017
Published 26 April 2017 Volume 2017:9 Pages 231—243
DOI https://doi.org/10.2147/CLEP.S127775
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Henrik Sørensen
Jens Dollerup,1 Jørgen Vestbo,2 Tarita Murray-Thomas,3 Alan Kaplan,4 Richard J Martin,5 Emilio Pizzichini,6 Marcia M M Pizzichini,6 Anne Burden,7 Jessica Martin,7 David B Price7,8
1Dollerup Medical Consultancy, Kongens Lyngby, Denmark; 2Division of Infection, Immunity and Respiratory Medicine, School of Biological Sciences, University of Manchester, Manchester, UK; 3Clinical Practice Research Datalink, Medicines and Healthcare products Regulatory Agency, London, UK; 4Family Physician Airways Group of Canada, Richmond Hill, ON, Canada; 5National Jewish Health, Denver, CO, USA; 6Federal University of Santa Catarina, Santa Catarina, Brazil; 7Observational and Pragmatic Research Institute Pte Ltd, Singapore; 8Centre for Academic Primary Care, University of Aberdeen, Aberdeen, UK
Background: Previous research suggests exposure to nicotine replacement therapy (NRT) may be associated with an increased risk of cardiovascular disease (CVD).
Methods: Using data from the United Kingdom’s Clinical Practice Research Datalink, this study aimed to evaluate CVD events and survival among individuals who attempted smoking cessation with the support of NRT compared with those aided by smoking cessation advice only. We studied CVD outcomes over 4 and 52 weeks in 50,214 smokers attempting to quit – 33,476 supported by smoking cessation advice and 16,738 with the support of NRT prescribed by their primary care physician. Patients were matched (2 smoking cessation advice patients:1 NRT patient) on demographic and clinical characteristics during a baseline year preceding their quit attempt. Cox proportional hazard regression, conditional negative binomial regression model, and conditional logistic regression were used to analyze data.
Results: Mean (standard deviation) population age was 47 (11.2) years; 51% were females. Time to first diagnosis of ischemic heart disease (IHD) among NRT and smoking cessation advice patients was similar within the first 4 weeks, but shorter for NRT patients over 52 weeks (hazard ratio [HR]: 1.35, 95% confidence interval [CI]: 1.03–1.77). A similar trend was observed for cerebrovascular disease (HR: 1.54, 95% CI: 1.08–2.19). NRT patients with a prior diagnosis of IHD or cerebrovascular disease had a higher rate of primary or secondary care consultations for IHD or cerebrovascular disease by 52 weeks (rate ratio: 1.50, 95% CI: 1.14–1.99). Patients prescribed NRT had a shorter survival time over 52 weeks, compared with those receiving advice only (HR: 1.39, 95% CI: 1.09–1.76).
Conclusion: Our findings suggest that treatment with NRT over 4 weeks does not appear to have an impact on cardiovascular risks. However, a longer follow-up period of 52 weeks resulted in an increase in cardiovascular events for patients prescribed NRT, compared with those receiving smoking cessation advice only.
ENCePP registration ENCePP/SDPP/4238
Keywords: smokers, cardiovascular, risk, nicotine replacement therapy, smoking cessation advice
Background
Tobacco smoking is the second leading risk factor for disease globally,1 killing approximately six million people each year.2 The World Health Organization European Region has one of the highest proportions of deaths caused by tobacco in the world.3 In the United Kingdom (UK), statistics from 2013 estimated that smoking caused around 80,000 deaths in adults aged >35 years, accounting for 17% of all deaths.4
Given the substantial harm caused by smoking, public health policies have focused on tobacco control measures.2 There have been large reductions in the prevalence of smoking on a global scale; however, owing to population growth the number of smokers has increased.5 According to the World Health Organization , 21% of the global population aged ≥15 years smoked tobacco in 2012,6 amounting to 1.1 billion smokers in the world, more than at any time in history.7 In the UK, figures from 2014 reported that one in six adults were smokers, about 10 million, of whom 22% were males and 17% females.8
Guidelines in the UK recommend that all smokers have their smoking status recorded at every medical consultation and are offered smoking cessation advice.9 The aim of nicotine replacement therapy (NRT) is to reduce both the motivation to smoke and withdrawal symptoms, by temporarily replacing the nicotine from cigarettes, thereby facilitating the transition from cigarettes toward abstinence.10 Evidence supports the effectiveness of NRT for smoking cessation, and many guidelines recommend it as a first-line treatment for people seeking pharmacological treatment.10–12 In 2011, NRT was the most common smoking cessation intervention “prescribed” in England.13
The literature reports conflicting results as to the safety of NRT among high-risk patients. Anecdotal evidence has highlighted the incidence of cardiovascular events in patients with unstable coronary syndromes.14–17 Conversely, a randomized controlled trial (RCT) conducted by Joseph et al,18 found no significant increase in cardiovascular events in two high-risk groups with cardiovascular disease (CVD) when NRT patch users were compared with placebo patch users. A systematic review and meta-analysis of adverse events associated with NRT, including 92 RCTs and 28 observational studies, concluded that the use of NRT was associated with a variety of side effects, including chest pain and heart palpitations.19 A more recent meta-analysis of RCTs reported an elevated risk of less serious cardiovascular events with NRT, but concluded that there was no clear evidence of harm with NRT.20
Evidence on the relationship between NRT and cardiovascular events is largely derived from RCTs, which frequently have strict eligibility criteria and a tendency to exclude patients at high risk of vascular events and vascular comorbidities. Evidence from observational studies is more limited, but one study involving 33,247 patients prescribed NRT, concluded that the use of NRT was not associated with an increase in the risk of myocardial infarction (MI), stroke, or death when used in a real-world routine care setting.21
Further real-world effectiveness studies are needed to assess the safety profile of NRT in patients with, or at high risk of, CVD. We hypothesized that patients exposed to NRT (NRT patients) are at a higher risk of CVD compared with patients receiving smoking cessation advice only (smoking cessation advice patients).
The aim of this study was to compare CVD events, at 4 and 52 weeks respectively, in NRT patients (individuals attempting smoking cessation with the aid of NRT as any, or a combination of, nasal spray, transdermal patches, inhaler or gum and tablets) compared with those in smoking cessation advice patients, in a representative UK primary care population.
Methods
Study design and data source
This historical, matched cohort database study comprised a 1-year baseline characterization period and a 1-year outcome evaluation period on either side of an index date. The index date was defined as the time at which patients received either smoking cessation advice only or a first prescription of NRT.
Data were extracted from the Clinical Practice Research Datalink (CPRD), the world’s largest validated computerized database of anonymized longitudinal medical records for primary care.22,23 At the time of this study, the CPRD comprised of ~10.6 million patients from >590 primary care practices throughout the UK. Records are derived from a widely used general practice software system and contain prescribing and coded diagnostic and clinical information as well as information on tests requested, laboratory results, smoking cessation advice recorded by general practitioners (GPs) using specific Read codes,24 and referrals made at or following on from each consultation.25
The study protocol was developed in collaboration with an independent steering committee and approved by the CPRD’s Independent Scientific Advisory Committee (protocol number 09_096R) prior to data extraction. The study protocol was publicly registered with the European Network of Centers for Pharmacoepidemiology and Pharmacovigilance (ENCePP; registration number ENCePP/SDPP/4238). The study period ran from January 2000 to the end of December 2009. Patient consent was not required due to the retrospective nature of this study.
Characteristics of participants
Eligible patients were current smokers during the baseline year, aged 18−75 years, who sought smoking cessation advice from their GP and had at least 1 year of up-to-standard data (as defined by the CPRD) prior to and following their quit date (ie, index date) or up to the time of death if death occurred within the outcome period. The outcome period is defined as up to 4 and/or 52 weeks post index date. End of observation occurred at the practice last collection date, patient transfer out date, outcome diagnosis date, end of the study period (4 and/or 52 weeks), or the study end date.
Patients were excluded if they were exposed to NRT or any other pharmacological smoking cessation interventions during the year preceding the index date. Patients in the smoking cessation advice group, who received NRT or any other pharmacological smoking cessation interventions during the outcome period, were excluded, as were patients in the NRT group who switched to other (non-NRT) pharmacological smoking cessation interventions during the outcome period. Switching between different NRT products, or use of multiple NRT products, was permitted.
Patients in the NRT group received a first prescription for NRT as any, or a combination of, transdermal patches, nasal spray, gum, tablets, or inhaler at the index date. Patients who formed the group undertaking smoking cessation unaided by pharmacological interventions, only received smoking cessation advice. This group was defined to reflect, as closely as possible, the patients in the exposed group, with the main exception of note being the decision by their physician to provide smoking cessation advice/education only, rather than a pharmacological intervention, at the index date.
Study end points
The co-primary end points were 1) time to diagnosis of ischemic heart disease (IHD) and 2) time to diagnosis of cerebrovascular disease over a 4-week outcome period (immediately post index date). These were also evaluated over a secondary 52-week outcome period. The 4-week outcome period allowed us to observe any immediate cardiovascular events. The 52-week outcome period allowed us to assess cardiovascular events and mortality over a longer time period; furthermore, it gave us the opportunity to detect any seasonal variations in the prevalence of cardiovascular events.
Additional secondary end points included the number of consultations for IHD or cerebrovascular disease (GP consultations, inpatient admissions, and emergency department and outpatient attendances) and survival time (all-cause mortality, IHD-related death, cerebrovascular disease-related death) during the 4-week and 52-week outcome periods. By investigating the number of consultations, we hoped to capture not only new diagnoses, but also a picture of the level of health care resource utilization, such as reviews, monitoring, and acute events, as existing disease potentially worsened. A consultation was taken as a date in the consultation table that was not inpatient, outpatient, or emergency department visit. Specific consultation types were identified based on diagnostic (Read codes) entered on the date corresponding with a code list for IHD/cerebrovascular disease. Death dates were identified using Read-coded statement of deaths. The cause was inferred on the basis of Read codes recorded within a 7-day window of that event. The start of follow-up for end points of interest occurred from the index date of prescribed NRT and the smoking cessation advice date, respectively. The “time to” analyses assessment ran until the earliest of the specific outcome of interest or end of the outcome period.
Statistical analysis
To control potential confounding between comparator groups, patients in the smoking cessation advice group were matched to those in the NRT group on a 2:1 ratio based on sex, age (±5 years), hypertension diagnosis (on or at any time before the index date), CVD diagnosis (on or at any time before the index date), cerebrovascular disease diagnosis (on or at any time before the index date), IHD diagnosis (on or at any time before the index date), diabetes diagnosis ever (at any time in the records), and chronic obstructive pulmonary disease (COPD) diagnosis ever. Further information on the potential confounders evaluated in the study is available in the supplementary material.
Two-way comparisons between treatment groups using the reduced but matched datasets were carried out making minimal adjustments for other baseline confounders as necessary.
The proportion of patients with IHD/cerebrovascular disease diagnosis, proportion of deaths (all-cause mortality), and the number of primary and secondary care consultations due to IHD/cerebrovascular disease were compared using conditional logistic regression.
The time to diagnosis of IHD was analyzed using Cox proportional hazards model, with times censored at 4 or 52 weeks. Patients with a prior diagnosis of IHD were excluded from this analysis. The same method was used to analyze the time to diagnosis of cerebrovascular disease, but patients with a prior diagnosis of cerebrovascular disease were excluded from the analysis.
The total number of consultations for IHD or cerebrovascular disease during the 4- and 52-week outcome periods was investigated using a conditional negative binomial regression model (rate ratios) to obtain estimates of consultation/hospitalization rates relative to the control group. Where counts were low, a conditional logistic regression model (odds ratios) was used, with the outcome categorized as none versus any consultations. Patients with a prior IHD and/or cerebrovascular disease diagnosis were analyzed separately from those with no prior diagnosis of IHD or cerebrovascular disease.
All survival times, until death due to IHD, cerebrovascular disease, or any cause, were analyzed using Cox proportional hazards regression (hazard ratios) with time censored at 4 or 52 weeks. Proportional hazards were checked and met. All hazard ratios, odds ratios, and rate ratios were presented as NRT relative to cessation advice only. Read code lists were generated in conjunction with medical expert advice.
All analyses were carried out using IBM Statistical Package for the Social Sciences (SPSS) Statistics version 21 (IBM SPSS Statistics, Feltham, Middlesex, UK), Statistical Analysis System version 9.3 (SAS Institute, Marlow, Buckinghamshire, UK), and Microsoft Office Excel 2007 (Microsoft Corp., Redmond, WA, USA). Statistical significance was set at P<0.05.
Results
The unmatched cohort consisted of 57,920 patients, of whom 40,799 received smoking cessation advice, and 17,121 were prescribed NRT. The mean (standard deviation [SD]) age was 48 (11.5) years; 48% were female (Figure S1). After matching for sex, age (±5 years), hypertension diagnosis, CVD diagnosis, cerebrovascular disease diagnosis, IHD diagnosis, diabetes diagnosis, and COPD diagnosis, there were a total of 50,214 patients (Figure S2) – 33,476 received smoking cessation advice only; 16,738 received their first prescription of NRT. The mean (SD) age of the matched cohort was 47 (11.2) years; 51% were female (Tables 1 and 2).
At 4 weeks post index smoking cessation attempt, there was no difference between NRT patients and smoking cessation advice patients in terms of the primary outcomes of time to first IHD (unadjusted hazard ratio [HR] 95% confidence interval [CI]: 1.08 [0.56–2.06]) and cerebrovascular disease diagnosis (unadjusted HR [95% CI]: 1.00 [0.34–2.93]) or the secondary outcomes survival time and odds of primary and secondary care consultations for IHD or cerebrovascular disease (Table 3).
By week 52, the adjusted HR (95% CI) for time to first diagnosis of IHD was higher for NRT patients compared with smoking cessation advice patients: 1.35 (1.03–1.77). Compared with smoking cessation advice patients, at 52 weeks there was also a shorter time to first diagnosis of cerebrovascular disease in NRT patients (unadjusted HR [95% CI]: 1.54 [1.08–2.19], Table 3; Figure 1) and survival time (adjusted HR [95% CI]: 1.39 [1.09–1.76], Figure 1). Cerebrovascular disease-related mortality and IHD remained low for both groups throughout the 52-week secondary outcome period. Among patients with a prior diagnosis of IHD or cerebrovascular disease, those on NRT therapy had a higher rate of primary or secondary care consultations for IHD or cerebrovascular disease over 52 weeks (adjusted rate ratio [RR]: 1.50, 95% CI: 1.14–1.99) compared with those receiving smoking cessation advice (Figure 1).
Discussion
This study of a real-world population found no significant differences in the primary end points of time to first IHD and cerebrovascular disease diagnosis at 4 weeks between patients attempting to give up smoking with the assistance of prescribed NRT or via smoking cessation advice alone. However, prescription of NRT to assist smoking cessation (compared with smoking cessation advice) was associated with a higher risk of IHD and cerebrovascular disease after 1 year, perhaps owing to cumulative exposure. Moreover, patients prescribed NRT to aid their quit attempt had a higher mortality in the following year than those patients supported with smoking cessation advice only. Furthermore, we found an increased rate of primary and secondary care consultations for IHD and cerebrovascular disease for patients receiving NRT when a prior diagnosis of IHD or cerebrovascular disease was made.
Our results of increased IHD and cerebrovascular diagnoses by week 52 for patients taking NRT (HR: 1.35, 95% CI: 1.03–1.77), and low instances of cardiovascular death, broadly support a recent meta-analysis.20 They reported an elevated risk of cardiovascular events with NRT that was driven by less serious events such as tachycardia, but concluded that NRT did not appear to be associated with more serious cardiovascular events. A study using the The Health Improvement Network (THIN) general practice database, included 33,247 patients taking NRT, and investigated acute MI, acute stroke, and death for each patient during exposed and unexposed time periods. The authors reported that although the incidence increased before exposure and decreased after exposure to NRT in the period of 56 days before and after first NRT prescription, NRT was not associated with an increase in risk of MI, stroke, or death.21 This is in agreement with our data that during short periods of evaluation of 4 weeks, there is no difference in cardiovascular incidence after exposure to NRT. However, our study examined the long-term effects of NRT and found a higher risk of IHD and cerebrovascular disease. Nevertheless, GP practices may contribute patient data to both CPRD and THIN, resulting in overlap of some patients between databases. Information about which practices contribute to either database is not publicly available; therefore, specific methods to identify overlap need to be applied to the data to exclude these patients.
Furthermore, Hubbard et al21 used a within-patient comparison, which could account for the differences observed compared with the current study, as well as looking only at outcomes up to 8 weeks of treatment. A second database study of 663 smokers with acute coronary syndrome that compared NRT versus no NRT reported no differences after 1 year for death, MI, repeat revascularization, or rehospitalization for angina, congestive heart failure, or arrhythmia.26 However, the authors acknowledge, as a limitation of the study, a lack in the number of patients needed to achieve 90% power, which could explain the difference in findings between that and the current study. This highlights the need for larger longer studies to evaluate the long-term effects of NRT.
An explanation for the differences in all-cause mortality between treatment groups in the current study could be prescribing practices, with GPs preferentially prescribing NRT in patients with other smoking-related diseases, such as lung cancer. These patients could be more likely to be prescribed NRT, as this intervention has been shown to increase the likelihood of quitting by 50%–70%.10 An alternative explanation is that the NRT cohort consisted of heavier smokers with a greater illness burden, who therefore had higher all-cause mortality compared with those receiving advice only. However, this study was designed primarily to investigate the safety profile of NRT, and therefore treatment groups were not matched on other diseases. As such, we do not have cause of death information beyond that for IHD and cerebrovascular disease. Patients with other diseases were not excluded in order to include as wide a population as possible who were undertaking smoking cessation.
NRT is a widely used smoking cessation pharmacotherapy, owing to the low possibility of abuse and potential for dependence.27 Bupropion sustained release was the first nonnicotine pharmacological treatment approved for smoking cessation.28 Originally designed as an antidepressant in the US, it is the least prescribed smoking cessation treatment.11,13 There is evidence that bupropion is more effective than a placebo, but only weak evidence to suggest superiority over NRT.12 However, there is evidence of a potentially protective effect for the risk of major cardiovascular events with bupropion, although the reason for this is not well understood.20 Varenicline, a competitive nicotine receptor antagonist, has been available as a smoking cessation treatment since 2006.29 It has been suggested that varenicline may be more effective than NRT.29
Our study adds real-world data to the existing evidence from RCTs on the safety profile of NRT in patients with, or at a higher risk of, cardiovascular events. Our findings of increased cardiovascular events with NRT in high-risk patients indicate that further investigation is needed into both the safety of NRT and alternatives to NRT treatment in this group of smokers.
Strengths of the current study include the use of data on real-life patients from a large, high-quality source, the CPRD, which is well described and has previously been used in respiratory research.23 Furthermore, compared with many of the RCTs investigating NRT as a smoking cessation treatment, this study has a larger patient population and longer follow-up period.
Limitations
The current study has a number of limitations. Firstly, there is lack of data on over-the-counter (OTC) purchases, particularly in light of the fact that most NRT in the UK is used without advice, and purchased OTC rather than by prescription.30 However, there is evidence that most patients who use OTC NRT do not exceed the recommended time of 12 weeks,31 allowing us to infer that our secondary outcome period of 52 weeks is less likely to be affected by potential OTC use. On the other hand, the results indicate that the potential excess use of OTC NRT in the control group may be counteracting the observed difference between the groups. A further consideration is that patients taking NRT could potentially be exposed to elevated nicotine levels if they continue to smoke while taking NRT. However, owing to the flat dose–cardiovascular response relation for nicotine, the effects of cigarette smoking in addition to NRT are likely to be similar to those of smoking alone.32 Data on hospitalizations (emergency department attendance, inpatient admissions, and outpatient attendance) were limited because Hospital Episode Statistics–linked data were not available; as a result, hospitalizations were ascertained using information from GP records. There was no matching or adjustment for the severity of the smoker (cigarettes per day, pack years or Fagerstrom test), and thus, there is the potential that those in the NRT group were heavier smokers than those in the advice group. We did not analyze the time period of NRT use, and patients were only required to initiate on NRT to be included in the NRT group of the study. Our data did not provide information on the utilization of NRT, but only that it was prescribed, and we can only assume that NRT patients took the treatment as intended, making this an “intention to treat” analysis. We also lack information on details such as family history, a potentially influential factor as to whether a patient decides to seek cessation treatment with NRT. Furthermore, we do not know the rates of smoking cessation in each group; we can speculate that it was higher in the NRT group; however, this might not be the case. Compliance with NRT is likely to be higher in the prescription group as patients will have a clearer understanding of the benefit of the therapy owing to the advice of health care professionals. The Charlson comorbidity index (CCI) score was higher in the NRT group, which could lead us to assume that increased morbidity initially drove patients to seek treatment with NRT, and could therefore explain the worsened end points.
Confounding by indication may affect our results; GPs may assess patients with indications of poorer health, such as indicated by a high CCI score, or those with a higher risk of future poor outcomes, and prescribe NRT rather than advice. If higher risk patients were more likely to be given NRT, we may have overestimated the effect of NRT on outcomes. Confounding by indication is more of a concern in studies such as ours where initiators of treatment are compared with non-initiators as supported by Schneeweiss et al.33 A comparison between similar treatments would minimize this bias. The more similar the compared treatments are, the less potential there is for unmeasured confounding.
Matched analysis was conducted to achieve balance of covariates between the cohorts. Adjusted analysis was carried out to minimize confounding. However, as we did not randomly assign patients to either NRT or advice, some bias may remain.
Both smoking and nicotine treatment have been found to increase heart rate and blood pressure.34–37 The hemodynamic effects of smoking have been linked to nicotine, with heart rate found to increase with intravenous nicotine, nicotine nasal sprays, and nicotine chewing gum.38–40 Nicotine was found to affect coronary artery constriction even at doses as low as 4 mg.41 These effects cause an increase in myocardial work and oxygen demand and result in impaired blood flow and oxygen supply to the heart. However, transdermal nicotine was found to have a lesser acute hemodynamic effect than smoking.42 Although there are not much data available on the effect of transdermal nicotine on coronary blood flow, Benowitz et al42 suggest that transdermal nicotine in smoking cessation treatment of patients with coronary heart disease is likely to be safer than cigarette smoking.
Further observational research is needed to provide insights into the effect of NRT on cardiovascular events. Data on nicotine exposure, which could be extracted through linked pharmacy and GP data, would be insightful. Extending the outcome period to longer than 1 year would increase the event numbers and allow a greater window to observe any potential associations. It would also be beneficial to obtain hospitalization data to ascertain whether increases in consultation rates are limited to GP consultations or also applicable to hospitalizations. A sub-analysis by NRT product would add meaningful information in this space. Further investigation into the use of nonnicotine alternatives is needed. The present study lacked the numbers to conduct such an analysis.
Conclusion
Although treatment with NRT during a short period (4 weeks) does not appear to have an impact on cardiovascular risks, a longer follow-up period of 52 weeks resulted in an increase in cardiovascular events for patients prescribed NRT, compared with those receiving smoking cessation advice only. In view of the ongoing global public health risk of cigarette smoking, there is an urgent need to investigate the safest treatments available for patients attempting smoking cessation.
Acknowledgments
The authors would like to thank Julie von Ziegenweidt, Daina Lim, and Muzammil Ali, who assisted with the analysis. Many thanks to Alison Chisholm for contribution to the study design and critical review of the manuscript, Derek Skinner for preparation of data for analysis, Rosalind Bonomally, and Martina Stagno d’Alcontres for medical writing. The study data were provided by the CPRD without charge (via a Medical Research Council study grant). The analysis was conducted by the Observational and Pragmatic Research Institute Pte Ltd, in collaboration with the Respiratory Effectiveness Group (REG), and funded by the Observational and Pragmatic Research Institute Pte Ltd. Manuscript costs were covered by the REG.
Author contributions
All authors contributed to the study design and formulation of the research question, and reviewed the manuscript at all stages of drafting. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
JD was formerly employed at MSD, Pfizer, and GSK, and was a contractor with Teva and Gilead. JV has received honoraria from AstraZeneca, Boehringer Ingelheim, Chiesi pharmaceuticals, GlaxoSmithKline, and Novartis for advising and presenting, none of it related to smoking cessation products. AK is either on the advisory board or speakers bureau for Astra Zeneca, Boehringer Ingelheim, Griffols, GSK, Johnson and Johnson, Meda, Merck Frosst, Novartis, Pfizer, Purdue, and Teva. RJM was a consultant for Teva and AstraZeneca, has received travel support from REG, is on the Genentech and Boehringer Ingelheim advisory boards, and has received research grants from NHLBI and MedImmune. AB and JM were employed by Observational and Pragmatic Research Institute Pte Ltd, which receives funding from the UK National Health Service, British Lung Foundation, Aerocrine, AKL Ltd, AstraZeneca, Boehringer Ingelheim, Chiesi, Meda, Mundipharma, Napp, Novartis, Pfizer, REG, Takeda, Teva Pharmaceuticals, Theravance, and Zentiva. DBP has board membership with Aerocrine, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Meda, Mundipharma, Napp, Novartis, and Teva Pharmaceuticals; consultancy with Almirall, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Meda, Mundipharma, Napp, Novartis, Pfizer, Teva Pharmaceuticals, and Theravance; grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from the UK National Health Service, British Lung Foundation, Aerocrine, AKL Ltd, AstraZeneca, Boehringer Ingelheim, Chiesi, Meda, Mundipharma, Napp, Novartis, Pfizer, REG, Takeda, Teva Pharmaceuticals, Theravance, and Zentiva; payment for lectures/speaking engagements from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Meda, Merck, Mundipharma, Novartis, Pfizer, Skyepharma, Takeda, and Teva Pharmaceuticals; payment for manuscript preparation from Mundipharma and Teva Pharmaceuticals; payment for the development of educational materials from Mundipharma and Novartis; stock/stock options from AKL Ltd, which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd, UK, and 74% of Observational and Pragmatic Research Institute Pte Ltd, Singapore; received payment for travel/accommodation/meeting expenses from Aerocrine, AstraZeneca, Boehringer Ingelheim, Mundipharma, Napp, Novartis, and Teva Pharmaceuticals; funding for patient enrollment or completion of research from Chiesi, Novartis, Teva Pharmaceuticals, and Zentiva; and peer reviewer for grant committees of the Medical Research Council, Efficacy and Mechanism Evaluation program, and Health Technology Assessment. The authors report no other conflicts of interest in this work.
References
Horton R. GBD 2010: understanding disease, injury, and risk. Lancet. 2012;380(9859):2053–2054. | ||
World Health Organization. WHO Report on the Global Tobacco Epidemic: Enforcing Bans on Tobacco Advertising, Promotion and Sponsorship. Geneva, Switzerland: WHO; 2013. | ||
World Health Organization. Data and Statistics. Geneva, Switzerland: WHO; 2016. Available from: http://www.euro.who.int/en/health-topics/disease-prevention/tobacco/data-and-statistics. Accessed February 3, 2016. | ||
Health and Social Care Information Centre. Statistics on Smoking: England, 2014. West Yorkshire, UK: HSCIC; 2014. | ||
Ng M, Freeman MK, Fleming TD, et al. Smoking prevalence and cigarette consumption in 187 countries, 1980–2012. JAMA. 2014;311(2):183–192. | ||
World Health Organization. Prevalence of tobacco use. Global Health Observatory (GHO) data; 2016. Available from: http://www.who.int/gho/tobacco/use/en/. Accessed February 3, 2016. | ||
Eriksen MP. The Tobacco Atlas. 5th ed. Atlanta, GA: The American Cancer Society, Inc.; 2015. | ||
ASH. Smoking Statistics: Who Smokes and How Much. ASH; 2015. Available from http://ash.org.uk/information-and-resources/fact-sheets/smoking-statistics-who-smokes-and-how-much/. Accessed January 4, 2016. | ||
Raw M, McNeill A, West R. Smoking cessation guidelines for health professionals. A guide to effective smoking cessation interventions for the health care system. Health Education Authority. Thorax. 1998;53 (Suppl 5):S1–S19. | ||
Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;11:CD000146. | ||
West R, McNeill A, Raw M. Smoking cessation guidelines for health professionals: an update. Health Education Authority. Thorax. 2000;55(12):987–999. | ||
Woolacott NF, Jones L, Forbes CA, et al. The clinical effectiveness and cost-effectiveness of bupropion and nicotine replacement therapy for smoking cessation: a systematic review and economic evaluation. Health Technol Assess. 2002;6(16):1–245. | ||
Langley TE, Huang Y, McNeill A, Coleman T, Szatkowski L, Lewis S. Prescribing of smoking cessation medication in England since the introduction of varenicline. Addiction. 2011;106(7):1319–1324. | ||
Dacosta A, Guy JM, Tardy B, et al. Myocardial infarction and nicotine patch: a contributing or causative factor? Eur Heart J. 1993;14(12):1709–1711. | ||
Najem B, Houssiere A, Pathak A, et al. Acute cardiovascular and sympathetic effects of nicotine replacement therapy. Hypertension. 2006;47(6):1162–1167. | ||
Mathew TP, Herity NA. Acute myocardial infarction soon after nicotine replacement therapy. QJM. 2001;94(9):503–504. | ||
Ottervanger JP, Festen JM, de Vries AG, Stricker BH. Acute myocardial infarction while using the nicotine patch. Chest. 1995;107:1765–1766. | ||
Joseph AM, Norman SM, Ferry LH, et al. The safety of transdermal nicotine as an aid to smoking cessation in patients with cardiac disease. N Engl J Med. 1996;335(24):1792–1798. | ||
Mills EJ, Wu P, Lockhart I, Wilson K, Ebbert JO. Adverse events associated with nicotine replacement therapy (NRT) for smoking cessation. A systematic review and meta-analysis of one hundred and twenty studies involving 177,390 individuals. Tob Induc Dis. 2010;8:8. | ||
Mills EJ, Thorlund K, Eapen S, Wu P, Prochaska JJ. Cardiovascular events associated with smoking cessation pharmacotherapies: a network meta-analysis. Circulation. 2014;129(1):28–41. | ||
Hubbard R, Lewis S, Smith C, et al. Use of nicotine replacement therapy and the risk of acute myocardial infarction, stroke, and death. Tob Control. 2005;14(6):416–421. | ||
Williams T, van Staa T, Puri S, Eaton S. Recent advances in the utility and use of the General Practice Research Database as an example of a UK Primary Care Data resource. Ther Adv Drug Saf. 2012;3(2):89–99. | ||
CPRD [homepage on the Internet]. Available from: http://www.cprd.com/home/. Accessed July 18, 2016. | ||
Mukherjee M, Gupta R, Farr A, et al. Estimating the incidence, prevalence and true cost of asthma in the UK: secondary analysis of national stand-alone and linked databases in England, Northern Ireland, Scotland and Wales-a study protocol. BMJ Open. 2014;4(11):e006647. | ||
Tate AR, Beloff N, Al-Radwan B, et al. Exploiting the potential of large databases of electronic health records for research using rapid search algorithms and an intuitive query interface. J Am Med Inform Assoc. 2014;21(2):292–298. | ||
Woolf KJ, Zabad MN, Post JM, McNitt S, Williams GC, Bisognano JD. Effect of nicotine replacement therapy on cardiovascular outcomes after acute coronary syndromes. Am J Cardiol. 2012;110(7):968–970. | ||
West R, Hajek P, Foulds J, Nilsson F, May S, Meadows A. A comparison of the abuse liability and dependence potential of nicotine patch, gum, spray and inhaler. Psychopharmacology (Berl). 2000;149(3):198–202. | ||
Hurt RD. New medications for nicotine dependence treatment. Nicotine Tob Res. 1999;1 (Suppl 2):S175–S179; discussion S207–S210. | ||
Huang Y, Lewis S, Britton J. Use of varenicline for smoking cessation treatment in UK primary care: an association rule mining analysis. BMC Public Health. 2014;14:1024. | ||
Dobbie F, Hiscock R, Leonardi-Bee J, et al. Evaluating Long-term Outcomes of NHS Stop Smoking Services (ELONS): a prospective cohort study. Health Technol Assess. 2015;19(95):1–156. | ||
Shaw JP, Ferry DG, Pethica D, Brenner D, Tucker IG. Usage patterns of transdermal nicotine when purchased as a non-prescription medicine from pharmacies. Tob Control. 1998;7(2):161–167. | ||
Benowitz NL, Gourlay SG. Cardiovascular toxicity of nicotine: implications for nicotine replacement therapy. J Am Coll Cardiol. 1997;29(7):1422–1431. | ||
Schneeweiss S, Patrick AR, Sturmer T, et al. Increasing levels of restriction in pharmacoepidemiologic database studies of elderly and comparison with randomized trial results. Med Care. 2007;45(10 Suppl 2):S131–S142. | ||
Beere PA, Glagov S, Zarins CK. Retarding effect of lowered heart rate on coronary atherosclerosis. Science. 1984;226(4671):180–182. | ||
Benowitz NL, Kuyt F, Jacob P 3rd. Influence of nicotine on cardiovascular and hormonal effects of cigarette smoking. Clin Pharmacol Ther. 1984;36(1):74–81. | ||
Cellina GU, Honour AJ, Littler WA. Direct arterial pressure, heart rate, and electrocardiogram during cigarette smoking in unrestricted patients. Am Heart J. 1975;89(1):18–25. | ||
Groppelli A, Giorgi DM, Omboni S, Parati G, Mancia G. Persistent blood pressure increase induced by heavy smoking. J Hypertens. 1992;10(5):495–499. | ||
Benowitz NL, Jacob P 3rd, Jones RT, Rosenberg J. Interindividual variability in the metabolism and cardiovascular effects of nicotine in man. J Pharmacol Exp Ther. 1982;221(2):368–372. | ||
Benowitz NL, Porchet H, Sheiner L, Jacob P 3rd. Nicotine absorption and cardiovascular effects with smokeless tobacco use: comparison with cigarettes and nicotine gum. Clin Pharmacol Ther. 1988;44(1):23–28. | ||
Sutherland G, Russell MA, Stapleton J, Feyerabend C, Ferno O. Nasal nicotine spray: a rapid nicotine delivery system. Psychopharmacology (Berl). 1992;108(4):512–518. | ||
Kaijser L, Berglund B. Effect of nicotine on coronary blood-flow in man. Clin Physiol. 1985;5(6):541–552. | ||
Benowitz NL, Fitzgerald GA, Wilson M, Zhang Q. Nicotine effects on eicosanoid formation and hemostatic function: comparison of transdermal nicotine and cigarette smoking. J Am Coll Cardiol. 1993;22(4):1159–1167. |
Supplementary material
Confounding factors
Owing to the potential for confounding demographic and comorbid factors, initial analysis identified key baseline confounders, and the outcome analyses utilized appropriate statistical methods (logistical regression and matching), to minimize confounding.
Potential confounders examined at, or closest to, the index date were
Age
Sex
Height
Body mass index (BMI)
Potential confounders that were examined irrespective of when they occurred relative to the index date were confounding diagnoses including
Diabetes
COPD
Rhinitis
Hypertension
Cardiovascular disease
Ischemic heart disease (IHD, subset of cardiovascular disease)
Cerebrovascular disease (subset of cardiovascular disease)
Angina (subset of IHD)
Myocardial infarction (subset of IHD)
Other important unrelated comorbidities were expressed using the CCI. This was calculated on the basis of an algorithm of weighted Read code diagnosis codes in the year prior to the index date.
Potential confounders that were examined the year prior to the index date included cholesterol measurements
Blood pressure
Drug therapies
General practice consultations
Outpatient attendances
Inpatient admissions
Accident and emergency admissions
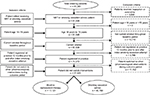  | Figure S1 Patient flow diagram for the unmatched NRT and smoking cessation advice cohorts. Abbreviation: NRT, nicotine replacement therapy. |
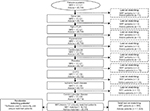  | Figure S2 Patient flow diagram for the matched NRT and smoking cessation advice cohorts. Note: Advice refers to smoking cessation advice. Abbreviation: NRT, nicotine replacement therapy. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




