Back to Journals » International Journal of General Medicine » Volume 15
Cardiac Abnormalities of People Living with HIV: A Comparative Study Between HAART Experience and Treatment Naïve Groups in Ghana
Authors Owusu I K , Wiafe YA, Opoku S , Anto EO , Acheamfour-Akowuah E
Received 16 March 2022
Accepted for publication 13 June 2022
Published 29 June 2022 Volume 2022:15 Pages 5849—5859
DOI https://doi.org/10.2147/IJGM.S366688
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Isaac Kofi Owusu,1 Yaw Amo Wiafe,2 Stephen Opoku,2 Enoch Odame Anto,2 Emmanuel Acheamfour-Akowuah1
1Department of Medicine, School of Medicine and Dentistry, College of Health Sciences, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana; 2Department of Medical Diagnostics, College of Health Sciences, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana
Correspondence: Isaac Kofi Owusu, Email [email protected]
Purpose: This study determined electrocardiographic and echocardiographic abnormalities of people living with HIV (PLWHIV); comparing the findings of PLWHIV on HAART versus treatment naïve groups.
Patients and Methods: In a prospective cross-sectional study, we recruited 157 PLWHIV on Highly Active Antiretroviral Therapy (HAART) and 28 HAART naïve PLWHIV. Clinical examination, electrocardiography and echocardiography were performed on study participants at the Komfo Anokye Teaching Hospital (KATH) in Kumasi, Ghana. Sociodemographic data and information about the use of HAART or otherwise was obtained. The Chi and Fisher Exact tests were used to find the significance of difference in proportions of abnormalities between PLWHIV on HAART and treatment naïve groups. Statistical analyses were performed on SPSS version 25.0 and GraphPad Prism version 8.0. P-values less than 0.05 were considered to be statistically significant.
Results: Echocardiographic abnormalities in the HAART and treatment naïve groups were 54.1% and 60.7%, respectively. Electrographic abnormalities in the HAART and treatment naïve groups were 45.9% and 50%, respectively. Sinus bradycardia was the most prevalent ECG abnormality in the treatment naïve. Nonspecific T-wave changes (36.1%) and sinus tachycardia (30.6%) were the most common ECG abnormalities seen in HAART treated group. The common echocardiographic abnormalities were pulmonary hypertension (22.7%), pericardial effusion (22.2%) and left ventricular systolic dysfunction (17.8%). There was no significant difference in the proportions of echocardiographic abnormalities between PLWHIV on HAART and the treatment naïve groups (p > 0.05).
Conclusion: Cardiac abnormalities are common in PLWHIV regardless of treatment with HAART. Echocardiographic and electrographic assessments are highly recommended for all PLWHIV.
Keywords: Ghana, echocardiographic abnormalities, electrocardiographic abnormalities, HIV, treatment naïve
Introduction
Human immunodeficiency virus (HIV) infection is a global epidemic currently affecting about 38 million people worldwide.1 About 25 million people living with HIV (PLWHIV) are on highly active antiretroviral therapy (HAART), whilst over 12 million people are not receiving HAART.1 Despite the immense reduction in morbidity and mortality achieved by the introduction of HAART, about 820,000 people died of HIV-related complications in 2020.1,2
Cardiovascular disease is reported as one of the main underlying causes of death in PLWHIV affecting about 61% of PLWHIV.3,4 Cardiac abnormalities in PLWHIV are often unrecognized and may only be detected at autopsy with only about 10% incidence of symptomatic heart failure.5–7 Routine electrocardiogram (ECG) has therefore been suggested for early detection of cardiac abnormalities in PLWHIV.8 While some existing studies attribute the prevalence of cardiac disorders in PLWHIV to the increased survival achieved by HAART, a number of studies have also reported cardiac abnormalities in treatment naïve people.9 Okoye and Anyabolu reported 70% of electrocardiographic (ECG) abnormalities in treatment naïve PLWHIV in Enugu, Nigeria.10 Olusegun-Joseph et al also reported 78% echocardiography abnormalities in treatment naïve people in the same region of Nigeria.11 Comparing ECG findings in HAART treated versus treatment naïve PLWHIV, Njoku et al reported 93% versus 73% ECG abnormalities in PLWHIV on HAART versus treatment naïve PLWHIV, respectively.12 However, a study in South Africa reported normal ECG and echocardiographic findings in most PLWHIV on HAART and treatment naïve.13
This study was done to determine ECG and echocardiographic abnormalities seen in PLWHIV on HAART and the treatment naïve group in Kumasi, Ghana.
Materials and Methods
Study Design and Setting
A prospective cross-sectional study was conducted at the HIV clinic of Komfo Anokye Teaching Hospital (KATH), Kumasi, Ghana. The facility has over 1000 bed capacities and serves as a referral center for other hospitals in the middle and northern belts of Ghana. KATH is the second largest teaching hospital in Ghana, and it is one of the biggest centres for the care of PLWHIV.
Participants Selection and Sampling
In this study, we recruited 157 registered PLWHIV aged 16 years and above who were receiving HAART at the KATH HIV clinic. Twenty-eight (28) treatment naïve PLWHIV who presented for treatment at the hospital over a period of three months were approached for voluntary participation. We excluded patients with prior history of cardiac disorders including those with congenital heart disease, and those who did not consent to participate.
Ethical Consideration
Ethical clearance (CHRPE/22/10) was obtained from the Committee on Human Research Publication and Ethics of the Kwame Nkrumah University of Science and Technology, Kumasi, Ghana. A thorough explanation of the study protocol and assurance of anonymity was made to the subjects. Written informed consent was also sought from participants and healthcare management before data and sample collection. All methods were carried out in accordance with the Helsinki Declaration.
Data Collection and Measurement of Echocardiographic and Electrographic Parameters
Standardized and structured questionnaires were used to obtain socio-demographic characteristics, disease history and physical examination findings of all participating PLWHIV. Physical examination performed by the study physician included general assessment, pulse (rate, rhythm, volume and the character), the blood pressure, the apex beat, auscultation for the heart sounds (S1, S2, S3 and S4) and murmurs.
A resting 12 lead ECG was obtained from each participant according to standard procedure and evaluated by the study physician/cardiologists. The ECGs were examined for the heart rate, the rhythm, electrical conduction abnormalities, chamber enlargement, arrhythmias, and other abnormalities.
Transthoracic echocardiography was performed using an eLogic GE ultrasound machine equipped with a 3.5 MHz sector probe. Study participants were examined in the left lateral decubitus position and the procedure followed the joint European Association of Echocardiography and American Society of Echocardiography guidelines.15 Left ventricular (LV) mass was calculated using the autopsy validated Devereux formula16 and indexed to the body surface area to determine LV mass index (LVMI). Left ventricular hypertrophy (LVH) was considered to be present when LVMI was >104 g/m2 in women and >116 g/m2 in men. LV end-diastolic and systolic volumes were measured using modified Simpson’s biplane method and were used to calculate ejection fraction, stroke volume and cardiac output as currently recommended.15
Statistical Analysis
Data from the questionnaires were entered into a Microsoft Excel (2016) spreadsheet and exported to SPSS Windows version 25.0 and GrapPad Prism version 8.0 for statistical analysis. Descriptive analysis of baseline parameters was performed. Frequencies and percentages were calculated for categorical variables. For continuous data, measures of central tendency using median (interquartile range) and mean were calculated; and measures of spread using standard deviation and range were also calculated. The Chi and Fisher Exact tests were used to find the significance in difference in proportions of abnormalities between HAART-treated PLWHIV and HAART naïve PLWHIV or between Efavirenz (EFF)-based and Nevirapine-based (NVP) regimen. The Mann Whitney U-test was used to assess the significance of differences in ECG and echocardiographic parameters between HAART-treated PLWHIV and HAART naïve PLWHIV or between Efavirenz (EFF)-based and Nevirapine-based (NVP) regimen. P-values less than 0.05 were considered to be statistically significant for all analyses.
Results
A total of 185 PLWHIV, consisting of 157 patients on HAART and 28 HAART naïve patients were included in the statistical analysis. The mean (±SD) age of the study participants was 50.5 (±9.8) years. The majority of the study participants were females (74.1%), more than 40 years (49.2%), had completed basic education (67.0%) and were engaged in some form of employment (78.9%). There was no significant difference in the gender distribution, age category, employment status and educational level between PLWHIV on HAART and the treatment naïve group (p > 0.05). Also, there was no significant difference in weight, height, body mass index (BMI), respiratory rate and temperature between PLWHIV on HAART and treatment the naïve group (p > 0.05). Table 1 displays the sociodemographic characteristics of the study groups.
 |
Table 1 Sociodemographic Characteristics of Study Participants |
Echocardiographic and Electrocardiographic Parameters by Study Groups and HAART Regimen
In general, there was no significant difference in echocardiographic parameters between PLWHIV on HAART and the treatment naïve group (p > 0.05). In addition, there was no significant difference in echocardiographic parameters between PLWHIV who were on efavirenz– based (EFV-based) regimen and those that took nevirapine-based (NVP-based) regimen (p > 0.05) (Table 2).
 |
Table 2 Echocardiographic Parameters Stratified by Study Groups and ART Regimen |
As shown in Table 3, although not significant, heart rate, PR interval (PR-I) and QRS complex duration (QRSD) were increased in PLWHIV on HAART compared to the treatment naïve group (87 (77–96.5) beats per minute vs 82.5 (68.75–95.75) beats per minute, 168 (144.0–183.0) ms vs 157 (140.5–173.5) ms and 88 (82.0–98.0) ms vs 87 (82.0–95.0) ms respectively). From Table 4, heart rate was increased amongst PLWHIV on efavirenz– based therapy than their counterparts who were on nevirapine-based therapy (88 (77–97) beats/minute vs 86 (76–94) beats/minute). The opposite was true for the PR-I and QRSD (168 (141.5–182) ms vs 170 (148–192) ms and 86 (81.5–98.0) ms vs 90 (82–100) ms). However, none of the variables were statistically significant between the two groups (p > 0.05).
 |
Table 3 Distribution of Electrocardiographic (ECG) Parameters Amongst PLWHIV on HAART and HAART- Naïve Subjects |
 |
Table 4 Distribution of Electrocardiographic (ECG) Parameters Based on Antiretroviral Regimen |
Prevalence of Echocardiographic Abnormalities Among Study Participants
The prevalence of echocardiographic abnormalities was 54.1% for PLWHIV on HAART, and 60.7% for the treatment naïve group, as shown in Figure 1. The common echocardiographic abnormalities seen among all the study population were pulmonary hypertension (22.7%), pericardial effusion (22.2%) and left ventricular systolic dysfunction (17.8%). There was no significant difference in the proportions of echocardiographic abnormalities between PLWHIV on HAART and the treatment naive (p > 0.05). Furthermore, there were no significant differences in the proportions of echocardiographic abnormalities between participants who were on EFV-based regimen and those who took NVP-based regimen (Table 5).
 |
Table 5 Echocardiographic Abnormalities Stratified by Study Groups and ART Regimen |
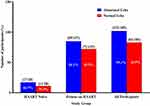 |
Figure 1 Echocardiographic abnormalities among study participants. |
Prevalence of Electrocardiographic Abnormalities Among Study Groups
As shown in Figure 2, 53.5% of the total participants had normal ECG whilst 46.5% had abnormal ECG. A similar trend was observed for PLWHIV on HAART, as more PLWHIV had normal ECG (54.1%) than those who had abnormal ECG (45.9%). The treatment naïve group, however, had equal distribution of ECG findings.
 |
Figure 2 Distribution of ECG abnormalities stratified by HAART status. |
Nonspecific T-wave changes (36.1%) and sinus tachycardia (30.6%) were the most common abnormalities seen in the participants (Table 6). All the other abnormalities were less than 10%. Also, apart from left ventricular hypertrophy (LVH), non-specific T-wave changes and sinus tachycardia which were higher in the efavirenz-based regimen group, all other abnormalities were more prevalent in the nevirapine-based regimen group than the efavirenz-based regimen group. None of the results was statistically significant between the two groups (p > 0.05).
 |
Table 6 Electrocardiographic (ECG) Abnormalities Stratified by HAART Status and Antiretroviral Regimen |
Table 6 summarizes the Spearman rho correlations of echocardiographic and ECG parameters. A negatively weak significant correlation was observed between QRS axis and LVIDd and LVIDs (r = - 0.165, p-value = 0.025 and r = - 0.157, p-value= 0.033 respectively). A weak positive correlation was also observed between PR-I and FS which was significant (r = 0.133, p-value = 0.042). A similarly weak but negative correlation, which was also significant, was also observed between PR-I and AR (r=−0.133. p-value = 0.032).
Discussion
The current study investigated ECG and echocardiographic abnormalities among PLWHIV on HAART and treatment naïve PLWHIV. In this study, echocardiographic abnormalities among PLWHIV on HAART and the treatment naïve group were found to be 54.1%, and 60.7%, respectively. This is similar to the findings of Oluwabunmi et al, who reported 52% echocardiographic abnormalities among PLWHIV on HAART and 60% echocardiographic abnormalities among treatment naïve PLWHIV in Enugu, Nigeria.17 Olusegun-Joseph et al also reported a higher prevalence of echocardiographic abnormalities among treatment naïve PLWHIV (78%) than HIV negative controls (16%).11 On the contrary, Roozen et al reported very low prevalence (<10%) of echocardiographic abnormalities among PLWHIV on HAART, treatment naïve PLWHIV and HIV negative controls in South Africa.13
On the other hand, the prevalence of ECG abnormalities was 46.5% among PLWHIV; with non-specific T-wave changes (36.1%) and sinus tachycardia (30.6%) being the most common abnormalities. This prevalence of ECG abnormalities is in harmony but lower than the report of Njoku et al12 and Okoye et al,10 in studies done in Nigeria; where 73% and 70% were reported among the HAART naïve PLWHIV, respectively, and a higher prevalence of 93% was reported by Njoku et al in the HAART treated group.
In this study, we found no significant difference in the proportions of echocardiographic abnormalities between treatment naïve PLWHIV and PLWHIV on HAART. Furthermore, there were no significant difference in the proportions of echocardiographic abnormalities between patients on EFV-based regimen and those on NVP-based regimen (Table 5). Our finding is comparable to that of Oluwabunmi et al, who found a similar proportion of echocardiographic abnormalities between treatment naïve PLWHIV and PLWHIV on HAART in Nigeria.17 In the same study, they reported no significant difference in echocardiographic abnormalities between participants on protease inhibitor (PI)-based and non-PI-based regimen.17
With respect to the distribution of abnormalities, non-specific T-wave changes (36.1%) and sinus tachycardia (30.6%) were the most common ECG abnormalities among the participants, which is comparable to the findings of Oluwabunmi et al, who reported that sinus tachycardia was the most prevalent ECG abnormality (64%) seen in this PLWHIV.17 Again, Oluwabunmi et al, in Nigeria, reported T-wave inversion to be the most prevalent ECG abnormality amongst PLWHIV in their study (23%), followed closely by sinus tachycardia (21%).17 This high prevalence of sinus tachycardia in the treatment naïve arm could be explained by anxiety, excessive sympathetic stimulation from autonomic imbalance, dehydration in subjects with chronic diarrhoea, anaemia and increased metabolic demand in febrile illnesses as heart rate is known to increase in such conditions.8 However, Kabwe et al, in Zambia, reported that sinus bradycardia was found in about 7% of the PLWHIV in their study. 17,18
Apart from left ventricular hypertrophy, non-specific T-wave changes and sinus tachycardia which were higher in the efaverenz-based regimen group, all other ECG abnormalities were more prevalent in the nevirapen-based regimen group than the efaverenz-based regimen group. This finding is also similar to the study by Oluwabunmi et al, who also found LVH, sinus tachycardia and T-wave inversion to be higher in non-PI-based regimen than in PI-based regimen.17
In this study, except for IVS that was significantly higher in PLWHIV on HAART than the treatment naïve, there was no significant difference in the other echocardiographic parameters between treatment naïve PLWHIV and those on HAART. In addition, there was no significant difference in echocardiographic parameters between participants who were on efaverenz-based regimen and those that took nevirapen-based regimen. Oluwabunmi et al reported similar echocardiographic parameters between treatment naïve PLWHIV and those on HAART.17 In another Nigerian study, Olusegun-Joseph et al reported more echocardiographic abnormalities in treatment naïve PLWHIV than healthy controls.11 Several studies have associated increased echocardiographic abnormalities with increased subclinical atherosclerosis among PLWHIV.9,10 Increased echocardiographic abnormalities have been associated with increased mortality among PLWHIV.11 Studies have shown that cardiovascular abnormalities in HIV infection are often clinically quiescent and may be attributed to disorder in other systems.12–16 Our current study, which confirms no significant difference in the findings between PLWHIV on HAART and the treatment naïve, implies that cardiac abnormalities are not significantly due to HAART. Our current study further confirms no significant difference in the rate of echocardiographic and ECG abnormalities between different regimens of HAART. This is an additional confirmation that cardiac abnormalities in PLWHIV are not significantly due to HAART. PLWHIV on HAART are therefore not at higher risk of cardiac abnormalities due to treatment. The higher rate of echocardiographic and ECG abnormalities in both HAART and naïve groups suggests that cardiac abnormalities are mostly due to the virus. Routine echocardiography and ECG are therefore recommended for all PLWHIV to improve management and quality of life.
Our study had a few limitations, and significantly amongst them is the low number of treatment naïve PLWHIV (n = 28). This was inevitable because most PLWHIV were on HAART as per the current guidelines.17 Our study findings were based on conventional 2D echocardiography which is less sensitive than 3D echocardiography. However, conventional 2D is still the most widely available in many clinics.
Conclusion
Cardiac abnormalities were found to be over 55% prevalent in PLWHIV, regardless of their HAART status. There is no significant difference in the rate of cardiac abnormalities in PLWHIV on HAART and treatment naïve PLWHIV, making HAART an unlikely cause of cardiac abnormalities in PLWHIV.
What is Already Known on This Topic?
- That cardiovascular disease is one of the main causes of death in PLWHIV.
- That cardiac abnormalities are often detected late.
What Does This Study Add?
- That PLWHIV could benefit from routine ECG and echocardiography.
- That being on HAART does not increase the risk of cardiac abnormalities and may even reduce the prevalence of cardiac abnormalities.
Acknowledgment
The authors would like to express their sincere gratitude to the staff and the study participants at the HIV clinic of the Department of Medicine, Komfo Anokye Teaching Hospital, Kumasi, Ghana, for their support. Without their co-operation, this study would not have been done.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in relation to this work.
References
1. UNAIDS. Global AIDS Update - Seizing the moment -Tackling entrenched inequalities to end epidemics; 2020.
2. UNAIDS. Global AIDS update 2016; 2016.
3. Barbaro G, Di Lorenzo G, Grisorio B, Barbarini G. INVESTIGATORS4 GIPLSCDPADA. Cardiac involvement in the acquired immunodeficiency syndrome: a multicenter clinical-pathological study. AIDS Res Hum Retroviruses. 1998;14(12):1071–1077. doi:10.1089/aid.1998.14.1071
4. Islam F, Wu J, Jansson J, Wilson D. Relative risk of cardiovascular disease among people living with HIV: a systematic review and meta‐analysis. HIV Med. 2012;13(8):453–468. doi:10.1111/j.1468-1293.2012.00996.x
5. Kaul S, Fishbein MC, Siegel RJ. Cardiac manifestations of acquired immune deficiency syndrome: a 1991 update. Am Heart J. 1991;122(2):535–544. doi:10.1016/0002-8703(91)91013-D
6. Chaudhary S, Apurva SK, Reddy DH, et al. A study of cardiovascular abnormalities in HIV positive patients in a tertiary care hospital in Northern India. J Assoc Physicians India. 2017;65(12):24–29.
7. Fisher SD, Lipshultz SE. Epidemiology of cardiovascular involvement in HIV disease and AIDS. Ann N Y Acad Sci. 2001;946(1):13–22. doi:10.1111/j.1749-6632.2001.tb03900.x
8. Myerson M, Kaplan-Lewis E, Poltavskiy E, Ferris D, Bang H. Prolonged QTc in HIV-infected patients: a need for routine ECG screening. JIAPAC. 2019;18:2325958219833926. doi:10.1177/2325958219833926
9. Reimer Jensen A, Ramalho S, Claggett B, Biering-Sorensen T, Shah A. P5419 Influence of HIV infection on cardiac structure and function in the era of HAART: a systematic review and meta-analysis of case-control studies. Eur Heart J. 2018;39(suppl_1):
10. Okoye IC, Anyabolu EN. Electrocardiographic abnormalities in treatment-naïve HIV subjects in south-east Nigeria. Cardiovasc J Afr. 2017;28(5):315–318. doi:10.5830/CVJA-2017-013
11. Olusegun-Joseph D, Ajuluchukwu J, Okany C, Mbakwem A, Oke D, Okubadejo N. Echocardiographic patterns in treatment-naïve HIV-positive patients in Lagos, south-west Nigeria: cardiovascular topic-online article. Cardiovasc J Afr. 2012;23(8):1–6. doi:10.5830/CVJA-2012-048
12. Njoku P, Ejim E, Anisiuba B, Ike S, Onwubere B. Electrocardiographic findings in a cross-sectional study of human immunodeficiency virus (HIV) patients in Enugu, south-east Nigeria. Cardiovasc J Afr. 2016;27(4):252–257. doi:10.5830/CVJA-2016-007
13. Roozen GV, Meel R, Peper J, et al. Electrocardiographic and echocardiographic abnormalities in urban African people living with HIV in South Africa. PLoS One. 2021;16(2):e0244742. doi:10.1371/journal.pone.0244742
14. Fox MP, Rosen S. A new cascade of HIV care for the era of “treat all”. PLoS Med. 2017;14(4):e1002268. doi:10.1371/journal.pmed.1002268
15. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7(2):79–108. doi:10.1016/j.euje.2005.12.014
16. Jafary FH. Devereux formula for left ventricular mass–be careful to use the right units of measurement. J Am Soc Echocardiogr. 2007;20(6):783. doi:10.1016/j.echo.2007.02.034
17. Oluwabunmi AA, Rumokere AM, Sotonye D-M. Pattern of electrocardiographic and echocardiographic abnormalities among HIV patients in Port Harcourt, Nigeria. Science. 2020;6(1):15–24.
18. Kabwe L, Lakhi S, Kalinichenko S, Mulenga L. Prevalence of subclinical cardiovascular disease in healthy HIV infected patients at the University Teaching Hospital in Lusaka, Zambia. Med J Zambia. 2016;43(1):12–23.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
