Back to Journals » Journal of Experimental Pharmacology » Volume 13
Cardioprotective Effect of Crude Extract and Solvent Fractions of Urtica simensis Leaves on Cyclophosphamide-Induced Myocardial Injury in Rats
Authors Tesfaye BA , Berhe AH , Wondafrash DZ , Berhe DF
Received 6 October 2020
Accepted for publication 3 February 2021
Published 16 February 2021 Volume 2021:13 Pages 147—160
DOI https://doi.org/10.2147/JEP.S270038
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bal Lokeshwar
Bekalu Amare Tesfaye, Abera Hadgu Berhe, Dawit Zewdu Wondafrash, Derbew Fikadu Berhe
Department of Pharmacology and Toxicology, School of Pharmacy, Mekelle University, Mekelle, Ethiopia
Correspondence: Derbew Fikadu Berhe Email [email protected]
Background: Globally, cardiovascular diseases (CVDs) are becoming the major cause of death. Urtica simensis is one of endogenous plant which treats a wide range of disease conditions including heart diseases. However, there is limited information on safety and efficacy of the plant.
Objective: To evaluate the in vitro antioxidant, the in vivo cardioprotective activity of crude extract and solvent fractions of Urtica simensis leaves on cyclophosphamide-induced myocardial injury.
Methods: The cardioprotective activity of the crude extract, aqueous and hexane fraction of Urtica simensis leaves was evaluated based on anatomical, biochemical and histopathological methods. The in vitro antioxidant activity of the plant was also assayed in terms of free radical scavenging activity (RSA).
Results: Crude extract and solvent fractions of Urtica simensis significantly prevented the deleterious effect of cyclophosphamide on body weight (P< 0.001), heart weight to body ratio (P< 0.01), cardiac biomarkers including troponin I (P< 0.01), alanine transaminase (ALT) (P< 0.001), aspartate aminotransferase (AST) (P< 0.01) and lipid profiles including triglycerides (P< 0.001) and total cholesterol (P< 0.01). The histopathological study confirmed presence of necrosis, oedema and haemorrhage on cyclophosphamide alone-treated groups while the 200mg/kg and 400mg/kg of the crude extract and aqueous fraction showed normal cardiocytes. The antioxidant assay of Urtica simensis plant exhibited free radical scavenging activity of inhibitory concentration of 50% (IC50) for the crude extract with the values of 63.27μg/mL, aqueous fraction with the values of 136.38μg/mL and hexane fraction with the values of 258.70μg/mL.
Conclusion: Crude extract and solvent fractions of Urtica simensis leaves have cardioprotective activities. The cardioprotective effect could be attributed to the antioxidant activity of the plant extracts. However, this requires further in-depth understanding.
Keywords: cardioprotective, cardiac biomarkers crude extract, cyclophosphamide, solvent fractions, Urtica simensis
Introduction
Cardiovascular diseases are the major global concerns as there is a sharp rise in prevalence rate in the last two decades.1–3 Globally, 31% of world populations died of CVDs which is an estimated 17.9 million people. More than 75% of CVDs deaths occur in developing countries that have limited access to medication. Stroke and heart attack were responsible for four out of five CVDs deaths.4,5 In 2017, nearly 10.9% of all deaths in Ethiopia were caused by CVDs.6 Even the available medications have the number of side effects and efficacy problems.
The loss of muscular or nervous function of the heart including myocarditis, ischemia and degeneration called myocardial injury.7 Acute myocardial injury may occur in a variety of cardiac and non-cardiac illnesses as a consequence of myocardial oxygen supply-demand mismatch. A direct or indirect injury is occurred by infection, acute left ventricular failure and anticancer drugs.8,9 Myocardial injury is developed to MI if the ischemia persists for greater than 20 minutes. In addition, myocardial injury sometimes associated with a proinflammatory and prothrombotic state due to embolization of platelet aggregates and thrombus from silent vulnerable plaque.7
The cardiotoxicity effects of cyclophosphamide consist of acute, dose-dependent cardiac damage morphologically characterized by necrosis, haemorrhage, oedema and consequently the fibrosis of myocytes which usually occurred within 10 days of administration.10–12
In the market, several classes of medicines and their combinational drugs are available to treat CVDs.13 However, overall treatment is expensive and causes side effects from minimal to fatal outcomes.14 For centuries, the nettle plants have been used in folk medicine to cure a wide range of ailments including inflammatory diseases and chronic illness.15
Urtica simensis is an endemic Ethiopian plant that belongs to the nettle plant family called Urticaceae. Urtica simensis has unpleasant stinging hairs which are located under the stems and lower leaf surface. The whole plant is covered with stinging hairs. The plant is one meter tall, dioecious, erect and non-branched herbal nettles. The leaves are opposite simple, stipules fused and interpetiolar 0.5–1 cm long. The leaves bases are slightly cordate, the apex broadly acute and the margins of the leaves are also serrated. The flowers Urtica simensis are unisexual and regular, while the fruit is about 2 mm long.16,17
The plant grows mostly in the northern part of Ethiopia commonly known in local language as Amei’e (Tigrigna)18 and Samma (Amharic).19 Various ethnobotanic reported that different parts of Urtica simensis are used for the treatment of gonorrhoea, wound infections, stomach ache and heart diseases in Ethiopia.20–22
Despite its use as food and traditional medicine to treat different diseases including cardiac illness, pharmacological investigation into its cardioprotective effect has not been carried out. This study set out to partly address the gap by using cyclophosphamide-induced rat myocardial injury model.
Materials and Methods
Chemicals and Instruments
Analytical grade chemicals and solvents were used. Cyclophosphamide (cycloxan, India), active ingredient enalapril (Ethiopia), ascorbic acid (India), ethanol (Alpha Chemika, Mumbai, India) (France), cotton gauze (Ethiopia), hexane (Carlo Erba Reagents SAS, Val de Reuil, France), DPPH ((2, 2-diphenyl-2-picrylhydrazyl) (Germany)), 10% formalin (Albert Rose Chemicals IP Ltd, Ahmedabad, India), ethyl acetate (India), distilled water, normal saline 0.9%, paraffin wax, xylene, hematoxylin, and eosin were purchased from local suppliers in Ethiopia.
Collection, Identification, and Preparation of Plant Materials
The Urtica simensis leaves were collected from Farta Woreda South Gondar Zone of Amhara Regional State, which is 660 kilometres north of Addis Ababa. The plant was then identified and authenticated by a botanist and the sample specimen was deposited in Herbarium unit of Department of Biology, College of Computational and Natural Science, the University of Gondar for future reference with voucher number of 001BAT/2011.
Experimental Animals
The study was conducted with male SD rats (weight 335–402g). The rats were bought from the Ethiopian public health institute. All the animals were kept in a plastic cage. The rats were fed rodent pellets, water ad libitum and acclimatized for one week to the experimental environment. The care and handling of animals were according to international guidelines for the use and maintenance of experimental animals (OCED, 2008).
The study was approved by the Health Research Ethics Review Committee (HRERC) at College of Health Sciences, Mekelle University with reference number of ERC1535/2018.
Methods
Crude Extraction of Plant Material
The collected Urtica simensis leaves were gently washed with tap water to eliminate any dead matter and other contaminants. The leaves were then air-dried under the shade at room temperature of the laboratory of the Department of Pharmacology and Toxicology, School of Pharmacy, Mekelle University. The dried leaves were then pulverized with a grinder to a coarse powder. A coarsely powdered plant leaves from Urtica simensis were weighed and extracted using soxhlet apparatus with 70% ethanol as a solvent for 48 hours at 80°C in one to five solvent-to-powder ratios. The filtrate was then dried in an oven at a temperature of 40°C. The heat-dried extract was kept in a desiccator and then stored in a refrigerator until further use.
Solvent Fractionation
Separatory funnel was used for the procedure of solvent-solvent fractions. A total of 110g of the Urtica simensis leaves powder of crude extract was dissolved in 550 mL of distilled water. Then, extraction was performed successively using solvents of increasing polarity, starting from 550mL hexane three times then the same amount of ethyl acetate three times. After collecting the hexane and ethyl acetate fractions the remaining residue was an aqueous fraction.
The fractions were then concentrated using oven dryer at 40°C. Airtight container wrapped with the aluminum foil was used to keep the dried powder and stored in a refrigerator at 4°C until further use.
Percentage Yield of Crude Extract and Solvent Fractions of Urtica simensis
The Percentage Yield of the Crude Extract
% Yield= (Weight of extracts obtained)/(Weight of powder used for extraction) ×100.
The Percentage Yield of the Fractions
% Yield= (Weight of fractions obtained)/(Weight of crude extracts used) ×100.
Acute Oral Toxicity Test
Acute oral toxicity of the crude extract of Urtica simensis and the solvent fractions were evaluated in five healthy, nulliparous and non-pregnant female SD rats. The weight of the rats was from 300 to 320g of age two months as per the organization for economic cooperation and development (OECD) of 425 guidelines. The different doses of extracts were assessed based on fasting body weight and volume administered was determined based on OECD guideline that states 1 mL/100 g of body weight of the animal. Accordingly, a single oral dose of 2000mg/kg extract was given for a single female rat (after overnight fasting); after administration of drug orally using oral gavage, the animal was supervised individually for the first 4 hours then periodically follow up during the first 24 hours. The first rat was survived and the study was repeated on the rest four female rats; with each rat observed for 14 days to assess the safety of the extracts. The observation included gross changes such as loss of appetite, hair erection, lacrimation, tremors, convulsions, salivation, diarrhea, and death.23
In vitro Antioxidant Activity
Free radical scavenging activity was evaluated by using a method of DPPH assay24 (Shen et al, 2010). A three mL of extracts with five different concentrations range from 12.5µg/mL to 200µg/mL was mixed with one mL of 0.1mM DPPH in 95% ethanol solution and the mixture was kept in dark and cool place for 30minutes. With UV spectrophotometry, the absorbance of the control, each extract and ascorbic acid solutions at 517 nm were recorded against blank solution (95% ethanol). The process was triplicated for each concentration.
The DPPH radical reduction was calculated as the following equation.
RSA (%) =((Ao-A1))/A0 X 100
Where A0 = control absorbance (DPPH with 95% ethanol), A1 is the absorbance of the sample/reference.
The average values of triplicate numbers from reading obtained were plotted against concentration (μg/mL) of sample dilutions and final results were expressed as IC50 values (concentration of samples required to scavenge 50% of DPPH radicals).24
Grouping and Dosing of Animals
Cardioprotective activity of the crude and solvent fractions of Urtica simensis leaves were assessed on SD rats with cyclophosphamide-induced myocardial injury. SD rats (n = 60) weighing from 335 to 402gram randomly divided into three controls and seven treatment groups with six rats each. Treatment group rats were weighed and pretreated daily with respective test extracts. The calculated test doses were Urtica simensis leaves extracts of 100,200 and 400mg/kg and two fractionated doses (200 and 400mg/kg) diluted in 2% tween 80. Treatment duration was 10 days then after on 11th-day rats were injected cyclophosphamide 200mg/kg via i.p.
For the comparison, there were additional three groups: one for the positive control (EN 10 mg/kg, diluted in 2% tween 80, daily po for ten days before the cardiac injury at 11th day by cyclophosphamide 200mg/kg i.p), another for negative control (the group that was not treated with any drug except the infliction of cardiac injury by cyclophosphamide 200mg/kg) and a third for normal control (1mL/100g of 2% tween 80 was administered for 10 days). All treatment doses and vehicles were given to SD rats based on their daily calculated weight.
Collection of Blood Samples and Plasma Preparation
After 11 days of treatment and injury infliction, on the final day (day 12) of treatment, blood was collected from the retro-orbital plexus of the animals into heparinized tubes after giving ketamine 100mg/kg i.p. The plasma was prepared by centrifuging blood samples for 15 min at 3500 rpm using a bench centrifuge. The clear supernatant was used for the estimation of troponin I, AST, ALT and lipid profiles using BTS-350 semi-automatic analyzer.25,26
Determination of Cardiac Marker Enzymes
4mL of blood samples were taken and plasma was separated by centrifugation at 3500 RPM for 5 minutes. The level of troponin I,27 AST and ALT were measured using BTS-350 semi-automatic analyzer.28
Lipid Profiles
2mL of plasma total cholesterol and triglycerides were analyzed and compared to each experimental group.10
Surgical Removal of the Heart
The rats were sacrificed by cervical dislocation. The thoracic cavity exposed after the diaphragm of the SD rats cut by a trans-abdominal incision. Then, the thorax was cut open on both sides following cartilage attachment to ribs and the heart was exposed, elevated by cradling it gently in the fingertips to avoid contusion injury. Immediately after excision, the heart was placed into a beaker containing a normal saline 0.9% solution for washing purposes. After washing, the heart was put on the filter paper to remove moisture and then the heart was weighed for calculating relative heart weight to body weight. The heart was then collected in a container containing 10% formalin and transferred to deep freeze (−80°) until further experiment.29
Measurement of Body Weight and Heart Weight
Each SD rat of body weight and heart weight was measured by the digital weighing balance to assess daily weight gain or loss.27
Heart Weight to Body Weight Ratio
To calculate the heart weight to body weight ratio, the heart weight was divided into the bodyweight of the rat. The values were expressed in g/g.30
Histopathological Studies
The heart of each rat was preserved in a 10% formalin solution for fixation. Then, a portion of heart tissue was taken, dehydrated in different grades of ethanol (40%, 70%, 80%, 95%, and 100%) and cleared with xylene using an automatic processing machine. Additionally, xylene was cleared by paraffin wax using an automatic tissue processing machine. Then, the tissues were embedded with paraffin wax and blocked in the coronal plane. Section of 4–5μm thickness of the tissue was made using microtome and stained with hematoxylin and eosin dye and histological observations were made under a light microscope.31
Statistical Analysis
Data entered and analyzed with SPSS version 22. The data obtained in the study were tabulated and expressed as mean ± standard errors of the mean (SEM). The statistical analysis was carried out using one-way analysis of variance (ANOVA) followed by Tukey post hoc test to compare variations among groups, where P-value <0.05 was considered as statistically significant.
Results
Percentage Yield of the Urtica simensis Extract and Solvent Fractions
For the crude extraction of the plant, 1.2 kilograms of Urtica simensis leaves powder was used. The actual yield of the plant was 150grams. 12.5% (w/w) was the percentage yield of the plant. The crude extract colour was dark green. The fine powder was formed after drying in an oven and then, homogenization was done using mortar and pestle.
The percentage yield of each solvent fraction was also determined. There was variation in the colour of the fractions as shown in Table 1.
 |
Table 1 Percentage Yield and Physical Properties of Crude Extract and Solvent Fractions of Urtica simensis |
Acute Oral Toxicity Study
Acute oral toxicity study revealed that 70% ethanolic extract and solvent fractions of Urtica simensis leaves at a dose of 2000 mg/kg did not produce any behavioral changes including activity, hair texture, pupil size, and feeding. Death was not observed at the limit dose of the extract. Therefore, the lethal dose 50 (LD50) of the extracts was considered to be greater than 2000 mg/kg.
In vitro Antioxidant Activity Assays
The crude extract showed the highest free radical scavenging activity with a 50% inhibition concentration of 63.2µg/mL in comparison to the aqueous fractions (136.38µg/mL) and hexane fractions (258.7µg/mL). Hexane fractions produced weak antioxidant activity in comparison to the other extracts and ascorbic acid as showed in Figure 1. The maximum percentage of inhibition was observed at high concentration of ascorbic acid (86%) followed by the crude extract (76.07%), aqueous fractions (60.67%) and hexane fractions (39.76%) at the maximum concentration of 200µg/mL (Table 2).
 |
Figure 1 DPPH scavenging activity of ascorbic acid and Urtica simensis crude extract and solvent fractions % RSA-percentage radical scavenging activity, IC50-50% inhibition concentration. |
 |
Table 2 DPPH Scavenging Activity of Crude Extract and Solvent Fraction of Urtica simensis |
Cardioprotective Activity of the Crude Extract and Solvent Fractions of Urtica simensis Leaves
Effect of the Crude Extract and Solvent Fractions of Urtica simensis on the Rats’ Body Weight
Rats administered with cyclophosphamide showed a significant reduction in final body weight relative to initial weight compared to the normal control group (P<0.01) Table 3. Moreover, the 100mg/kg, 200mg/kg crude extract, all the administered doses of aqueous and hexane fractions showed that a significant weight loss compared to the normal control group (P<0.05). However, pretreatment of the rats with the standard drug (EN 10mg/kg) and 400mg/kg crude extract of Urtica simensis weight loss was insignificant compared with the normal control group.
 |
Table 3 The Influence of Plant Extracts on Body Weight |
Effect of the Crude Extract and Solvent Fractions of Urtica simensis on Heart Weight to Body Weight Ratio
Compared to the normal control group, cyclophosphamide administration caused a significant increase in heart weight to body weight ratio (P<0.001), Table 4. However, pretreatment of the rats with standard drug, all the doses of crude extract and solvent fractions of Urtica simensis leaves showed a significant decrease in heart weight to body weight ratio compared to the cyclophosphamide-treated group (P<0.001).
 |
Table 4 The Effect of Crude Urtica simensis and Solvent Fractions on Heart Weight to Body Weight Ratio |
Effect of the Crude Extract and Solvent Fractions of Urtica simensis on Cardiac Biomarkers
As shown in Table 5 troponin I level was significantly increased among the rats administrated with cyclophosphamide compared to the normal control group (P<0.01). However, pretreatment of the rats with the 200mg/kg dose of the crude extract and all doses of aqueous fractions of Urtica simensis decreased troponin I level significantly (P<0.05). Besides, the EN-treated and 400mg/kg crude extract reduced the troponin I level significantly (p<0.01).
 |
Table 5 Effect of Crude Extract and Solvent Fractions of Urtica simensis on Cardiac Biomarkers |
An elevation in plasma ALT value was noticed in the cyclophosphamide-treated group compared to the normal control group (P<0.001). The EN, crude extract and solvent fractions reduced plasma ALT value at all the administered doses relative with the negative control group (P<0.001). Nevertheless, the 200mg/kg and 400mg/kg hexane fraction treated rats showed an elevated ALT level as compared with the EN-treated and normal control group (P<0.05).
The plasma AST value was elevated in cyclophosphamide-administered group relative to the normal control group (P<0.01). The crude extract of 400mg/kg and the EN-treated group reduced the plasma AST level in comparison with the negative control group (P<0.01). In addition, there is a decreased plasma AST level in the 200 and 400mg/kg aqueous fraction in comparison with the negative control group (P<0.05).
Effect of the Crude Urtica simensis and Solvent Fractions on Lipid Profiles
Triglycerides were increased significantly among the rats administered with cyclophosphamide relative to the normal control group (P<0.001). However, the EN-treated, crude extract and an aqueous fraction of Urtica simensis administered at a dose of 200 and 400mg/kg showed a decreased level of triglyceride (P<0.001) compared to the cyclophosphamide-treated group (Table 6). Furthermore, the 100mg/kg crude extract and the 200 and 400mg/kg hexane fractions also reduced the triglycerides level in relative to the cyclophosphamide-treated group of P<0.01 and P<0.05, respectively.
 |
Table 6 Effect of Crude Extract and Solvent Fractions of Urtica simensis on Lipid Profiles |
An elevation in plasma total cholesterol value was observed in the cyclophosphamide-treated compared with the normal control group (P<0.01). However, the crude extract and aqueous fractions reduced plasma total cholesterol value at 400mg/kg administered doses in comparison to the toxic group (P<0.01). The EN-treated group, 200mg/kg crude and aqueous extracts reduced the plasma total cholesterol with P<0.05 level of significance. On the other hand, the hexane fraction treated groups are also showed total cholesterol reduction though it was insignificant statistically.
Histopathological Finding
The cardioprotective activity of the crude extract and solvent fractions of Urtica simensis leaves were confirmed by the histopathologic examination of the cardiac tissues of control and treated animals. As showed in Figure 2, the cardiac tissues in the normal control group showed normal morphological architecture with no cellular necrosis, interstitial space oedema and haemorrhage (A). However, the cardiac tissues of rats administered with only cyclophosphamide showed necrotic cardiocytes, haemorrhage and oedema (B). The histopathologic examination of animal groups treated with the 100mg/kg of crude extract and 200 and 400mg/kg of hexane fractions revealed that there were more necrotic heart tissues and some hemorrhagic cells compared to the rats found in the normal control group. However, the cardiac tissues of the rats treated with EN, 200 and 400mg/kg of crude extract and aqueous fractions of the plant showed that there were more normal cardiocytes and regeneration of cardiac cells were observed.
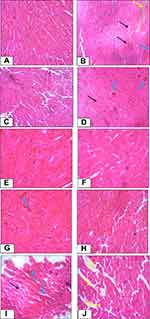 |
Figure 2 Histopathological changes of cardiac tissue of control and experimental rats. |
Discussion
To our best knowledge, this is the first report, which aimed to study the cardioprotective effect of Urtica simensis leaves extracts on cyclophosphamide-induced MI in an experimental animal model.
The major findings for this study were the level of cardiac enzymes (troponin I, ALT and AST) and lipid profiles including triglycerides and total cholesterol elevated on cyclophosphamide-treated groups while the crude and solvent fractions of Urtica simensis especially aqueous fractions treated groups reversed the problem. In addition, cyclophosphamide treatment caused the body weight reduction and heart weight elevation, which was expressed as heart weight to body weight ratio elevation and the problem was tackled by all the plant extract and solvent fractions. This finding was further consolidated by histopathological results which showed the deleterious effect of cyclophosphamide on heart tissue and the problem was improved in the plant extract and aqueous fraction treated groups. In addition, the crude extract showed the highest IC50 value followed by the aqueous and hexane fraction of Urtica simensis based on DPPH assay method.
The antioxidant activity of the Urtica simensis was done based on the DPPH assay method. This assay is the widely used method to determine the radical scavenging activity of different plant samples due to its stability, simplicity and fast process.32 The DPPH is a purple colored, stable free radical having maximum absorbance at 517 nm. In the presence of free radical scavenger, there is a reduction in the absorbance measurement and color change of DPPH from purple to light yellow. The extent of reduction in absorbance measurement is an important indicator of the free radical scavenging power of the extracts. The different extracts and fractions were compared for their antioxidant activity based on their IC50. The lower IC50 value indicates that the extracts have high radical scavenging activity.24
The crude extract and solvent fractions of the plant showed a dose-dependent free radical scavenging activity; by which the crude extract showed a strong antioxidant activity with IC50 of 63.27µg/mL compared to the aqueous and hexane fractions. The antioxidant activity of the crude extract of Urtica simensis is much better than that of aqueous and hexane fractions. A similar finding was also stated in another study.33 The antioxidant activity may be related to the presence of secondary metabolites including flavonoids, polyphenols, and tannins. These similar reasons were also reported in Karou et al; Patel Chirag et al; Seifu et al; Enayati et al34–37 who reported the free radical scavenging activity of these secondary metabolites. The variation in antioxidant activity of the plant extracts could be due to a difference in the amount and kind of phytochemicals present in the crude extract and solvent fractions of the Urtica simensis. Thus, the highest free radical scavenging activity of the crude extract could be due to the ability of the solvent (70% ethanol) to extract non-polar, medium polar and polar phytochemicals that can act synergistically. This reason is also stated in other studies in which the highest free radical scavenging activity was associated with the presence of polar and non-polar components in the plant of crude extract.37 The hexane fractions exhibited the lowest antioxidant activity with IC50 of 258.7µg/mL compared with ascorbic acid and other extracts. The possible reason might be due to the presence of low level of phenolic compounds in the hexane fractions, and this is in good line with the Khan et al (2012) study.38 Previous studies have reported that the secondary metabolites exhibit to antioxidant, anti-inflammatory antibacterial and anti-diabetic activity.39,40
In the present study, there was a significant loss in the final body weight relative to the initial weight in the cyclophosphamide-treated group when compared with the normal control group. In addition, experimental groups, except that treated 400mg/kg crude extract and standard drug, significantly reduced their weight compared with normal control groups. The weight loss observed in the present study may be the cyclophosphamide harms the appetite center in the hypothalamus or gastrointestinal tract which causes anorexia in the experimental rats. However, groups which administered the standard drug and 400mg/kg did not show statistically significant weight reduction relative to the control groups. This could be related with the abundant presence of phenolic compounds in 400mg/kg crude extract gave it the potential for best RSA activity which in return challenge the deleterious effect of cyclophosphamide on appetite.27,41
This study also found that the administration of cyclophosphamide produced a significant increase in the relative heart weight to body weight ratio of the rats when compared with the normal control group. This might be due to the direct or indirect participation of cyclophosphamide-metabolites especially acrolein which causes damage directly or indirectly via oxidative stress to the heart tissue leading to cytoplasmic vacuolization, myocyte disruption oedema and fibrosis.27,41 Nevertheless, the crude extract and the solvent fractions of Urtica simensis have significantly decreased heart weight to body weight ratio in all treatment groups compared to the negative control group. Accordingly, similar results were reported in preceding.27,41,42
The plasma troponin I, AST and ALT levels are one of the basic parameters for detecting cardiac injury; however, troponin I is most sensitive and specific to the heart. These cardiac biomarkers were elevated during myocardial insult. The reason is that these enzymes were abundantly found in the heart and were leaked into the blood as a result of membrane degradation and disruption of cardiac muscle cells. Thus, the increase in cardiac enzymes is significant indications of cellular leakage and loss of functional integrity of the cell membrane of the heart.11,43,44
It is known that apart from cardiac injury, AST and ALT are also released in the response to liver injury. However, AST tends to be more specific to heart injury, whereas ALT is more specific to liver damage. Thus, it is vital to calculate the ratio of AST and ALT to know which organ is the more pertinent source. In heart muscle injury, the AST level is higher than ALT and the AST/ALT ratio will be more than one.45 In the current study, the troponin I, AST and ALT level were significantly increased among the rats administrated with cyclophosphamide compared to the normal control group. Most importantly, AST/ALT of cyclophosphamide-treated group was greater than one which implied the damage was more pronounced on the heart than the liver. The high levels of cardiac enzymes in the plasma were seen to return to the nearly normal profile after treatment with the crude extract and solvent fractions of Urtica simensis. Pretreatment of the rats with the EN, crude extract and solvent fractions of Urtica simensis leaves halted the toxic effect of cyclophosphamide on the heart by reducing the elevated levels of these enzymes.
Hence, suppression of the elevated plasma levels of troponin I, AST and ALT by the crude extract and solvent fractions especially the aqueous fraction of Urtica simensis towards the respective normal value is an indication of stabilization of plasma membrane as well as repair of cardiac tissue damages caused by cyclophosphamide. The secondary metabolites of this plant including polyphenols, flavonoids, and tannins might help to maintain membrane integrity, thereby limiting the leakage of these enzymes. This finding is consistent with what has been found in previous studies by Asiri, (2010); Şekeroğlu et al; Afroos et al; Omole et al27,41,46,47, which showed that the cardioprotective activity of the plant extract is due to plasma membrane stabilization effect and repair of cardiomyocyte damage.
The elevation of total cholesterol and triglycerides plasma levels in the cyclophosphamide-treated group indicate cyclophosphamide may be interfering with biosynthesis or metabolism of lipids. Cyclophosphamide impaired secretion of heart lipoprotein lipase (LPL) which results in an elevation of total cholesterol and triglycerides levels of lipid markers. Lipoprotein lipase is an enzyme which degrades triglycerides to fatty acid.10,43,47,48 Treatment with Urtica simensis crude and aqueous extracts showed a reduction in plasma lipid profile levels in a dose-related fashion. The lipid-lowering effect of this plant may be due to the secondary metabolite of polyphenols that can bind with bile acids to increase their excretion, inhibit hepatic cholesterol biosynthesis and inducing LPL enzyme. However, the hexane fractions showed a weak activity regarding total cholesterol reduction. This may indicate that the active constituents of the plant are polar compounds which could not extract by the hexane solvent. These findings agreed with the ideas of Sudharsan et al; Afroos et al; Paul et al and Tsegaye et al40,41,49,50.
The activity of the crude extract and solvent fractions of Urtica simensis on biochemical results were also consolidated by the histopathologic findings. The section of the normal control rats’ heart showed normal heart architecture while the section cyclophosphamide-treated group presented cardiac necrosis, interstitial oedema and haemorrhage of the myocardium. This may be a result of the formation of free radicals and oxidative stress induced by cyclophosphamide-metabolites including acrolein. The haemorrhage, oedema and necrosis were also seen in the 100mg/kg crude extract and all doses of hexane fractions. However, these severe pathological changes were lesser in the rats administered with the different doses of the crude extract, aqueous fractions and the standard drug followed by administration of cyclophosphamide. This indicates that pretreatment of the rats with the plant extracts, aqueous fraction and EN has the potential to prevent cardiotoxicity caused by cyclophosphamide. Therefore, the histopathologic findings revealed protective activity of the crude extract and aqueous fractions of Urtica simensis against cyclophosphamide-induced MI which is in good line with the results of the preceding studies.42,43,51,52
Sharma et al (2001) explained that the antioxidant activity of the plant extracts might be one of the possible mechanisms for their cardioprotective activity; and their antioxidant activity can be explained by direct trapping of the oxygen species, chelation of transition metals involved in the process of free radical formation and prevention of the peroxidation process by reducing ROS including hydrogen peroxides, superoxide radicals, and alkoxy radicals.53
In the previous study, preliminary phytochemical screening of the crude extract and solvent fractions of Urtica simensis revealed the presence of flavonoids, polyphenols, and tannins.39 Therefore, the cardioprotective activity of the crude extract and solvent fractions of Urtica simensis may be due to the presence of phytochemicals such as polyphenols, alkaloids, flavonoids, polyphenols and tannins which posses’ cardioprotective activity either alone or in combination. These findings are in accordance with other previous studies that showed the cardioprotective activity of these phytoconstituents.14,37,54,55
The mechanism of these phytochemicals for their cardioprotective activity is mainly due to their free radical scavenging activity; as free radicals are responsible for oxidative stress and cardiac injury in cyclophosphamide-induced cardiotoxicity. Besides, phenolic compounds and flavonoids inhibit low-density lipoprotein peroxidation, platelet aggregation and maintain cell membrane stability or could protect leakage of the cell membrane due to damage by cyclophosphamide-metabolites as evidenced by a reduction in the cardiac biomarkers. These findings collaborate with Adegbola et al (2017) and Cook and Samman, (1996) studies.56,57 Moreover, the anti-inflammatory effect of the secondary metabolites including polyphenols, tannins and flavonoids could be the meant for their cardioprotective activity. This reasoning is also stated with other studies which have shown the anti-inflammatory activity of these phytochemicals.57–59
The anti-inflammatory activity of the plant extracts can be also one of the probable mechanisms as cyclophosphamide-toxicity produces inflammatory mediators such as monocytes, neutrophils and cytokines (interleukin-6 and tissue necrosis factor alpha). This explanation is similar in other studies, in which anti-inflammatory activity could be the possible mechanism of medicinal plants for their cardioprotective activity in cyclophosphamide-induced cardiac injury.60
This study is the first report on the cardioprotective effect of Urtica simensis extracts on cyclophosphamide-induced MI which can be considered as the main strength of the study. However, the limitations of this study were that the MI inducing drug, cyclophosphamide has the potential to damage organs other than the heart. Since the designed experimental model was protective, treatment model can be done for the future.
Conclusion and Recommendation
The results of the present study revealed that the crude extract and solvent fractions of Urtica simensis have significant cardioprotective activity in most parameters with a dose-dependent manner. The crude extract has the most significant protective effect against deleterious effect of cyclophosphamide in body weight, heart weight, lipid profiles and cardiac biomarkers followed by the aqueous fraction. The hexane fraction was the least active one. The cardioprotective effect might possibly be mediated by the antioxidant polyphenolic components (tannins and flavonoids) of the leaves. However, Isolation of the compounds is recommended to identify the specific phytoconstituents that were responsible for the cardioprotective activity of Urtica simensis crude extract and fractions. The finding should be substantiated by more relevant models that could inflict direct cardiac damage; including ex vivo ischemia/reperfusion model and endogenous antioxidant enzymes test. Moreover, proteomic analysis is important to investigate the protein expression level.
Abbreviations
CVD, cardiovascular diseases; DPPH, 2, 2-diphenyl-1-picrylhydrazyl; EN, enalapril; IC50, inhibitory concentration 50%; LD50, lethal dose 50%; LPL, lipoprotein lipase; MI, myocardial infarction; OECD, Organization for Economic Cooperation and Development; ROS, reactive oxygen species; RSA, radical scavenging activity; SD, Sprague Dawley.
Data Sharing Statement
The original data used to support the findings of this study are available from the corresponding author upon request.
Acknowledgments
We are most grateful to Dr. Henok Desalegn for reading and interpretation of histopathological findings. We would also like to acknowledge Mekelle University, Mekelle, Ethiopia for financial support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Mr Abera Hadgu Berhe reports grants from Mekelle University, outside the submitted work. The authors report no other potential conflicts of interest in this work.
References
1. Parikh H, Tripathi CB, Shah P, Pharm VGM, Goyal RK. Investigation of the cardioprotective effects of Crataegus oxycantha and its molecular mechanism. Curr Res Cardiol. 2015;2(4):161–167.
2. Patil SS, Naikwade NS, Shikalgar TS. The cardioprotective effect of argemone mexicana on isoproterenol induced cardiotoxicity in rats. Eur J Pharm Med Res. 2018;5(6):367–375.
3. Thounaojam MC, Jadeja RN, Karn SS, et al. Experimental and toxicologic pathology cardioprotective effect of Sida rhomboidea. Roxb extract against isoproterenol induced myocardial necrosis in rats. Exp Toxicol Pathol. 2011;63(4):351–356. doi:10.1016/j.etp.2010.02.010
4. WHO. Cardiovascular Diseases in the world; 2019. Available from: https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds).
5. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146–e603. doi:10.1161/CIR.0000000000000485
6. Ali S, Misganaw A, Worku A, et al. The burden of cardiovascular diseases in Ethiopia from 1990 to 2017: evidence from the Global Burden of Disease Study. Int Health. 2020;1–9. DOI:10.1093/inthealth/ihaa069
7. Chapman AR, Adamson PD, Mills NL. Assessment and classification of patients with myocardial injury and infarction in clinical practice. Heart. 2017;103(1):10–18. doi:10.1136/heartjnl-2016-309530
8. Mythili Y, Sudharsan PT, Varalakshmi P. Lipoic acid ameliorates cyclophosphamide induced cardiac mitochondrial injury. Toxicology. 2005;215:108–114. doi:10.1016/j.tox.2005.07.001
9. Frencken JF, Donker DW, Spitoni C, et al. Myocardial injury in patients with sepsis and its association with long-term outcome. Circ Cardiovasc Qual Outcomes. 2018;11(2):1–9. doi:10.1161/CIRCOUTCOMES.117.004040
10. Chakraborty M, Bhattacharjee A, Kamath JV. Cardioprotective effect of curcumin and piperine combination against cyclophosphamide-induced cardiotoxicity Results: conclusion. Indian J Pharmacol. 2017;49(1):65–70. doi:10.4103/0253-7613.201015
11. Fatani AG, Darweesh Q, Rizwan L, Aleisa M, Al-Shabanah OA, Sayed-Ahmed MM. Carnitine deficiency aggravates cardiotoxicity in rats. Exp Chemother. 2010;56:71–81. doi:10.1159/000298822
12. Gharib MI, Burnett AKU. Chemotherapy-induced cardiotoxicity: current practice and prospects of prophylaxis. Eur J Heart Fail. 2002;4:235–242. doi:10.1016/S1388-9842(01)00201-X
13. Countries M. Access to medications for cardiovascular diseases in low- and middle-income countries. Circulation. 2017;133(21):2076–2085. doi:10.1161/CIRCULATIONAHA.115.008722.Access
14. Chandrashekar BS, Prabhakara S, Mohan T, et al. Characterization of Rubia cordifolia L. root extract and its evaluation of cardioprotective effect in Wistar rat model. Indian J Pharmacol. 2018;50(1):12–21. doi:10.4103/ijp.IJP
15. Atanassova MS, Aslam MS, Sharma S. Studies on nutritional facts of spring herbs collected from Bulgarian market. J Public Health. 2018;1(3):15–20.
16. Grubben GJH, Denton OA. Plant Resources of Tropical Africa 2. Vegetables. Wageningen: PROTA Foundation; 2004.
17. Gebrehiwot K, Hundera K. Species composition, plant community structure and natural regeneration status of belete moist evergreen montane forest, Oromia Regional state, Southwestern Ethiopia. Momona Ethiop J Sci. 2019;6(1):97. doi:10.4314/mejs.v6i1.102417
18. Asfaw Z. Ethnobotanical studies on traditional medicinal plants used to treat livestock and human ailments in Tigray Region, Ethiopia. Adv J Biol Sci Res. 2015;3(2):8–36.
19. Yohannis SW, Asfaw Z, Kelbessa E. Ethnobotanical study of medicinal plants used by local people in Menz Gera Midir District, North Shewa Zone, Amhara Regional State, Ethiopia. J Med Plant Res. 2018;12(21):296–314. doi:10.5897/JMPR2018.6616
20. Alemayehu G, Asfaw Z, Kelbessa E. Ethnobotanical study of medicinal plants used by local communities of Minjar-Shenkora District, North Shewa Zone of Amhara Region, Ethiopia. J Med Plants Stud. 2015;3(6):1–11.
21. Chekole G, Asfaw Z, Kelbessa E. Ethnobotanical study of medicinal plants in the environs of Tara-gedam and Amba remnant forests of Libo Kemkem District, northwest Ethiopia. J Ethnobiol Ethnomed. 2015;11(1):1–38. doi:10.1186/1746-4269-11-4
22. Maryo M, Nemomissa S, Bekele T. An ethnobotanical study of medicinal plants of the Kembatta ethnic group in Enset-based agricultural landscape of Kembatta Tembaro (KT) Zone. Asian J Plant Sci Res. 2015;5(7):42–61.
23. OECD. OECD Guidelines for the Testing of Chemicals/Section 4: Health Effects Test No. 425: Acute Oral Toxicity: Up-And-Down Procedure. OECD Publishing; 2008.
24. Shen Q, Zhang B, Xu R, Wang Y, Ding X, Li P. Antioxidant activity in vitro of the selenium-contained protein from the Se-enriched Bi fi dobacterium animalis 01. Anaerobe. 2010;16(4):380–386. doi:10.1016/j.anaerobe.2010.06.006
25. Olayinka ET, Ore A, Ola OS, Adeyemo OA. Ameliorative effect of gallic acid on cyclophosphamide-induced oxidative injury and hepatic dysfunction in rats. Med Sci. 2015;3:78–92. doi:10.3390/medsci3030078
26. Yin J, Xie J, Guo X, Ju L, Li Y, Zhang Y. Plasma metabolic profiling analysis of cyclophosphamide-induced cardiotoxicity using metabolomics coupled with UPLC/Q-TOF-MS and ROC curve. J Chromatogr B. 2016;1033:428–435. doi:10.1016/j.jchromb.2016.08.042
27. Omole JG, Ayoka OA, Alabi QK, et al. Protective effect of kolaviron on cyclophosphamide-induced cardiac toxicity in rats. J Evid Based Integr Med. 2018;23(1):1–11. doi:10.1177/2156587218757649
28. Liu Y, Tan D, Shi L, Liu X, Zhang Y, Tong C. Blueberry anthocyanins-enriched extracts attenuate cyclophosphamide-induced cardiac injury. PLoS One. 2015;10(7):1–18. doi:10.1371/journal.pone.0127813
29. Prasad EM, Mopuri R, Islam S, Devi L. Cardioprotective effect of Vitex negundo on isoproterenol-induced myocardial necrosis in wistar rats: a dual approach study. Biomed Pharmacother. 2016;85:601–610. doi:10.1016/j.biopha.2016.11.069
30. Alhumaidha KA, Saleh DO, Abd MA, Fattah E, El-eraky WI, Moawad H. Cardiorenal protective effect of taurine against cyclophosphamide-induced toxicity in albino rats. Can J Physiol Pharmacol. 2016;94:131–139. doi:10.1139/cjpp-2015-0138
31. Srivastav RK, Siddiqui HH, Mahmood T, Ahsan F. Evaluation of cardioprotective effect of silk cocoon (Abresham) on isoprenaline-induced myocardial infarction in rats. Avicenna J Phytomed. 2013;3(3):216–223.
32. Aam E, Mirghani MES, Kh M, Na K, Mz A. Challenges of extraction techniques of natural antioxidants and their potential application opportunities as anti-cancer agents. Heal Sci J. 2018;12(5):596. doi:10.21767/1791-809X.1000596
33. Jing L, Ma H, Fan P, Gao R, Jia Z. Antioxidant potential, total phenolic and total flavonoid contents of Rhododendron anthopogonoides and its protective effect on hypoxia-induced injury in PC12 cells. BMC Complement Altern Med. 2015;15(1):1–12. doi:10.1186/s12906-015-0820-3
34. Karou D, Dicko MH, Simpore J, Traore AS. Antioxidant and antibacterial activities of polyphenols from ethnomedicinal plants of Burkina Faso. Afr J Biotechnol. 2005;4(20):823–828. doi:10.5897/AJB09.1302
35. Halligudi N, Pathak S, Kamboj DS. Antioxidant activity of herbal plants: a recent review. J Drug Discov Ther. 2013;8(1):1–8.
36. Enayati A. Antioxidant activity and cardioprotective effect of potentilla reptans L. via Ischemic Preconditioning (IPC). Res J Pharmacogn. 2019;6(1):19–27. doi:10.22127/rjp.2018.80367
37. Sintayehu B, Asres K, Raghavendra Y. Radical scavenging activities of the leaf extracts and a flavonoid glycoside isolated from Cineraria abyssinica Sch. Bip. exA. Rich. J Appl Pharm Sci. 2012;2(4):44–49. doi:10.7324/JAPS.2012.2407
38. Khan RA, Khan MR, Sahreen S, Ahmed M. Evaluation of phenolic contents and antioxidant activity of various solvent extracts of Sonchus asper (L.) Hill. Chem Cent J. 2012;6(1):12. doi:10.1186/1752-153X-6-12
39. Seifu T, Mehari B, Atlabachew M, Chandravanshi B. Polyphenolic content and antioxidant activity of leaves of Urtica simensis grown in Ethiopia. Lat Am Appl Res. 2017;40:35–40.
40. Activity A, Rich A, Tsegaye W, Urga K, Asres K. Antidiabetic activity of Samma (Urtica simensis Hochst. ex. A. Rich.) in streptozotocin-induced diabetic mice. Ethiop Pharm J. 2014;27:75–82. doi:10.4314/epj.v27i2.58265
41. Karale S, Kamath JV, Kamath JV. Cardioprotective effect of Mentha longifolia against cyclophosphamide induced cardiotoxicity in rats: a biochemical, electrocardiographic and histopathological study. Int J Pharm Pharm Sci. 2016;8(9):214–217. doi:10.22159/ijpps.2016v8i9.13004
42. Ogunsanwo OR, Asenuga ER. Biochemical and electrocardiographic studies on the beneficial effects of gallic acid in cyclophosphamide-induced cardiorenal dysfunction. J Complementary Integr. 2017;14(3). doi:10.1515/jcim-2016-0161
43. Swamy AHMV, Patel UM, Koti BC, Gadad PC, Patel NL, Thippeswamy AHM. Cardioprotective effect of Saraca indica against cyclophosphamide induced cardiotoxicity in rats: a biochemical, electrocardiographic and histopathological study. Indian J Pharmacol. 2013;45(1):44–48. doi:10.4103/0253-7613.106434
44. Gunes S, Sahinturk V, Karasati P, Sahin IK. Cardioprotective effect of selenium against cyclophosphamide-induced cardiotoxicity in rats. Biol Trace Elem Res. 2016;3–10. doi:10.1007/s12011-016-0858-1
45. Weng SF, Kai J, Guha IN, Qureshi N. The value of aspartate aminotransferase and alanine aminotransferase in cardiovascular disease risk assessment. Open Heart. 2015;2(1):e000272. doi:10.1136/openhrt-2015-000272
46. Şekeroğlu V, Aydın B, Şekeroğlu ZA. Viscum album L. extract and quercetin reduce cyclophosphamide-induced cardiotoxicity, urotoxicity and genotoxicity in mice. Asian Pacific J Cancer Prev. 2011;12:2925–2931.
47. Asiri YA. Probucol attenuates cyclophosphamide- induced oxidative apoptosis, p53 and Bax signal expression in rat cardiac tissues. Oxid Med Cell Longev. 2010;3(5):308–316. doi:10.4161/oxim.3.5.13107
48. Nagi MN, Al-shabanah OA, Hafez MM, Sayed-ahmed MM. Thymoquinone supplementation attenuates cyclophosphamide-induced cardiotoxicity in rats. J Biochem Mol Toxicol. 2011;25(3):135–142. doi:10.1002/jbt
49. Sudharsan PT, Mythili Y, Selvakumar E, Varalakshmi P. Lupeol and its ester inhibit alteration of myocardial permeability in cyclophosphamide administered rats. Mol Cell Biochem. 2006;292(1–2):39–44. doi:10.1007/s11010-006-9171-1
50. Paul S, Das S, Tanvir EM, et al. Protective effects of ethanolic peel and pulp extracts of Citrus macroptera fruit against isoproterenol-induced myocardial infarction in rats. Biomed Pharmacother. 2017;94:256–264. doi:10.1016/j.biopha.2017.07.080
51. Song Y, Zhang C, Wang C, et al. Ferulic acid against cyclophosphamide-induced heart toxicity in mice by inhibiting NF- κ B pathway. Evid Based Complement Altern Med. 2016;2016:1–8. doi:10.1155/2016/1261270
52. Avci H, Epikmen ET, Ipek E, et al. Protective effects of silymarin and curcumin on cyclophosphamide-induced cardiotoxicity. Exp Toxicol Pathol. 2017;69(5):317–327. doi:10.1016/j.etp.2017.02.002
53. Sharma M, Kishore K, Gupta SK, Joshi S, Arya DS. Cardioprotective potential of Ocimum sanctum in isoproterenol induced myocardial infarction in rats. Mol Cell Biochem. 2001;225:75–83. doi:10.1023/A:1012220908636
54. Rahman M, Islam B, Biswas M, Alam AHMK. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res Notes. 2015;8:621. doi:10.1186/s13104-015-1618-6
55. Peer PA, Trivedi PC, Nigade PB, Ghaisas MM, Deshpande AD. Cardioprotective effect of Azadirachta indica A. Juss. on isoprenaline induced myocardial infarction in rats. Int J Cardiol. 2008;126:123–126. doi:10.1016/j.ijcard.2007.01.108
56. Cook NC, Samman S. Flavonoids—chemistry, metabolism, cardioprotective effects, and dietary sources. Elsevier Sci Inc. 1996;7:66–76.
57. Adegbola P, Aderibigbe I, Hammed W, Omotayo T. Antioxidant and anti-inflammatory medicinal plants have potential role in the treatment of cardiovascular disease: a review. Am J Cardiovasc Dis. 2017;7(2):19–32.
58. Oguntibeju OO. Medicinal plants with anti-inflammatory activities from selected countries and regions of africa. J Inflamm Res. 2018;11:307–317. doi:10.2147/JIR.S167789
59. Park M, Kim M. Analysis of antioxidant and anti-inflammatory activities of solvent fractions from rhynchosia nulubilis cultivated with Ganoderma lucidum mycelium. Prev Nutr Food Sci. 2017;22(4):365–371. doi:10.3746/pnf.2017.22.4.365
60. Iqubal A, Iqubal MK, Sharma S, et al. Molecular mechanism involved in cyclophosphamide-induced cardiotoxicity: old drug with a new vision. Life Sci. 2019;218:112–131. doi:10.1016/j.lfs.2018.12.018
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
