Back to Journals » International Journal of Nanomedicine » Volume 18
Carbon Nanomaterial-Based Hydrogels as Scaffolds in Tissue Engineering: A Comprehensive Review
Authors Stocco TD , Zhang T, Dimitrov E, Ghosh A, da Silva AMH , Melo WC, Tsumura WG , Silva ADR , Sousa GF , Viana BC , Terrones M, Lobo AO
Received 24 August 2023
Accepted for publication 12 October 2023
Published 27 October 2023 Volume 2023:18 Pages 6153—6183
DOI https://doi.org/10.2147/IJN.S436867
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor R.D.K. Misra
Thiago Domingues Stocco,1 Tianyi Zhang,2 Edgar Dimitrov,2 Anupama Ghosh,3 Alessandro Marcio Hakme da Silva,1 Wanessa CMA Melo,4 Willian Gonçalves Tsumura,1 André Diniz Rosa Silva,5,6 Gustavo F Sousa,6 Bartolomeu C Viana,6 Mauricio Terrones,2 Anderson Oliveira Lobo6
1Bioengineering Program, Scientific and Technological Institute, Brazil University, São Paulo, SP, Brazil; 2Pennsylvania State University, University Park, PA, USA; 3Department of Chemical and Materials Engineering (DEQM), Pontifical Catholic University of Rio de Janeiro, Rio de Janeiro, Brazil; 4FTMC, State Research institute Center for Physical Sciences and Technology, Department of Functional Materials and Electronics, Vilnius, Lithuanian; 5FATEC, Ribeirão Preto, SP, Brazil; 6Interdisciplinary Laboratory for Advanced Materials (LIMAV), BioMatLab Group, Materials Science and Engineering Graduate Program, Federal University of Piauí (UFPI), Teresina, PI, Brazil
Correspondence: Anderson Oliveira Lobo, Tel +55 86 981115013, Email [email protected]; [email protected]
Abstract: Carbon-based nanomaterials (CBNs) are a category of nanomaterials with various systems based on combinations of sp2 and sp3 hybridized carbon bonds, morphologies, and functional groups. CBNs can exhibit distinguished properties such as high mechanical strength, chemical stability, high electrical conductivity, and biocompatibility. These desirable physicochemical properties have triggered their uses in many fields, including biomedical applications. In this review, we specifically focus on applying CBNs as scaffolds in tissue engineering, a therapeutic approach whereby CBNs can act for the regeneration or replacement of damaged tissue. Here, an overview of the structures and properties of different CBNs will first be provided. We will then discuss state-of-the-art advancements of CBNs and hydrogels as scaffolds for regenerating various types of human tissues. Finally, a perspective of future potentials and challenges in this field will be presented. Since this is a very rapidly growing field, we expect that this review will promote interdisciplinary efforts in developing effective tissue regeneration scaffolds for clinical applications.
Keywords: carbon, biomaterial, nanotechnology, tissue engineering, scaffold
Introduction
Nanomaterials have gained considerable attention in various biomedical applications over the past decade owing to their fascinating physicochemical properties.1,2 For example, the reduced sizes of nanomaterials allow them to be efficiently internalized into cells by penetrating through cellular membranes or via endocytosis.3 Owing to effects of quantum confinement, a variety of nanomaterials display dissimilar physical properties when compared to their bulk counterparts, such as different optical absorption and emission with engineering aiming for applications in biosensing and bioimaging.4 In terms of therapeutic applications, nanoparticles, nanotubes, and nanosheets possess an ultra-high surface area that enables an increased drug-loading capacity, and thus they have been widely applied in nanomedicine.5 In addition, some nanomaterials (eg, gold nanoparticles and carbon nanotubes) can also exhibit strong plasmonic effects that enhance the photothermal conversion efficiency. As a result, nanomaterials have also been used as agents for photodynamic therapy (PDT) and photothermal therapy (PTT).6,7
Among the large family of nanomaterials, carbon-based nanomaterials (CBNs) constitute an important member which has shown tremendous potential in diverse applications for both diagnostics and therapeutics.3,8 CBNs can display diverse structures ranging from zero-dimensional quantum dots, one-dimensional tubular structure (carbon nanotubes), two-dimensional (2D) sheets (graphene), and three-dimensional (3D) sponges and hydrogels, which are constructed by sp2- and sp3-hybridized carbon bonds. The varied structures and chemical bonds within CBNs also lead to a full spectrum of physicochemical properties ranging from metallic carbon nanotubes (CNTs) and graphene to semiconducting carbon quantum dots (CQDs) and nanodiamonds to insulating graphene oxide (GO).9,10 A summary of different types of CBNs will be provided in the following section. Furthermore, as-prepared CBNs can also be functionalized with different types of molecules and functional groups through either covalent or noncovalent interactions.4 A series of functional properties of CBNs can be tuned by surface functionalization, including but not limited to biocompatibility, dispersibility, and surface reactivity. Due to this functionalization process, many modified CBNs can display low toxicity in biological systems and can be applied in many biomedical applications with minimal adverse effects.11
Carbon-based nanomaterials (CBNs) have found pivotal applications in the realm of tissue engineering, a strategic approach aimed at repairing and replacing defective human tissues.12,13 Within this domain, CBNs boast attributes such as high mechanical strength, biocompatibility, chemical stability, and electrical conductivity.14 Notably, the integration of CBNs into hydrogel matrices introduces a transformative dimension to their potential, especially when CBNs transition from being mere fillers to becoming the central component of the hydrogel structure.15
While there exists a breadth of literature reviewing the application of CBNs in biomedical sciences,14–17 the novelty of our work lies in its emphasis on hydrogels where carbon-based nanomaterials serve as the primary matrix component, not just an ancillary filler. This distinction is pivotal, as traditional reviews often regard CBNs as secondary elements in polymer-based hydrogel matrices. Here, we underscore the significant advancements and challenges associated with these unique carbon nanomaterial-based hydrogels.
In this comprehensive review, we start by offering an overview of the CBN family, spanning from zero- to three-dimensional systems. This is followed by a presentation of the main findings related to the primary applications of CBNs in core areas of tissue engineering. Finally, we showcase studies that have prominently employed carbon nanomaterial-based hydrogels as scaffolds for tissue regeneration. Critical discussions on future directions, challenges, understanding action mechanisms, and interaction with diverse cellular systems round off our exploration. Our aspiration is that shedding light on the nuanced application of CBNs in hydrogel frameworks will catalyze further research and innovation in tissue engineering scaffolds.
Overview of Carbon-Based Nanobiomaterials
As discussed above, CBNs include a wide range of materials that can be classified by dimensionality from zero-dimensional (0D) CQDs, fullerenes and nanodiamonds, one-dimensional (1D) CNTs, to two-dimensional (2D) graphene, as well as their three-dimensional (3D) assemblies such as nanocarbon sponges and hydrogels (Figure 1). Despite their unique structures and geometrical shapes, many CBNs possess key features such as high mechanical strength and stiffness, the ability to interact with biomolecules such as nucleic acids and proteins, large surface area, and low cytotoxicity, which make them effective biomaterials for tissue engineering.18 In tissue regeneration, CBNs are usually used as either the matrix material or an additive in bioactive nanocomposites to improve their mechanical properties. Before reviewing the state-of-the-art applications of CBNs in tissue engineering, it is necessary to classify CBNs based on their structures and understand their physicochemical properties. Therefore, an overview of different types of bioactive CBNs is presented in this section.
 |
Figure 1 Different carbon-based nanomaterials used in tissue engineering approaches are categorized by dimensionality from 0D to 3D. |
Nanodiamonds (NDs)
Although graphite is the most thermodynamically stable form of carbon experimentally in its bulk state,19 this changes at the nanoscale because it includes a significant amount of surface energy. Especially in the dimension range smaller than 3–6 nm, tetrahedral hydrocarbons (higher diamondoids) exhibit greater stability than polyaromatic carbons, although the dangling sp3 bonds on the surface have to be either passivated by functionalization or stabilized by reconstruction into sp2 hybridized carbons.20 While a fixed structure cannot describe all nanodiamonds (NDs), a more general view can be considered as a nanoparticle of diameter 2–10 nm with an almost perfect diamond crystalline structure with the occasional presence of non-diamond carbon and nitrogen, with surface termination by a graphitic shell or amorphous carbon, saturated by oxygen-containing functional groups such as O=CH2, O–H, O–CH, O–C=O, –COOH, –C–O–C–, –C=O.21,22 Nanoscale diamond particles were obtained by detonation (explosion synthesis) in the USSR in the 1960s,23 but its usual production started in the late 1990s after a series of essential breakthroughs.10 NDs (4–5 nm) are usually synthesized by detonating explosive molecules in a closed chamber filled with water or inert gas and they are formed by homogeneous nucleation of the supersaturated carbon vapor by condensation and crystallization of liquid nanocarbon at this elevated pressure and temperature in this process.
NDs synthesized in this way contain plenty of impurities and are washed thoroughly with oxidizing agents such as mixtures of acids (solution method) or oxygen plasmas (gaseous method). NDs are more accessible to functionalize without compromising the core structure when compared to other graphitic nanoparticles. Generally, NDs purified by acid or oxygen plasma possess –OH and –COOH groups on the surface, which can undergo further functionalization with acyl chlorides yielding long alkyl chain terminated NDs24 and by silanization.25 Additionally, NDs can be modified by its superficial graphitic carbon through cycloaddition26 and diazonium reaction.24
Raman and infrared (IR) spectroscopies provide valuable information about NDs’ phase, composition, and surface terminations. They can also identify the nitrogen defects present in NDs, which leads to fluorescence properties exploited in quantum computing,27 high-resolution magnetic sensing,28 fluorescence resonance energy transfer,29 and medical imaging.30 These photoluminescent NDs, synthesized at high temperature and pressure, possess a small size (3–50 nm), the same scale of magnitude that other materials used in tissue engineering scaffolds, elevated photostability, bright multicolor fluorescence, comparable to semiconductor quantum dots with much better biocompatibility, non-toxicity effects, and rich surface chemistry, all suitable properties for in-vivo imaging applications.31 Apart from optical properties, NDs exhibit robust mechanical properties, high thermal conductivity, negligible conductivity, chemical stability, high surface areas, and tunable surface reactivity.32 In addition to the fact that NDs can self-assemble,33 they can also bind with various molecules, such as proteins, nucleic acids, and antibodies. This makes NDs very useful for a wide range of applications in lubrication, tribology, bioimaging, sensing, protein mimicry, drug delivery, tissue engineering, and filler material for nanocomposites.34 The surface properties of the NDs, such as topography, wettability, and surface charge, determine the binding capacity and the surface interaction mechanism. For example, oxygen-terminated NDs (O–NDs) exhibit better adhesion to human neural stem cells when compared to hydrogen-terminated NDs (H–NDs).35 The cytotoxicity of NDs can also be altered by suitable functionalization. For example, NDs with the surface, dominated by C=O, –OH, and –NH2 groups, show no cellular toxicity with several cell lines.36
Properties such as biocompatibility, mechanical property, stability, and chemical inertness, of NDs, make them a useful candidate for tissue engineering, especially for bone and neural tissues. Also, its surface tunability through surface charge and surface functional group modification makes it a carrier of practically any type of biomolecule.37 Nanodiamond coatings on biomedical implants can be achieved through scalable and reproducible synthetic methods, such as CVD, whose parameters can be changed to control the topography and surface properties of the NDs.38 The tunable surface hydrophilicity can also increase the osteoblast’s efficiency (a cell that secretes the matrix for bone formation) adhesion of the implant, making it more capable of bone tissue regeneration.39 When combined with other bioactive materials, such as hydroxyapatite, NDs can enhance their osteoinductive properties.40 Apart from bone tissue regeneration, NDs have also been used in neural tissue regeneration.41 Unlike osteoblast, NDs’ surface chemistry does not have much effect on neural growth, indicating that it involves more physisorption of cells on the NDs nanoparticles. Also, due to selectively enhanced neural tissue growth on NDs, microscale patterned NDs created by photolithography and etching can create patterned neural networks.42 In addition to using NDs as cell-adhering substrates, they have been used in coating prosthetic implants to improve their tribological properties and decrease their metal ion leaching behavior.43
Owing to their excellent hardness and surface functionality, NDs are also used as nanofillers of polymer composites, generally created by electrospinning, to make tissue scaffolds.44 In addition to their potential as nanofillers, NDs can work as a delivery platform for tissue cells, giving rise to multifunctional bioactive scaffolds capable of releasing therapeutic biomolecules.45 NDs-polymer composites exhibit homogeneity, mechanical and thermal stability, and chemical resistance with a capacity to load a large number of biological structures onto their surface. Therefore, they can be used in tissue engineering, implants, stents, biosensors, scaffolds for tissue engineering, and other biomedical devices.46 These polymers can be woven in mats and are suitable for wound healing applications because of their improved mechanical properties and hydrophobicity upon NDs incorporation.47 In addition, advanced multifunctional NDs-based nanocomposites, such as silver-loaded polycation functionalized NDs, have been used as filler in dental resin since its hardness and flexural strength increase due to the presence of NDs when compared with pure resins, whereas the presence of silver enhances the bactericidal property.48
Fullerenes
C60-Buckminsterfullerene, the most symmetrical molecule and the first symmetrical carbon nanomaterial, has a spherical cage-like structure formed by sp2 hybridized carbon. In 1996, the Nobel Prize in chemistry was awarded to Richard E. Smalley, Robert F. Curl, and Harold W. Kroto for its discovery.49 The general formulae of fullerenes can be written as C2n+20, where n represents the number of hexagons. For example, C60 possesses 20 hexagons and 12 pentagons, forming a truncated icosahedron with a diameter of approximately 1 nm with very high symmetry containing 30 twofold axes, 20 threefold axes, and 12 fivefold axes. C60 has been well characterized by Raman spectroscopy, UV–visible and IR spectroscopy, and C13-nuclear magnetic resonance.50 After its accidental discovery, its first large-scale synthesis was achieved in 1990, in which graphite was electrically heated in a helium atmosphere at low pressure in order to evaporate and recondense as soot. The soot was dispersed in organic solvents and separated to isolate C60 crystals.51 Subsequently, C60 was synthesized similarly by vaporizing other carbon precursors, eg, coal, or by other evaporation techniques (eg, arc-evaporation, laser ablation, and radio frequency plasma).52 Other important synthetic routes include the incomplete combustion of benzene in oxygen53 and microwave techniques.54
There has also been ample research on fullerenes-containing polymers or polyfullerenes. These are synthesized via the covalent linkage of fullerene units,55 organometallic fullerenes,56 and cross-linked polyfullerenes.57 One- or two-dimensional self-assembled fullerene-based nanostructures, such as C60 nanowhiskers58 and C60 hexagonal thin crystalline nanosheets,59 are considered fullerene-based nanostructures. Other fullerene composites can be designed by exploiting their charge transfer ability. For example, a hybrid hexagonal nanosheet containing C60 and ferrocene has been built using donor-acceptor interactions.60 A silver-fullerene nanocomposite was also prepared by synthesizing silver nanoparticles immersed in the C60 matrix by thermal co-deposition.61
Although the chemical and physical properties of C60 show great potential towards biological applications, its inherent hydrophobicity and water-insolubility need to be addressed by adequate functionalization.62,63 In addition, surface functionalization can change the carbon hybridization from sp2 to sp3, releasing strain and making the fullerenes more stable. Interestingly, surface functionalization also reduces the cytotoxicity of fullerenes.64 The double bonds present in fullerenes can be utilized to facilitate functionalization in order to generate surface-modified fullerenes, such as amine (–NH2), hydroxyl (–OH), and carboxyl (–COOH) fullerenes. Another approach to functionalizing fullerenes is via the utilization of their electron-deficient nature in order to promote cycloaddition reactions.65 Other attempts to overcome the hydrophobicity of fullerenes have been microencapsulation between special carriers such as cyclodextrins66 or calixarenes67 and suspension with the help of co-solvents. For example, stepwise and slow addition of solvents with increasing polarity, such as benzene, tetrahydrofuran, acetone, then water, followed by the evaporation of all the non-aqueous, low-boiling solvents, can lead to water-soluble C60.68 Attempts for dissolving fullerenes include the covalent attachment of water-soluble polymers69 or surfactants.70
When C60 is excited (visible light), it rapidly decays, producing several reactive oxygen species (ROS) in the environment through an electron transfer pathway. These ROS are responsible for the antibacterial activity of fullerenes and their cytotoxicity.71 However, the toxicity of fullerenes depends on the preparation method as well as the chemical modification. Cytotoxicity induced by C60 in vitro tests with alveolar macrophages was found to be quite low compared to other carbon nanostructures, such as single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs).72 In addition, due to dissimilar fullerene-cell wall interactions, C60 has more effect on gram-positive bacteria than gram-negative ones.73 This interaction depends on the surface electrostatic nature of fullerenes. For example, hydroxyl (–OH), carboxyl (–COOH), and amine (–NH2) functionalized fullerenes show a different effect on E. coli and S. oneidensis.74 C60 can also scavenge free radicals without being consumed due to the presence of several double bonds within the fullerene cage, and it is called a “free radical sponge”.
Although the present work focuses on tissue engineering, it is worth highlighting a series of biomedical applications of Fullerene. Polyhydroxylated fullerenes, known as fullerenols, are used for cancer treatments due to their non-toxicity and biocompatibility.75 In order to prepare multifunctional biomedical systems, a combination of C60 and iron oxide nanoparticles are used in photodynamic therapy, drug delivery, and magnetic resonance imaging (MRI).76 More interestingly, C60 exhibits antiviral activity against human immunodeficiency virus (HIV) since C60 can be introduced into the catalytic cavity of HIV protease, which is the primary enzyme of HIV.77 Due to their hydrophilic nature, –OH, –COOH, and –NH2 functionalized fullerenes can act as effective mediators between biosensor electrodes and recognition sites and improve the efficiency of the biosensors.78 Due to their radical scavenging properties, fullerenes can also act as neuroprotective agents.79 Gadolinium (Gd), a metal used for MRI, generates several discomforts in patients; however, endohedral Gd-fullerenol, which is less toxic due to its biocompatibility, has been used as an MRI contrast agent.80 Because of its free radical scavenging properties described above, fullerenes can also be used in moisturizers and acne-removing creams.81
Adequate functionalization of fullerenes can lead to water solubility as well as other favorable properties, such as fluorescence or cell selectivity, which can open up the possibility of using fullerene (itself or with any other material) as a tissue scaffold material.82 The use of fullerene in bone tissue engineering has been done in recent years.83 Fullerene-coated carbon nanofibers-based microarrays, prepared using a metallic nanomask, could increase the adhesion and proliferation of osteoblastic MG-63 cells.84 Pure fullerene (C60) films can be deposited following a similar technology which showed enhanced human osteoblast-like MG-63 cell regeneration, rendering it a promising material in bone tissue engineering, especially as a bioactive coating of bone implants.85 Fullerene molecules can also be combined with other materials, such as compounds of Ti, Co, or Ni, to form binary fullerene-metal composites with promising biomedical applications.86 For example, C60/Ti composite films were fabricated by co-deposition through the molecular beam epitaxy method and utilized in enhanced human osteoblasts-like MG-63 or U-2 OS cell growth.87 Apart from bone tissue, fullerenes can be used in adipose tissue engineering as highly hydroxylated fullerene can inhibit the oxidative stress in adipose tissues through antioxidation, owing to its free radical scavenging properties.88
Carbon Quantum Dots (CQDs)
CQDs are amorphous or crystalline carbon quasi-spherical nanoparticles of size below 10 nm, with predominantly graphitic or turbostratic sp2 hybridized carbon with diamond-like sp3 hybridized carbon insertions. This unique structure may contain 5–50% oxygen, mainly on the surface as -COOH groups, making CQDs soluble in water, with possibilities of further functionalization and surface passivation, thus altering their physicochemical properties.89,90
Graphene quantum dots (GQDs) constitute a variation of CQDs and consist of graphene sheets with lateral dimensions less than 100 nm and a few-stacked layers (1–10 layers).91 Similar to graphene, GQDs have a large surface area with a π–π conjugated network with –COOH and –OH groups functionalized at the edges. The excellent electron donating and accepting properties of GQDs make them good candidates in photo-detection and solar cell applications, whereas their good conductivity makes them useful in electrochemical biosensors.92 In addition, both CQD and GQDs exhibit better chemical and photo-inertness with tunable luminescence, low cytotoxicity, and good biocompatibility compared to toxic inorganic metal oxide-based semiconductor quantum dots, thus making them a better choice for bio-applications.93
CQDs can be prepared mainly by the top-down and bottom-up approaches. While the top-down approach could be more attractive because of the abundance of raw materials, large-scale production, and the inherent oxidative reaction conditions leading to surface functionalization, it has some disadvantages, such as low yield and less control over the structure of the final products. These top-down approaches can be further classified as chemical or physical methods. The first discovery of CQDs was reported during the purification of SWCNTs through electrophoresis in 2004.94 Subsequently, CQDs were obtained by electrochemical methods using graphite as electrodes in an alkaline or aqueous medium.95 In addition, graphene electrodes have been used to produce GQDs with 1–3 graphene layers.96 Combustion of carbon-rich precursors and the subsequent purification can result in CQDs.97 However, monodispersed CQDs can be synthesized using molecular sieves, such as mesoporous silica or porous carbon as support.98 Acid-oxidation of carbon-rich precursors, such as coal, has also prepared GQDs.99 Alternatively, hydrothermal conversion and microwave/ultrasonic irradiation of carbon-rich green precursors, such as carbohydrate molecules, can produce a clean synthesis of CQDs.100,101 Some other methods, such as ozonation, H2O2 mediated photo-Fenton reaction, and oxygen plasma treatments of GO, have also been used to synthesize CQDs and GQDs. Apart from these chemical methods, CQDs can be obtained by physical treatments such as arc discharge, laser ablation, and plasma treatments of carbon targets at high temperature and pressure conditions. On the other hand, while the bottom-up methods can control the shape, size, and properties of synthesized CQDs, the complexity of the procedure is challenging, and the general hydrophobic synthetic condition may lead to agglomeration of the resultant CQDs. One example of this approach is Ruthenium catalyzed cage-opening of fullerenes, where structural transitions between graphene and fullerene are utilized to obtain geometrically well-defined GQDs.102 Other bottom-up approaches, such as cyclodehydrogenation of polyphenylene precursors, stepwise oxidative condensation reactions of carbon precursors, such as glucose can be used.103 These top-down and bottom-up approaches can induce size-control and band-gap tuning of the resulting CQDs since these two are responsible factors for their optical properties.
CQDs have also been prepared from biocompatible and natural precursors such as citric acid due to their cost-effectiveness, flexible designability, and straightforward synthesis routes.104 However, depending on the applications, the synthesis strategy could be modified to achieve surface functionalization, such as amine or aryl functionalization or polyethylene glycol passivation.105 The cytotoxicity of CQDs varies as a function of their surface molecules.106 Furthermore, different effects on the cancer cell stages have been reported depending on the surface physicochemical characteristics of modified CQDs with neutral, anionic, and cationic surface functionalities.107
Another way to modify CQDs’ properties is by doping with other elements, such as nitrogen108 or fluorine.109 Novel, non-toxic N-doped chitosan-based CQDs have been synthesized using amino acids where the quantum yield (QY) can be varied by changing the amino acid.110 Biocompatible red-emitting magnesium–nitrogen-doped CQDs were obtained by carbonization of a leaf extract.111 CQDs are essential for their photoluminescence (PL) properties, which are tunable by varying the size of the CQDs, utilizing quantum confinement in conjunction with surface modifications to generate emissive trap sites. The PL properties vary with their size; the PL wavelength increases with the increasing size of CQDs as the band gap decreases. However, CQDs show strong electrochemical luminescence (ECL), which is solely a surface-state phenomenon. So, it had been surmised that the most intense PL bands of CQDs are due to their core inherent band gap, which is size-dependent, but the less intense PL bands may be due to surface traps. The CQDs show stable PL and ECL responses over time, and their QY may vary with the synthesis method and the surface chemistry involved. One of the disadvantages of CQDs is their low QY (often less than 10%). However, passivation with organic polymers,112 doping, and reducing the CQDs can increase the QY to 50%.113
CQDs’ optical and electronic properties can be modified by changing their assembly in the solution or onto the substrate. For example, CQDs are assembled in a plane (face-on) or out-of-plane (edge-on) fashion by electrophoresis or other guiding techniques.114 CQDs can also show upconverted PL (UCPL), which is a multiphoton process involving low-energy photons (in the visible or IR region), thus providing a new way to develop novel photocatalysts combining CQDs and other materials. For example, for TiO2/CQDs systems, CQDs absorb visible light and then emit in shorter wavelength via up conversion, which excites TiO2 to generate electron-hole pairs that give rise to ROS, capable of dissociating larger organic molecules, eg, harmful dyes. This can even be exploited in photovoltaics.115 Some metal ions and other compounds, even some biomolecules, such as human immunoglobulin, can quench the PL of CQDs by facilitating non-radiative electron/hole recombination via an effective electron transfer method; thereby, CQDs can act as a sensor for those biomolecules.116 Gold-CQD composite materials exhibited surface-enhanced Raman scattering (SERS) for rhodamine 6G dye molecules due to their improved adsorption on the CQDs surface.117
Because of non-toxicity and excellent PL properties, CQDs are an attractive candidate for in vivo and in vitro imaging and nanomedicine applications.118,119 CQDs can be used as photosensitizers for photodynamic therapy.120,121 Smart multifunctional biomaterials can also be synthesized utilizing the unique properties of CQDs. For example, bioactive CQD/organosilica nanospheres exhibit visible-light emission for optical imaging, Near-Infrared photothermal activity used in photothermal therapy, and loading capacity for controlled drug delivery.122 A multifunctional core-shell structure composed of olive oil, Fe3O4, porous TiO2, and GQDs can be used in oil-soluble drug delivery as well as magnetic and fluorescence imaging.123
CQDs have also found remarkable applications as a tissue engineering material due to their engineered PL properties, biocompatibility, tunable surface functionalities, and cost-effective synthesis.124 Primarily, the enhancement of mechanical properties in bone regeneration scaffolds is attributed to efficient cellular interplay and the process of forming cross-links.125 For example, CQDs, combined with hydroxyapatite, show significantly increased cell proliferation, the activity of alkaline phosphatase as well as mineralization.126 Another attractive factor is the cheap and easy fabrication procedure. For example, a biological scaffold with enhanced bone regeneration activity containing CQDs, hydroxyapatite, and polyurethane had been synthesized from an eco-friendly one-pot hydrothermal synthesis employing sustainable, renewable bio-based starting materials: calcined eggshell and the water-based extract from a plant, C. esculenta corm (taro).127 Besides enhancing the mechanical properties, CQDs improve cell adhesion, proliferation, and anti-bacterial and anti-tumor properties.128 CQDs-based nanomaterials are also used in nerve tissue regeneration.129 Various metals, such as gold, silver, tin, zinc, and gallium, can be doped in CQDs through simple sonochemical processes, and the resulting doped CQDs have shown excellent biocompatibility against neuronal cells even in relatively higher concentrations.130 For example, gallium doped CQDs coated glass substrate was registered to be a very good supporting material for neuronal cells differentiation and growth compared to bare glass substrate.131 Also, γ-Fe2O3/CQDs magnetic nanoparticles were synthesized which, along with high fluorescence and very good biocompatibility, show efficacy to various neuronal manipulations, including cell labeling and magnetic force-driven controlled cell motility.132
Further details on the applications of CQDs, especially pertaining to their role in tissue engineering and regenerative medicine, can be found in two recently published reviews.133,134
Carbon Nanotubes (CNTs)
CNTs consist of one-dimensional (1D) tubular structures, which can be constructed by rolling up sp2-hybridized single- or multi-layered graphene sheets into a hollow, seamless cylinder. The rolling direction can be defined by a chiral vector, a pair of integers (n,m), and CNTs can be either metallic or semiconducting, depending on the tubes’ chirality.135–137 Experimentally, the synthesis of both SWCNTs and MWCNTs can be achieved by several methods, including laser ablation,138 arc discharge, and chemical vapor deposition (CVD). In the arc discharge process, CNTs are produced by creating an electrical arc between two graphite electrodes in an inert atmosphere.139 The discharge at the graphite anode generates a high temperature that sublimates graphite and leads to the deposition of CNTs on the cathode. This method usually yields MWCNTs, but it has been found that adding a metal catalyst in the anode and controlling the temperature gradient between the electrodes can also yield SWCNTs.140,141 An alternative method is to apply a laser pulse to a target containing graphite and metal catalysts, which leads to a similar effect as the electrical arcing and ablates the graphite at a high temperature to produce SWCNTs and MWCNTs.142 Nowadays, a commonly used technique for the scaled-up synthesis of CNTs is catalytic Chemical Vapor Deposition (CVD).143 In the CVD method, carbon-containing precursors (eg, methane, acetylene, or toluene) flow to the hot zone of a furnace inside a tube reactor. The precursor decomposes in the presence of transition metal catalysts (eg, Ni, Fe, Co, and Mo).144 The resulting morphologies (length, diameter, and crystallinity) of CVD-grown CNTs are controlled by temperature, precursor chemistry, precursor/carrier gas flow rates, catalyst, and growth time, among other experimental parameters.145 CVD has been reported to produce CNTs at gram-scale,146 and the individual tube length is usually much larger than the tube diameter, ranging from micrometer- even to meter-scale.147 The as-synthesized metallic and semiconducting CNTs have been employed as high-performance interconnects in integrated circuits and nanoelectronics such as field-effect transistors.148,149 The Young’s modulus and strength of high-quality CNTs are measured to be ~1 TPa and tens of GPa, respectively, higher than steel. This leads to CNTs in nanocomposites, where CNTs are employed as fillers to reinforce the mechanical strength and stiffness of the polymer matrix.150
The obtained CNTs after synthesis generally present a wide range of impurities such as amorphous carbon, graphitic nanoparticles, fullerenes, and particles from catalysts, which can be a problem during the use in tissue engineering, so it is necessary to improve the actual purification techniques besides development of new methods.151 Toxicity is another challenge in the application of those materials. CNTs show more significant toxicity than most other CBNs, second only to graphene, justifying the requirement of new treatment strategies to modify their length, surface and properties.152
Carbon Nanofibers (CNFs)
CNFs are linear filaments of sp2 hybridized carbon. Unlike conventional carbon fibers with a micrometer or larger diameters, CNFs are characterized by their much smaller diameters (~ 100 nm).153 In addition, CNFs should also be differentiated from CNTs, as CNFs can be viewed as stacks of carbon nanocones along the fiber axis, which do not have perfect structural configurations compared to uniform tubular CNTs. CNFs can be synthesized by a catalytic CVD process similar to the growth of CNTs, or by high-temperature carbonization of electrospun polymer nanofibers such as polyacrylonitrile (PAN).154,155 The as-synthesized CNFs exhibit high mechanical strength and modulus, and they are usually considered biocompatible as CNFs are high-purity carbon with low densities of impurities and functional groups. Thus, CNFs can be assembled into 3D web-like structures as scaffolds for bone tissue regeneration.156
Graphene and Its Derivatives
Since the successful isolation and identification of graphene in 2004, this two-dimensional (2D) form of sp2 hybridized carbon has gained tremendous research attention due to its unique properties, including one-atomic-layer thickness, remarkable mechanical properties (Young’s modulus of 1 TPa, and strength of 130 GPa), along with robust electrical and thermal conductive properties.140,157 Furthermore, GO can be synthesized on a large scale through partial oxidation and exfoliation of graphite crystals.158 This technique results in GO sheets that can be dispersed in water, presenting numerous oxygen-based functional groups on their surface. The synthetic GO materials can be further reduced to remove most surface functional groups, forming reduced graphene oxide (rGO) with properties akin to pristine graphene.159,160
Although the excellent structural and mechanical properties suggest that graphene and its derivatives described above possess the potential to be incorporated into scaffolds for tissue regeneration applications, the unmodified graphene, GO, and rGO can improve their performance by surface functionalization or modification.14,125 Surface functionalization is needed to mitigate the biocompatibility issue of graphene-based nanomaterials. Two main types of surface functionalization, covalent functionalization and non-covalent functionalization, are commonly applied. Covalent functionalization is usually achieved by reacting with oxygen functional groups on GO and rGO. Examples of covalently functionalized groups include aliphatic amines and amino acids,161 polyethylene glycol (PEG),162 and poly(vinyl alcohol) (PVA).163 Alternatively, non-covalent functionalization relies on the non-bonding interactions between 2D sheets and molecules, such as π-π, hydrophobic and electrostatic interactions.164,165 Both covalent and non-covalent functionalization are reported to reduce the cytotoxicity of graphene and its derivatives by attaching proper functional groups and/or molecules.11
Due to GO’s physicochemical versatility, it has recently been applied as a biomaterial for tissue engineering and drug delivery. The chemical structure of this compound, including carboxyl (−COOH), hydroxyl (−OH), and epoxy (−O−) moieties, increases its hydrophilic properties, which improves the bioavailability of this biomaterial.166 These plentiful oxygen-based functional groups can also serve as sites for functionalizing GO with a range of biomolecules, making possible the use of this graphene in drug delivery applications.167 GO is also an excellent candidate to be applied in connective tissue regeneration once it presents a sizeable π-conjugated structure and surface area (890 m2/g) that shows the potential to absorb proteins and adhere to cells. In addition, this vast surface area allows the GO to create a structure for managing stem cell activity without being invasive, contributing to the liberation of potent biological elements and transport of growth factors, DNA, and man-made proteins that can influence stem cell differentiation and multiplication.168–170 The electronic configuration of GO also facilitates additional chemical alterations that, together with maintenance of the sp2 bonding lattice, endows this compound to be applied in cardiac and nerve regeneration.171 Regarding GO’s mechanical properties, its robustness contributes to a matrix that can resist mechanical forces during bone and cartilage regeneration [165]. Therefore, GO exhibits a range of advantageous characteristics, such as exceptional bio-compatibility, substantial drug-holding capacity, electric conductivity, along with adjustable size and form.172
Carbon-Based Nanomaterials as Scaffolds for Tissue Regeneration
In tissue engineering, the purpose of scaffolds is to mimic the structure and function of the native extracellular matrix (ECM) to promote an optimal environment for cell adhesion, migration, proliferation, and differentiation and stimulate the growth of new tissue.173 Although different human tissues have distinct characteristics and specific needs within tissue engineering, in general, the design of an ideal scaffold should consider physicochemical and biological characteristics, including biocompatibility and biodegradability in a living organism, bioactivity, mechanical characteristics, microarchitecture, and porosity.174,175
Progress in the use of nanotechnology within the field of tissue engineering have revolutionized regenerative medicine, resulting in the synthesis of innovative scaffolds. In particular, due to their special chemical, physical, mechanical, electrical, optical, and biological properties, CBNs have played a prominent role in developing these scaffolds, and their application in various human tissues has been investigated.
Building upon the detailed discussions presented in the preceding section regarding individual carbon-based nanomaterials, it is imperative to provide a synthesized perspective for readers. To that end, we have consolidated the primary positive characteristics and challenges associated with each of these carbon-based nanomaterials concerning their application as scaffolds for tissue engineering, aimed at tissue regeneration. This summary is presented in Table 1, offering a succinct comparison that underscores the potential and hurdles of each material within the context of tissue engineering.
 |
Table 1 Overview of Selected Carbon-Based Nanomaterials Highlighting Their Key Advantages and Challenges for Use as Scaffolds in Tissue Engineering |
The following topics summarize the recent and main findings concerning the most common applications of CBNs in tissue engineering (Figure 2).
Bone Tissue Engineering Applications
Bone is a mineralized tissue with a hierarchical structure formed by cells and an ECM that consists predominantly of water, collagen (mainly type I), and a calcified matrix in which hydroxyapatite (HAp) is the most abundant component. As a fundamental member of the human musculoskeletal system, bones play an essential role in the mechanical support and movement of body segments, in addition to protecting organs, maintaining the homeostasis of key electrolytes, and hosting the bone marrow.176,177
Unlike other human tissues, bones exhibit a high level of regeneration and self-repair, and most of the time, Bone damage and breaks recover without the development of scar tissue.178 However, for fractures or large bone defects, bone healing fails. Thus, many people require skeletal reconstruction or bone defect replacement caused by conditions such as high-energy trauma, bone tumor resection, congenital diseases, and bone infection. Accordingly, these bone defects are managed based on bone graft, the second most commonly transplanted human tissue (after blood transfusion).179,180
Although autologous bone grafting or autografts still are the gold standard for repairing large bone injuries, they carry limitations such as high morbidity at the harvesting site, secondary damages, and low availability. In addition, allografts and xenografts are also options available in clinical practice. However, these methods have disadvantages, including the risk of disease transmission and graft rejection.176,181
In the search for better therapeutic strategies, tissue engineering has emerged as a potential approach for bone regeneration since it can overcome some limitations of bone grafts and enhance the healing processes of bone fractures and bone damage. More specifically, bone tissue engineering aims to induce the repair and regeneration of new tissues based on the synergistic interaction between cells, signals, and the biomaterial scaffold.176 A suitable scaffold for bone regeneration should be biodegradable, biocompatible, bioactive, osteoconductive, and osteoinductive, and have optimal mechanical properties.125,176
Carbon-based nanocomposites continue to be some of the most popular approaches to constructing scaffolds for bone regeneration. Their key benefits over other biomaterials include superior biocompatibility without harm to bone cells, biodegradability, robust mechanical characteristics, inherent antibacterial action, and remarkable impacts on gene expression and the multiplication of osteoblasts.125 In this context, several CBNs have been investigated for their use in the field of bone tissue reconstruction, highlighting graphene and its derivatives and CNTs. Here we describe current progress and the most recent findings of these nanomaterials for bone repair applications. An even more detailed reading on the CBNs in bone tissue engineering applications can be found in two recently published comprehensive reviews.124,125
Graphene itself has demonstrated its excellent ability to promote cellular osteogenic differentiation169,182,183 and has been used as filler in nanocomposite scaffolds for bone regeneration. The conjunction of graphene with bioactive glass,184 HAp,185 and HAp with natural polymer186 has been shown to significantly enhance the mechanical properties, proliferation, and cell differentiation of the scaffold.
However, the oxidized variant of graphene, known as GO, is more frequently employed in in tissue engineering applications. Besides the high adhesion ability, excellent osteogenic cell differentiation outcome, mineralization promotion, and improved mechanical strength, incorporation187 into polymer-based scaffolds188–191 demonstrates the potential of GO to improve the performance and interaction between biopolymers and bioceramics in composite scaffolds, by introducing strong electrostatic and π-π interactions.192–194 In addition, similar to GO, the incorporation of rGO improves mechanical properties and promotes osteogenic differentiation (Figure 3), thus enhancing the performance of bioceramics-based195–197 and polymer-based scaffolds.198
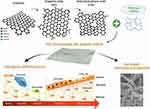 |
Figure 3 Schematic representation of the rGO incorporation polymer-based scaffold to increase the mechanical properties and promotes osteogenic differentiation. |
GO has shown potential as a useful platform for bone injuries, as it replicates both the chemical and mechanical attributes of bone. Moreover, it encourages the differentiation of stem cells, thereby aiding in the regeneration dependent on cells.168 For example, Wang et al linked hydroxyapatite to GO, demonstrated adherence and growth of mouse-derived mesenchymal stem cells (MSCs), and stimulated the osteogenic gene osteocalcin expression, thus promoting differentiation of MSCs into bone.199 Afterward, Fu et al incorporated poly(lactic-co-glycolic acid) (PLGA) into these compounds, which promoted the mechanical stability of the scaffolds, and induced alkaline phosphatase activity, gene expression related to osteogenesis, and mineral deposition.200 Boga et al documented that a fabricated scaffold, consisting of 3D cylindrical tricalcium phosphate (TCP) and alginic acid (AA) functionalized with GO, could offer a temporary structure for human osteoblast (hOB) cells to attach. The in vivo studies were realized in mice models. The findings revealed a satisfactory osteoinductive response following the non-covalent alteration of the graphene oxide (GO) surface using recombinant human bone morphogenetic protein 2 (rhBMP-2), a potent growth factor known for its remarkable capability to guide mesenchymal stem cells (MSCs) towards osteogenic specialization.201 Another important study performed by Xue et al202 demonstrated that graphene oxide (GO) facilitates the differentiation of bone marrow-derived mesenchymal stem cells (BMSCs) towards an osteogenic lineage. This effect is achieved by inducing the release of inflammatory cytokines and enhancing the expression of vascular endothelial growth factor (VEGF) pathway in human umbilical vein endothelial cells (HUVECs). These findings suggest that GO exhibits dual functionality in promoting both bone formation and the formation of new blood vessels, which are critical processes for successful bone regeneration. Thus, all these effects are correlated to the incorporation of graphene and GO, which simultaneously promotes differentiation and greatly improves the mechanical strength of biocomposites. A recent study by Wang et al aimed to evaluate a novel zwitterionic hydrogel incorporated with GO for bone tissue engineering.203 In vitro and in vivo tests showed that the hydrogel with GO greatly augmented the osteogenic differentiation of BMSCs in a dose-dependent manner, with 2 mg/mL GO being the optimum concentration. The incorporation of GO in the scaffold reduced its cytotoxicity and slowed down the release rate.
Another carbon-based nanomaterial of prominence in bone tissue engineering is CNT. Although it has already been investigated in isolation as the only component of the scaffold and has shown promising results in osteogenic differentiation, cell proliferation, and inhibition of osteoclast,204–206 the main potential of CNTs for bone regeneration is as a reinforcing agent in nanocomposite scaffolds. Nevertheless, in addition to the increase in mechanical performance, even with the incorporation of small CNTs concentrations, CNTs have shown an important role in improving cellular attachment, proliferation, and promoting osteogenic differentiation, both in conjunction with polymers207,208 or HAp209,210 or in scaffolds based on mixtures of polymers with Hap.211,212
Much less frequent in the literature than GO or CNT, fullerene-based scaffolds have also been investigated for bone tissue engineering. Although it has the disadvantages of only nonpolar carbon-carbon bonds, the ability to produce detrimental reactive oxygen species (ROS), and difficulty forming 3D porous scaffold,124,213 some benefits have been reported with the use of fullerenes for bone regeneration, mainly regarding the orientation of the adhesion and growth of osteoblastic cells.84,85,214 Fullerenes may also incorporate more hydrophobicity and roughness in a composite scaffold, increasing the ability to control the cell attachment.125
Fullerene’s greater ROS production also is useful for osteoporosis treatment. For this reason, hydrophilic bisphosphonate groups like hydroxyapatite (applicable in osteoporosis) were coupled with fullerene, preparing a tissue-vectored compound, namely C60(OH)16AMBP.215 This compound was able to contribute to osteoclast differentiation beginning with RANK-RANKL signals, as well as to help the treatment of osteoclast hyper-resorption on osteoarthritis.216 Therefore, it has been proved that fullerene can reduce joint destruction and suppress bone resorption caused by osteoclasts215 and that the fullerenols stimulate osteogenesis in bone marrow.217
NDs represent an alternative form of carbon-based nanobiomaterial applied to bone tissue regeneration. As a result of its intense fluorescence, a scaffold with NDs could be used to monitor the scaffold behavior in vivo during bone tissue growth.218 Furthermore, together with anti-inflammatory properties, studies have indicated that NDs promote cellular attachment and enhance cell proliferation, osteogenic activity, and mechanical properties when incorporated into polymeric matrices such as Poly-L-Lactic Acid (PLLA)219 and Poly-Lactic-co-Glycolic Acid (PLGA),220 making this carbon-based nanomaterial a promising biomaterial for the management of large bone defects.
Further, the unique optical and physical properties have encouraged using CQDs for bone tissue application. In addition to applications in bone crack detection, targeted bone imaging, and drug delivery,221–223 incorporating CQDs in polymers and bioceramics matrix has shown promising results in repairing bone defects. Among the main advantages of CQDs for bone regeneration described in the literature are the stimulation of osteogenic differentiation, good osteoblast adhesion and proliferation, low toxicity, simple fabrication methods, excellent mechanical properties, and the ability to improve the distribution of HAp in polymer matrix.125,128,224–226
Although further investigations are required to comprehensively explore the complete capabilities of CBNs for bone regeneration, mainly with regards to more complex in vivo assays, understanding of the mechanisms behind biological and physical changes, and clinical translation challenges, evidence points to the area of bone tissue engineering as one of the most promising for applications of these unique materials, mainly due to its admirable ability to stimulate osteogenic differentiation and provide exceptional mechanical properties.
Articular Cartilage and Osteochondral Tissue Engineering Applications
Articular cartilage is a load-bearing and viscoelastic connective tissue that overlays the end surfaces of bones in a synovial joint, reducing shear friction forces, increasing lubrication, bearing and transferring load, and thus performing an essential role in the articular function.227 Unfortunately, articular cartilage’s avascular structure and poor metabolic activity reflect a limited regenerative capacity. Consequently, damages in articular cartilage caused by trauma or degenerative diseases are irreparable and result in early osteoarthritis, usually accompanied by chronic pain and disability. Osteoarthritis, reported as the most common joint disease, represents the second leading cause of physical disability, with a vast socioeconomic impact worldwide.178,228,229
Although new approaches have been developed, the management options available in clinical practice today for cartilage injuries are deficient and have controversial results.227,230 Thus, effective therapies and methods for treating articular cartilage defects remain one of the biggest challenges in orthopedics. In this context, tissue engineering methods have emerged as a potential approach in medicine regenerative for cartilage repair. Particularly, this strategy has been focused on stimulating the regeneration of cartilaginous tissue through the combination of scaffolds, cells, and bioactive substances (eg, growth factors).227 The effectiveness of the cartilage tissue engineering approach is closely related to the design of biomaterial scaffolds able to support, guide, and stimulate tissue growth. Considering that the most important role of articular cartilage is to resist mechanical loads and absorb shocks, the engineered scaffold for cartilage regeneration should be able to mimic the mechanical properties of the native cartilage.231 In this sense, several researchers have developed scaffolds integrating natural or synthetic polymers with CBNs. Thus scientists, by using carbon-based materials, aim to produce scaffolds with sufficient mechanical strength and with conductive and chemical properties which facilitate the differentiation of MSCs towards a chondrogenic lineage by utilizing the electrical and mechanical cues provided by these materials.
Since CNTs have shown positive effects on chondrocyte adhesion, proliferation, and differentiation,232–234 CNTs have been used to create reinforced nanocomposites for articular cartilage regeneration. Markowski et al reported a substantial increase in mechanical properties (Young’s modulus and tensile strength) in Polylactic acid (PLA)-based nanofibrous scaffold when small concentrations of CNTs were incorporated (1% wt) without experiencing cytotoxicity and genotoxicity (Figure 4).235 Incorporating CNTs in bovine articular cartilage decellularized matrix also demonstrated a significant improvement in the mechanical properties of the scaffold without negatively affecting its biocompatibility.236 Recently, Mirmusavi et al evaluated the potential of combining Poly 3-hydroxybutyrate (P3HB), silk, chitosan, and CNTs for cartilage tissue engineering. In addition to increased tensile strength, the scaffold containing CNTs showed better bioactivity properties and provided a suitable environment for chondrocyte adhesion and proliferation.237 It is clear that, although load-bearing characteristics of articular cartilage depend closely on its mechanical properties, it is a soft tissue that has its environment much better mimicked by hydrophilic polymeric matrices with a high water content such as hydrogels.238 Thus, despite all the unique properties of CNTs, mainly of mechanical strength, in articular cartilage tissue engineering, this type of CBN is evidently more linked only as a reinforcement filler and not as the main component of scaffolds.
 |
Figure 4 The effect of the incorporation of CNT in PLA nanofibers on the mechanical properties (A) and cell viability (B), compared with pure PLA and PLA with gelatin (GEL). Adapted from Markowski J, Magiera A, Lesiak M, Sieron AL, Pilch J, Blazewicz S. Preparation and characterization of nanofibrous polymer scaffolds for cartilage tissue engineering. J Nanomater. 2015;2015:1–9. Creative Commons.235 **Statistical difference P < 0.01 when compared to control. |
In the same way, due to its exceptional biocompatibility and impressive mechanical characteristics, graphene has also been applied in cartilage tissue engineering. For example, Liao et al synthesized a hybrid scaffold based on methacrylated chondroitin sulfate (CSMA), methoxyl poly(ethylene glycol)-poly(ε-caprolactone)-acryloyl chloride (PECA) and GO (Figure 5).239 The results demonstrated a scaffold with porosity, swelling behavior, degradation rate, compression modulus, and biocompatibility with cartilage cells, suitable to mimic the native ECM of cartilage. Furthermore, in in vivo assays, the scaffolds showed good results for the repair of full-thickness cartilage damage. Shamekhi et al researched chitosan-based scaffolds reinforced with GO nanoparticles. In addition to the enhancement in scaffold stiffness and surface roughness with increasing GO content, the authors also reported an increase in the proliferation and improvement of the morphology of human articular chondrocytes.240
 |
Figure 5 Schematic representation showing the CSMA/PECA/GO hybrid scaffold with potential application in cartilage tissue engineering. To produce the scaffold, an aqueous solution containing PECA, CSMA, GO, and Ammonium persulfate (APS, used as the initiator agent) was heated at 60° C for 2 h. Then, after the incorporation of cells, the scaffold was inserted into a defect in rabbit articular cartilage, where it demonstrated an important regenerative capacity after 18 weeks. Reprinted from Liao J, Qu Y, Chu B, Zhang X, Qian Z. Biodegradable CSMA/PECA/Graphene porous hybrid scaffold for cartilage tissue engineering. Sci Rep. 2015;5(1):9879. Creative Commons.239 |
Although the mechanical properties of the scaffolds have not been carefully evaluated, Deliormanlı demonstrated the potential chondrogenic differentiation of mouse bone marrow MSCs (mBMSCs) in Polycaprolactone (PCL) scaffolds containing pristine graphene nanopowders. Furthermore, cells seeded onto the scaffolds with graphene showed greater cell viability than pure PCL scaffolds.241 The authors suggest that graphene-containing scaffolds’ increased protein adsorption capacity can explain these results. Graphene can readily interact with a wide range of proteins that influence cell proliferation and differentiation when adsorbed on the surface.242 In addition, graphene-containing scaffolds exhibit better electrical conductivity compared to pure PCL scaffolds. Greater electrical conductivity improves cell communication and increases scaffold adhesion and proliferation.243
Regarding NDs, Wang et al recently used functionalized NDs on their biodegradable scaffold for cartilage regeneration. Adding NDs in polyurethane (PU) composites demonstrated less toxicity, increased crystallinity, and a consequent considerable improvement in tensile properties.244 Although this single published study, NDs have also contributed in another way to treating defects in articular cartilage. Although it is not directly related to stimulating cartilage regeneration, it is worth mentioning that NDs have been used successfully as a long-term cell tracking tool for studying in vivo chondrogenesis without affecting cellular performance, such as proliferation and differentiation.245 Briefly, in this method, stem cells labeled with Fluorescent Nanodiamonds (FNDs) implanted in an animal model can be identified and quantified in the long-term, as well as detecting the chondrocyte-specific markers, such as type II collagen and aggrecan, by Fluorescence spectra of FNDs acquired using a Magnetically Modulated Fluorescence (MMF) spectrometer.246 Accurately tracking stem cells in vivo over the long term is one of the vital requirements in evaluating the efficacy and safety of stem cell treatments and one of the main challenges of regenerative medicine and tissue engineering.
About other types of CBNs less investigated for articular cartilage tissue engineering applications, newly doping CQDs into calcium phosphate has demonstrated the potential to positively affect the differentiation pathway and improve chondrogenesis.247 Concerning the fullerenes, although they have shown a capacity to inhibit inflammation and degeneration by down-regulation of chondrocyte catabolic activity,248–251 to our knowledge, to date, no research has been published towards the fullerene-based scaffold for cartilage regeneration. Combining the therapeutic effects of fullerene for inflammatory joint conditions with the positive results of cell performance found in studies with other types of cells and applications84,214,252 leads us to believe that fullerene may be a potential nanobiomaterial for use in the construction of scaffolds for articular cartilage tissue engineering, both as a filler and as a coating surface, however, for this, additional investigations specifically on articular cartilage, need to be carried out.
In many cases, the bone located just below the cartilage gets damaged, resulting in a defect known as an osteochondral lesion.253 For successful osteochondral tissue engineering, scaffolds should possess the capability to regenerate both the cartilage and the underlying subchondral bone, taking into account the distinct characteristics of each tissue, such as mechanical properties, chemical composition, and regeneration capacity.254,255 Therefore, composites that combine more than one type of biomaterial, frequently polymers, and bioceramics, have been considered since scaffolding based on a single type of matrix has shown a series of limitations for osteochondral defects.256–259
Recent studies, including CBNs on composite polymer-bioceramic scaffolds, have exhibited encouraging outcomes in the treatment of osteochondral lesions using these approaches. For example, Deliormanlı and Atmaca evaluated the biological behavior of osteoblastic MC3T3-E1 and chondrogenic ATDC5 cells to PCL/bioactive glass bilayered scaffold with graphene.260 For part of the articular cartilage of the osteochondral tissue, the authors used the graphene-containing PCL scaffolds, while the PCL/bioactive glass/Graphene layer was used to replicate the part of the subchondral bone. Higher cell viability, no cytotoxic effects, larger mineralized areas, and increased synthesis of glycosaminoglycans were observed on graphene-containing scaffolds compared with bare scaffolds, demonstrating the positive effects of graphene for this application. Encouraging findings have been achieved through the utilization of composite nanofibers consisting of Chitosan/Poly (vinyl alcohol) (PVA)/GO and biocompatible nanocomposite hydrogel films composed of chitosan/GO (CS/GO) for cartilage tissue engineering. These materials exhibit desirable toughness and strength properties.261,262 Certain scaffolds, comprising a combination of poly (ethylene glycol) methyl ether-ε-caprolactone acryloyl chloride (MPEG-PCL-AC), chondroitin sulfate (CSMA), and GO, have displayed favorable attributes related to chondrocyte morphology, biocompatibility, and the in vivo restoration of cartilage defects.239 Two studies conducted by the same research group have shown promising results regarding adhesion and gene expression of both chondrocytes and osteoblasts on porous Poly-d, l-lactic Acid (PDLLA)/Nanohydroxyapatite/CNT scaffold.263,264 The main findings of the studies suggest the potential of this nanocomposite for osteochondral tissue applications.
Finally, when it comes to joint cartilage regeneration, the recent trends in regenerative medicine are injectable hydrogels. This type of scaffold has the excellent ability to fill irregular tissue defects, requires minimally invasive procedures to be applied, and has reduced therapeutic costs.265 An ideal injectable hydrogel for articular cartilage should be able to preserve the viability of encapsulated cells during administration (low viscosity) and quickly polymerize in situ in a robust hydrogel with mechanical properties similar to native tissue, prerequisites that have not yet been fully resolved by current injectable hydrogels.265,266 In this scenario, in the future, the incorporation of CBNs should help to overcome these challenges of injectable hydrogels for the articular cartilage and subchondral defects, especially the problems related to mechanical behavior.
Skeletal Muscle Tissue Engineering Applications
While muscle tissue has some regenerative ability, extensive injuries or muscle volume loss require tissue engineering treatments. The primary method used is to design a scaffold that can promote muscle cells’ growth, alignment, and differentiation.267 In this context, carbon nanomaterials are either used directly as the scaffold material or as an additive or coating for some other biocompatible polymer scaffold to harness the benefits of its remarkable electrical conductivity, mechanical strength, and unique surface structure and chemistry of CBNs.268 The effectiveness of various scaffolds incorporating graphene, GO, rGO, and CNTs has been widely studied, typically using the model of C2C12 mouse myoblast cells. These models have shown that CBNs can improve conductivity, myotube formation, and myoblast differentiation.269
It is crucial to mimic the environment of the natural ECM to promote the regeneration of skeletal muscle cells, so the biomaterial used for the scaffold must mimic the ECM’s behavior. Adding graphene or GO to other biomaterials can enable better emulate these properties [300]. Furthermore, owing to their exceptional characteristics such as superior electrical conductivity (0.6 S/m), ultra low density, and exceptional flexibility, graphenated materials demonstrate tremendous potential as ideal cellular substrates for muscle tissue engineering applications.18 In the case of skeletal muscle, GO-based compounds promote attachment, proliferation, and differentiation of its precursor cells.270 Investigations involving scaffolds incorporating GO for skeletal muscle regeneration have conclusively demonstrated notable improvements in cell viability, aspect ratio, and the expression of myogenic marker genes CD56, myogenin, and desmin. These advancements were notably superior when compared to control surfaces such as glass and collagen.271 GO has been incorporated into several biomaterials already used in skeletal muscle engineering and shows a significant increase in the proliferation and attachment of C2C12 skeletal myoblasts, including GO/poly (lactic-co-glycolic acid, PLGA)/Collagen hybrid matrices,270 graphene oxide/polyacrylamide,272 graphene-containing poly(ε-caprolactone) (PCL) nanocomposites.273
Experiments that culture skeletal muscle myoblasts directly grown on graphene and its derivatives have shown increased differentiation of myoblasts and growth rate of myotubes. Furthermore, patterning graphene allows for control of growth location and improved myotube alignment.274 Likewise, GO and rGO substrates show an improvement of 1.5 to 2-fold in most measures of myoblast growth over growth on glass. Ku and Park propose that surface oxygen improves the uptake of proteins from the growth matrix.270 Due to the good electrical conductivity of CBNs, it is possible to further improve the growth and formation of myotubes through electrical stimulation. By applying an 8V, 1Hz signal during growth, Ahadian et al found a doubling in the myotube coverage area by a combination of electrical stimulation of the myocytes and thermal reduction of GO over unstimulated and un-reduced GO, in addition to improvements in myotube length, and myogenic gene expression.275 In order to overcome the surface area and structural limitations of 2D materials, graphene can be incorporated into 3D scaffold structures. For example, 3D graphene foams and polyurethane foams coated by GO have been shown to promote myogenic growth while assuming a structure that can more easily mimic a natural cell environment.276,277
Cardiac Tissue Engineering Applications
Cardiac disease is a leading worldwide cause of death, which places cardiac muscle tissue engineering as an important area of research.278 The importance of electrical signals to heart function and the large mechanical strain on cardiac tissue means that an ideal scaffold for tissue engineering should be conductive and contractile.279 Carbon nanomaterials such as CNTs and CNFs have been incorporated into polymer-based scaffolds to improve the scaffold’s electrical conductivity and mechanical properties and improve cardiomyogenesis. Furthermore, at the nanoscale, even individual nanotubes can direct the growth of cells and possess the potential to augment the differentiation of MSCs280,281 (Figure 6). Ren et al demonstrated that a sheet of aligned CNTs improves cardiomyocytes’ alignment and intercellular coupling, leading to an increased synchronization in spontaneous beating. These same sheets were applied as electrodes for flexible pacemakers on neonatal rat hearts.282 3D scaffolds constructed with CNTs incorporated into gelatin hydrogels have shown improved contraction and maturation of cardiomyocytes over gelatin scaffolds. In addition, once grafted into a rat host, the CNTs migrate from the scaffold and incorporate into the myocardium structure without cytotoxicity and improving regeneration.283 Another major complication with cardiac tissue regeneration is fibrosis, scarring due to excessive collagen generation by fibroblasts. Scaffolds constructed from a combination of PDMS and CNTs, and from Collagen and carbon nanohorns both show a decrease in the proliferation of fibroblasts, which can reduce the scar tissue generated.284,285
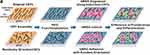 |
Figure 6 The conductivity of CNTs allows the differentiation of MSCs and promotes tissue architecture formation. Reprinted from Namgung S, Baik KY, Park J, Hong S. Controlling the growth and differentiation of human mesenchymal stem cells by the arrangement of individual carbon nanotubes. ACS Nano. 2011;5(9):7383–7390. Copyright © 2011 American Chemical Society.281 |
In the cardiac tissue engineering field, the scaffolds have been meticulously engineered to encompass a diverse spectrum of mechanical characteristics, enabling their application in the restoration of myocardial functionality. Promoting the generation of cytoskeletal structures and facilitating the formation of intercalated disks play vital roles in maintaining cardiac integrity and function. The incorporation of GO nanoparticles into oligo(poly(ethylene glycol) fumarate) (OPF) hydrogels has demonstrated the ability to enhance these essential factors.286 Recently, it has been observed that GO possesses antioxidant properties, allowing it to modulate inflammation and inflammatory polarization by reducing ROS within macrophages.287 Furthermore, GO can serve as a carrier for interleukin-4 plasmid DNA (IL-4 pDNA), facilitating the generation of reparative M2 macrophages for myocardial infarction (MI) treatment. In an in vitro study utilizing a synthetic complex termed the macrophage-targeting/polarizing GO complex, a decrease in ROS levels and inflammatory cytokine release from immune-stimulated macrophages was observed. This led to a reduction in inflammation, promotion of early differentiation into M2 macrophages, mitigation of fibrosis, and improvement in heart function in animal models of MI.168
Carbon-Based Hydrogel Scaffolds in Tissue Engineering
The effectiveness of regenerative medicine tissue engineering approaches relies significantly on the meticulous design of the scaffold. In this context, hydrogel scaffolds have captured considerable attention from researchers, attributed to their inherent resemblance to the structural composition of the native ECM.265 A hydrogel is a 3D network structure formed by strongly hydrophilic crosslinked polymers, able to hold huge amounts of water.288,289 Hydrogels are broadly divided into two classes according to the cross-linking type: physical or chemical. Hydrogels formed by physical crosslinking through non-covalent interactions, such as ionic interactions, hydrogen bonding, and van der Waals forces, provide hydrogels with viscoelastic behavior, generally called reversible gels.290
In contrast, chemically crosslinked hydrogels are formed from covalent bonds, resulting in strong interactions that are more resistant to mechanical forces and provide hydrogels with more elastic behavior.289,291 In addition, it is common for hydrogels to be classified as natural or synthetic, depending on their origin. In general, natural hydrogels, made of polymers obtained from natural sources, possess better biocompatibility and biodegradability, while synthetic hydrogels exhibit more tunable properties, low-cost and wide availability.288,292,293
Due to their hydrated and porous 3D structure, with high surface area and highly adjustable properties, hydrogels have emerged as one of the most promising and versatile classes of biomaterials for soft tissue engineering. Hydrogel-based scaffolds can be designed to provide an appropriate microenvironment similar to the ECM native allowing the diffusion of oxygen, nutrients, and biomolecules, thus supporting cell adhesion, migration, proliferation, and differentiation.289,294
In hydrogels, CBNs play two principal roles: as filler in polymer hydrogels with improved properties, such as conductivity and mechanical resistance,295–300 and as a central component of the hydrogel matrix, being a gelator to self-assemble into hydrogels. This topic is focused precisely on that last application, collectively called carbon-based hydrogels. Graphene appears as the most widely used carbon-based material for hydrogel construction. In fact, there is a lack of evidence in the literature on the formation of hydrogels by other CBNs.
Graphene-based hydrogels are 3D structures derived from their corresponding two-dimensional forms.301 The unique characteristics of graphene, such as high thermal and electrical conductivity, excellent mechanical performance, and large surface area, have made graphene hydrogels an important topic of research for various applications, eg, water treatment,302,303 supercapacitors,304–306 lithium-ion batteries,301,307 wearable sensors.308 In addition, as a result of its wettability, GO has shown cytocompatibility, and its presence can favor cell adhesion and proliferation.309 Thus, its biocompatibility, high swelling, softness, and elasticity resembling living tissues have attracted attention in tissue engineering.
To synthesize a graphene-based hydrogel, Lim et al applied a hydrothermal method.310 This simple and environmentally friendly methodology, originally described by Xu et al,303 can prepare a hydrogel from GO suspension using a single-step hydrothermal reduction process in which 2D graphene sheets are self-assembled in 3D macrostructures (Figure 7). These authors conducted in vitro studies to assess the viability of MG63 cells in the synthesized scaffold and found good biocompatibility and potential for biological applications of GO hydrogel.
Li et al designed an oxide-graphene-based stimulus-responsive hydrogel to synthesize a smart scaffold for dynamic control of cell release.311 This active cell scaffold was prepared by in-situ polymerization introducing GO into a monomer solution of Poly(N-isopropylacrylamide) (pNIPAAm). In this method, the polymerization occurs on the GO surface, leading to the composite hydrogels of GO and polymer.312 The developed hydrogel scaffold, which also included the addition of arginine-glycine-aspartic acid (RGD) to improve biocompatibility, was able to change its morphology in response to the photothermal effects resulting from the application of Near-Infrared (NIR) light. Briefly, when the NIR light is applied, the hydrogel undergoes a transition from a swollen hydrophilic state to a non-swollen hydrophobic state, releasing the cells seeded into the hydrogel together with the water (Figure 8).311
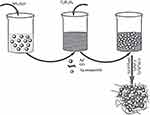 |
Figure 8 3D graphene oxide/Poly (N-isopropylacrylamide composite hydrogel responsive to near-infrared light for capturing and releasing cells on demand. Reprinted from Li W, Wang J, Ren J, Qu X. 3D graphene oxide-polymer hydrogel: near-infrared light-triggered active scaffold for reversible cell capture and on-demand release. Adv Mater. 2013;25(46):6737–6743.311 |
To fabricate a scaffold with antibacterial properties for wound repair, Fan et al fabricated a graphene/Ag composite hydrogel synthesized by the crosslinking reaction of graphene with acrylic acid and N, N ′-methylene bis-acrylamide313 (Figure 9). In addition to exhibiting excellent biocompatibility, strong antibacterial abilities, high swelling ratio, and adequate extensibility, in vivo studies indicated that the graphene/Ag hydrogel significantly accelerated the healing process of wounds in rats, indicating a potential for applications in wound dressing.
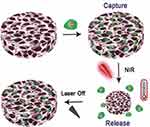 |
Figure 9 Scheme illustrating the synthesis of the graphene/Ag composite hydrogel. First, a solution of Ag(NH3)2OH is obtained by slowly adding NH3H2O to an AgNO3 solution. This resulting solution is poured into a GO solution, and a glucose solution is added (as a green, reducing agent). Then, crosslinking was achieved by incorporating acrylic acid and N, N ′-methylene bis-acrylamide. Reprinted from Fan Z, Liu B, Wang J, et al. A novel wound dressing based on Ag/Graphene polymer hydrogel: effectively kill bacteria and accelerate wound healing. Adv Funct Mater. 2014;24(25):3933–3943. © 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.313 |
In their study, Jing et al created a conductive hydrogel based on chitosan/dopamine (DA)/GO that, in addition to electrical conductivity, exhibited rapid self-healing and self-adhesive properties, high stability, strong mechanical and recovery behavior.314 The hydrogel was produced by introducing dopamine in the chitosan/GO solution, and following self-polymerization, it was able to form the chitosan/DA/GO composite hydrogel. Therefore, it is essential to highlight that incorporating GO increased the cell viability and proliferation of cardiomyocytes and human embryonic stem cell-derived fibroblasts.
Finally, regarding drug delivery applications of 3D scaffolds, recently, Bayón et al developed an electromagnetic-responsive graphene-based hydrogel.315 The graphene/diaminotriazine composite hydrogels, synthesized using graphene solutions dispersed in DMSO as a polymerization medium for diaminotriazine, revealed responsiveness with microwave irradiation (915 MHz), a frequency more suitable for the penetration into deeper tissues than 2.45 GHz, commonly applied to other microwave-responsive soft materials.316,317 As a result, the authors demonstrated a biocompatible scaffold that can promote the drug-controlled release on demand and potential uses in biomedical applications.
Outlook and Challenges
In summary, this paper offers an overview of the synthesis, intriguing properties, and surface functionalization approaches for 0D to 3D CBNs that enable their applications in tissue engineering. This review also summarizes recent progress using CBNs and, especially, CBN-based hydrogels as scaffolds for many typical tissue regeneration applications. Regarding CBN materials development, an important and challenging task is to perform a systematic cross-comparison regarding the effectiveness of different types of CBNs for certain types of tissue engineering applications. Since different tissues have diverse structures and involve different cell systems, such comprehensive comparison will identify not only the most promising CBNs and/or hydrogels that are currently available for the regeneration of specific tissues but also further trigger the design and functionalization of new CBN materials to tackle the challenges in this field.
In the future, we foresee that N-doped CBNs including N-doped CNTs, and graphene could result in faster and more effective tissue regeneration. This is due to the clear biocompatibility of N-doped species, which reduce the presence of oxygen radicals. The mechanism behind this is that N-doping introduces favorable electronic properties in the nanomaterials, facilitating the scavenging of reactive oxygen species (ROS) and thus decreasing oxidative stress in cells. As a result, several studies indicate that N-doped MWNTs promote (or keep constant) cell viability318,319 and are prone to biomolecules.320–322
Regarding the use of carbon-based hydrogels in tissue engineering, a current limitation is a lack of in vivo assays, particularly in long-term and human research. However, before taking this step, further in vitro studies with the direct application of these carbon-based hydrogels to other specific tissues may be necessary. Another critical challenge is the development of in situ-forming injectable hydrogels. As mentioned in the previous section, since minimally invasive procedures can administer and are able to fill tissue defects conformally, this type of scaffold has gained special attention in recent years. Nevertheless, one of the main disadvantages of this hydrogel remains the insufficient mechanical resistance to maintain the loads imposed on the tissues under physiological conditions. The synthesis of more robust carbon-based injectables hydrogels with adequate mechanical characteristics could have a considerable impact, especially for treating bones and cartilage that frequently have non-uniform tissue damage and need stiffness. In the case of anatomically well-defined structures (eg, knee menisci, intervertebral discs, and the heart), bioprinting has been one of the most promising strategies. As the development of new bioink formulations continues to be the main target of interest in this field, engineering novel carbon-based hydrogels and using them as bioink could also contribute significantly to the progress of this area.
Acknowledgments
A.O.L would like to thanks to National Council for Scientific and Technological Development (CNPq, grants #310883/2020-2 and #404683/2018-5).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Liu Y, Yu Q, Chang J, Wu C. Nanobiomaterials: from 0D to 3D for tumor therapy and tissue regeneration. Nanoscale. 2019;11(29):13678–13708. doi:10.1039/C9NR02955A
2. Feng W, Zhu X, Li F. Recent advances in the optimization and functionalization of upconversion nanomaterials for in vivo bioapplications. NPG Asia Mater. 2013;5(12):e75–e75. doi:10.1038/am.2013.63
3. Biagiotti G, Fedeli S, Tuci G, et al. Combined therapies with nanostructured carbon materials: there is room still available at the bottom. J Mater Chem B. 2018;6(14):2022–2035. doi:10.1039/C8TB00121A
4. Georgakilas V, Tiwari JN, Kemp KC, et al. Noncovalent functionalization of graphene and graphene oxide for energy materials, biosensing, catalytic, and biomedical applications. Chem Rev. 2016;116(9):5464–5519. doi:10.1021/acs.chemrev.5b00620
5. Shen S, Wu Y, Liu Y, Wu D. High drug-loading nanomedicines: progress, current status, and prospects. Int J Nanomedicine. 2017;12:4085–4109. doi:10.2147/IJN.S132780
6. Al-Hetty HRAK, Jalil AT, Alghazali MW, et al. Nanomaterials for combination cancer photothermal therapy. Emergent Mater. 2023;6(2):425–438. doi:10.1007/s42247-023-00464-5
7. Chen J, Ning C, Zhou Z, et al. Nanomaterials as photothermal therapeutic agents. Prog Mater Sci. 2019;99:1–26. doi:10.1016/j.pmatsci.2018.07.005
8. Patel KD, Singh RK, Kim H-W. Carbon-based nanomaterials as an emerging platform for theranostics. Mater Horizons. 2019;6(3):434–469. doi:10.1039/C8MH00966J
9. Huang X, Yin Z, Wu S, et al. Graphene-based materials: synthesis, characterization, properties, and applications. Small. 2011;7(14):1876–1902. doi:10.1002/smll.201002009
10. Mochalin VN, Shenderova O, Ho D, Gogotsi Y. The properties and applications of nanodiamonds. Nat Nanotechnol. 2012;7(1):11–23. doi:10.1038/nnano.2011.209
11. Guo X, Mei N. Assessment of the toxic potential of graphene family nanomaterials. J Food Drug Anal. 2014;22(1):105–115. doi:10.1016/j.jfda.2014.01.009
12. Ashammakhi N, GhavamiNejad A, Tutar R, et al. Highlights on advancing frontiers in tissue engineering. Tissue Eng Part B Rev. 2022;28(3):633–664. doi:10.1089/ten.teb.2021.0012
13. Zheng S, Tian Y, Ouyang J, Shen Y, Wang X, Luan J. Carbon nanomaterials for drug delivery and tissue engineering. Front Chem. 2022;10. doi:10.3389/fchem.2022.990362
14. Kandhola G, Park S, Lim J-W, et al. Nanomaterial-based scaffolds for tissue engineering applications: a review on graphene, carbon nanotubes and nanocellulose. Tissue Eng Regen Med. 2023;20(3):411–433. doi:10.1007/s13770-023-00530-3
15. Adorinni S, Rozhin P, Marchesan S. Smart Hydrogels meet carbon nanomaterials for new frontiers in medicine. Biomedicines. 2021;9(5):570. doi:10.3390/biomedicines9050570
16. Alam A, Zhang Y, Kuan H-C, Lee S-H, Ma J. Polymer composite hydrogels containing carbon nanomaterials—morphology and mechanical and functional performance. Prog Polym Sci. 2018;77:1–18. doi:10.1016/j.progpolymsci.2017.09.001
17. Maiti D, Tong X, Mou X, Yang K. Carbon-based nanomaterials for biomedical applications: a recent study. Front Pharmacol. 2019;9. doi:10.3389/fphar.2018.01401
18. Shin SR, Li Y-C, Jang HL, et al. Graphene-based materials for tissue engineering. Adv Drug Deliv Rev. 2016;105:255–274. doi:10.1016/j.addr.2016.03.007
19. Popov IV, Görne AL, Tchougréeff AL, Dronskowski R. Relative stability of diamond and graphite as seen through bonds and hybridizations. Phys Chem Chem Phys. 2019;21(21):10961–10969. doi:10.1039/C8CP07592A
20. Badziag P, Verwoerd WS, Ellis WP, Greiner NR. Nanometre-sized diamonds are more stable than graphite. Nature. 1990;343(6255):244–245. doi:10.1038/343244a0
21. Jabeen S, Kausar A, Muhammad B, Gul S, Farooq M. A review on polymeric nanocomposites of nanodiamond, carbon nanotube, and nanobifiller: structure, preparation and properties. Polym Plast Technol Eng. 2015;54(13):1379–1409. doi:10.1080/03602559.2015.1021489
22. Vlasov II, Shenderova O, Turner S, et al. Nitrogen and luminescent nitrogen-vacancy defects in detonation nanodiamond. Small. 2010;6(5):687–694. doi:10.1002/smll.200901587
23. Danilenko VV. On the history of the discovery of nanodiamond synthesis. Phys Solid State. 2004;46(4):595–599. doi:10.1134/1.1711431
24. Krueger A, Boedeker T. Deagglomeration and functionalisation of detonation nanodiamond with long alkyl chains. Diam Relat Mater. 2008;17(7–10):1367–1370. doi:10.1016/j.diamond.2008.01.033
25. Liang Y, Ozawa M, Krueger A. A general procedure to functionalize agglomerating nanoparticles demonstrated on nanodiamond. ACS Nano. 2009;3(8):2288–2296. doi:10.1021/nn900339s
26. Jarre G, Liang Y, Betz P, Lang D, Krueger A. Playing the surface game—Diels–Alder reactions on diamond nanoparticles. Chem Commun. 2011;47(1):544–546. doi:10.1039/C0CC02931A
27. Neumann P, Beck J, Steiner M, et al. Single-shot readout of a single nuclear spin. Science. 2010;329(5991):542–544. doi:10.1126/science.1189075
28. Balasubramanian G, Chan IY, Kolesov R, et al. Nanoscale imaging magnetometry with diamond spins under ambient conditions. Nature. 2008;455(7213):648–651. doi:10.1038/nature07278
29. Tisler J, Reuter R, Lämmle A, et al. Highly efficient FRET from a single nitrogen-vacancy center in nanodiamonds to a single organic molecule. ACS Nano. 2011;5(10):7893–7898. doi:10.1021/nn2021259
30. Chang Y-R, Lee H-Y, Chen K, et al. Mass production and dynamic imaging of fluorescent nanodiamonds. Nat Nanotechnol. 2008;3(5):284–288. doi:10.1038/nnano.2008.99
31. Mohan N, Chen C-S, Hsieh -H-H, Wu Y-C, Chang H-C. In vivo imaging and toxicity assessments of fluorescent nanodiamonds in caenorhabditis elegans. Nano Lett. 2010;10(9):3692–3699. doi:10.1021/nl1021909
32. Chaudhary A, Welch JO, Jackman RB. Electrical properties of monodispersed detonation nanodiamonds. Appl Phys Lett. 2010;96(24):242903. doi:10.1063/1.3446966
33. Huang H, Dai L, Wang DH, Tan L-S, Osawa E. Large-scale self-assembly of dispersed nanodiamonds. J Mater Chem. 2008;18(12):1347. doi:10.1039/b716676a
34. Xu J, Chow EK-H. Biomedical applications of nanodiamonds: from drug-delivery to diagnostics. SLAS Technol. 2023;28(4):214–222. doi:10.1016/j.slast.2023.03.007
35. Taylor AC, González CH, Miller BS, Edgington RJ, Ferretti P, Jackman RB. Surface functionalisation of nanodiamonds for human neural stem cell adhesion and proliferation. Sci Rep. 2017;7(1):7307. doi:10.1038/s41598-017-07361-y
36. Burleson T, Yusuf N, Stanishevsky A. Surface modification of nanodiamonds for biomedical application and analysis by infrared spectroscopy. J Achiev Mater Manuf Eng. 2009;37(2):258–263.
37. Whitlow J, Pacelli S, Paul A. Multifunctional nanodiamonds in regenerative medicine: recent advances and future directions. J Control Release. 2017;261:62–86. doi:10.1016/j.jconrel.2017.05.033
38. Grausova L, Kromka A, Burdikova Z, et al. Enhanced growth and osteogenic differentiation of human osteoblast-like cells on boron-doped nanocrystalline diamond thin films. PLoS One. 2011;6(6):e20943. doi:10.1371/journal.pone.0020943
39. Keremidarska M, Hikov T, Radeva E, Pramatarova L, Krasteva N. Effect of nanodiamond modification of siloxane surfaces on stem cell behaviour. J Phys Conf Ser. 2014;558:012056. doi:10.1088/1742-6596/558/1/012056
40. Hristova K, Pecheva E, Pramatarova L, Altankov G. Improved interaction of osteoblast-like cells with apatite–nanodiamond coatings depends on fibronectin. J Mater Sci Mater Med. 2011;22(8):1891–1900. doi:10.1007/s10856-011-4357-9
41. Thalhammer A, Edgington RJ, Cingolani LA, Schoepfer R, Jackman RB. The use of nanodiamond monolayer coatings to promote the formation of functional neuronal networks. Biomaterials. 2010;31(8):2097–2104. doi:10.1016/j.biomaterials.2009.11.109
42. Edgington RJ, Thalhammer A, Welch JO, et al. Patterned neuronal networks using nanodiamonds and the effect of varying nanodiamond properties on neuronal adhesion and outgrowth. J Neural Eng. 2013;10(5):056022. doi:10.1088/1741-2560/10/5/056022
43. Xie Y, Zhou J, Wei Q, et al. Improving the long-term stability of Ti6Al4V abutment screw by coating micro/nano-crystalline diamond films. J Mech Behav Biomed Mater. 2016;63:174–182. doi:10.1016/j.jmbbm.2016.06.018
44. Wang Z, Cai N, Zhao D, et al. Mechanical reinforcement of electrospun water-soluble polymer nanofibers using nanodiamonds. Polym Compos. 2013;34(10):1735–1744. doi:10.1002/pc.22577
45. Suliman S, Xing Z, Wu X, et al. Release and bioactivity of bone morphogenetic protein-2 are affected by scaffold binding techniques in vitro and in vivo. J Control Release. 2015;197:148–157. doi:10.1016/j.jconrel.2014.11.003
46. Hikov T, Mitev D, Radeva E, et al. Studying the influence of nanodiamonds over the elasticity of polymer/nanodiamond composites for biomedical application. J Phys Conf Ser. 2014;558:012060. doi:10.1088/1742-6596/558/1/012060
47. Mahdavi M, Mahmoudi N, Rezaie Anaran F, Simchi A. Electrospinning of nanodiamond-modified polysaccharide nanofibers with physico-mechanical properties close to natural skins. Mar Drugs. 2016;14(7):128. doi:10.3390/md14070128
48. Cao W, Wang X, Li Q, Ye Z, Xing X. Mechanical property and antibacterial activity of silver-loaded polycation functionalized nanodiamonds for use in resin-based dental material formulations. Mater Lett. 2018;220:104–107. doi:10.1016/j.matlet.2018.03.027
49. Kroto HW, Heath JR, O’Brien SC, Curl RF, Smalley RE. C60: buckminsterfullerene. Nature. 1985;318(6042):162–163. doi:10.1038/318162a0
50. Taylor R, Hare JP, Abdul-Sada AK, Kroto HW. Isolation, separation and characterisation of the fullerenes C60 and C70: the third form of carbon. J Chem Soc Chem Commun. 1990;(20):1423. doi:10.1039/c39900001423
51. Krätschmer W, Lamb LD, Fostiropoulos K, Huffman DR. Solid C60: a new form of carbon. Nature. 1990;347(6291):354–358. doi:10.1038/347354a0
52. Goodarzi S, Da Ros T, Conde J, Sefat F, Mozafari M. Fullerene: biomedical engineers get to revisit an old friend. Mater Today. 2017;20(8):460–480. doi:10.1016/j.mattod.2017.03.017
53. Kharlamov AI, Bondarenko ME, Kirillova NV. New method for synthesis of fullerenes and fullerene hydrides from benzene. Russ J Appl Chem. 2012;85(2):233–238. doi:10.1134/S1070427212020127
54. Ikeda T, Kamo T, Danno M. New synthesis method of fullerenes using microwave-induced naphthalene-nitrogen plasma at atmospheric pressure. Appl Phys Lett. 1995;67(7):900–902. doi:10.1063/1.114688
55. Sun D, Reed CA. Crystal engineering a linear polymer of C60 fullerene via supramolecular pre-organization. Chem Commun. 2000; 23:2391–2392. doi:10.1039/b007116l
56. Stephens PW, Bortel G, Faigel G, et al. Polymeric fullerene chains in RbC60 and KC60. Nature. 1994;370(6491):636–639. doi:10.1038/370636a0
57. Chiang LY, Wang LY, Kuo C-S. Polyhydroxylated C60 cross-linked polyurethanes. Macromolecules. 1995;28(22):7574–7576. doi:10.1021/ma00126a042
58. Shrestha LK, Shrestha RG, Hill JP, Ariga K. Self-assembled fullerene nanostructures. J Oleo Sci. 2013;62(8):541–553. doi:10.5650/jos.62.541
59. Sathish M, Miyazawa K. Size-tunable hexagonal fullerene (C 60) nanosheets at the liquid−liquid interface. J Am Chem Soc. 2007;129(45):13816–13817. doi:10.1021/ja076251q
60. Wakahara T, Sathish M, Miyazawa K, et al. Preparation and optical properties of fullerene/ferrocene hybrid hexagonal nanosheets and large-scale production of fullerene hexagonal nanosheets. J Am Chem Soc. 2009;131(29):9940–9944. doi:10.1021/ja901032b
61. Singhal R, Agarwal DC, Mishra YK, et al. Synthesis, characterizations, and thermal induced structural transformation of silver-fullerene C60 nanocomposite thin films for applications in optical devices. J Appl Phys. 2010;107(10):103504. doi:10.1063/1.3366709
62. Siringan MJ, Dawar A, Zhang J. Interactions between fullerene derivatives and biological systems. Mater Chem Front. 2023;7(11):2153–2174. doi:10.1039/D3QM00004D
63. Nakamura E, Isobe H. Functionalized fullerenes in water. The first 10 years of their chemistry, biology, and nanoscience. Acc Chem Res. 2003;36(11):807–815. doi:10.1021/ar030027y
64. Sayes CM, Fortner JD, Guo W, et al. The differential cytotoxicity of water-soluble fullerenes. Nano Lett. 2004;4(10):1881–1887. doi:10.1021/nl0489586
65. Mateo-Alonso A, Bonifazi D, Prato M. Functionalization and applications of [60]fullerene. In: Carbon Nanotechnology. Elsevier; 2006:155–189. doi:10.1016/B978-044451855-2/50010-3
66. Andersson T, Nilsson K, Sundahl M, Westman G, Wennerström O. C 60 embedded in γ-cyclodextrin: a water-soluble fullerene. J Chem Soc, Chem Commun. 1992;(8):604–606. doi:10.1039/C39920000604
67. Atwood JL, Koutsantonis GA, Raston CL. Purification of C60 and C70 by selective complexation with calixarenes. Nature. 1994;368(6468):229–231. doi:10.1038/368229a0
68. Scrivens WA, Tour JM, Creek KE, Pirisi L. Synthesis of 14C-labeled C60, its suspension in water, and its uptake by human keratinocytes. J Am Chem Soc. 1994;116(10):4517–4518. doi:10.1021/ja00089a067
69. Tabata Y, Murakami Y, Ikada Y. Antitumor effect of Poly(Ethylene Glycol)-modified fullerene. Fuller Sci Technol. 1997;5(5):989–1007. doi:10.1080/15363839708013312
70. Da Ros T, Prato M. Medicinal chemistry with fullerenes and fullerene derivatives. Chem Commun. 1999;(8):663–669. doi:10.1039/a809495k
71. Rondags A, Yuen WY, Jonkman MF, Horváth B. Fullerene C 60 with cytoprotective and cytotoxic potential: prospects as a novel treatment agent in dermatology? Exp Dermatol. 2017;26(3):220–224. doi:10.1111/exd.13172
72. Jia G, Wang H, Yan L, et al. Cytotoxicity of carbon nanomaterials: single-wall nanotube, multi-wall nanotube, and fullerene. Environ Sci Technol. 2005;39(5):1378–1383. doi:10.1021/es048729l
73. Fang J, Lyon DY, Wiesner MR, Dong J, Alvarez A. Effect of a fullerene water suspension on bacterial phospholipids and membrane phase behavior. Environ Sci Technol. 2007;41(7):2636–2642. doi:10.1021/es062181w
74. Tang YJ, Ashcroft JM, Chen D, et al. Charge-associated effects of fullerene derivatives on microbial structural integrity and central metabolism. Nano Lett. 2007;7(3):754–760. doi:10.1021/nl063020t
75. Lichota A, Krokosz A. Fullerenols in therapy and diagnosis of cancer. Med Pr. 2016;67(6):817–831. doi:10.13075/mp.5893.00466
76. Shi J, Yu X, Wang L, et al. PEGylated fullerene/iron oxide nanocomposites for photodynamic therapy, targeted drug delivery and MR imaging. Biomaterials. 2013;34(37):9666–9677. doi:10.1016/j.biomaterials.2013.08.049
77. Friedman SH, DeCamp DL, Sijbesma RP, Srdanov G, Wudl F, Kenyon GL. Inhibition of the HIV-1 protease by fullerene derivatives: model building studies and experimental verification. J Am Chem Soc. 1993;115(15):6506–6509. doi:10.1021/ja00068a005
78. Afreen S, Muthoosamy K, Manickam S, Hashim U. Functionalized fullerene (C 60) as a potential nanomediator in the fabrication of highly sensitive biosensors. Biosens Bioelectron. 2015;63:354–364. doi:10.1016/j.bios.2014.07.044
79. Dugan LL, Lovett EG, Quick KL, Lotharius J, Lin TT, O’Malley KL. Fullerene-based antioxidants and neurodegenerative disorders. Parkinsonism Relat Disord. 2001;7(3):243–246. doi:10.1016/S1353-8020(00)00064-X
80. Tóth É, Bolskar RD, Borel A, et al. Water-soluble gadofullerenes: toward high-relaxivity, pH-responsive MRI contrast agents. J Am Chem Soc. 2005;127(2):799–805. doi:10.1021/ja044688h
81. Inui S, Aoshima H, Nishiyama A, Itami S. Improvement of acne vulgaris by topical fullerene application: unique impact on skin care. Nanomed Nanotechnol Biol Med. 2011;7(2):238–241. doi:10.1016/j.nano.2010.09.005
82. Gholami A, Hashemi SA, Yousefi K, et al. 3D nanostructures for tissue engineering, cancer therapy, and gene delivery. J Nanomater. 2020;2020:1–24. doi:10.1155/2020/1852946
83. Bacakova L, Kopova I, Stankova L, et al. Bone cells in cultures on nanocarbon-based materials for potential bone tissue engineering: a review. Phys Status Solidi. 2014;211(12):2688–2702. doi:10.1002/pssa.201431402
84. Bacakova L, Grausova L, Vacik J, et al. Improved adhesion and growth of human osteoblast-like MG 63 cells on biomaterials modified with carbon nanoparticles. Diam Relat Mater. 2007;16(12):2133–2140. doi:10.1016/j.diamond.2007.07.015
85. Kopova I, Bacakova L, Lavrentiev V, Vacik J. Growth and potential damage of human bone-derived cells on fresh and aged fullerene C60 films. Int J Mol Sci. 2013;14(5):9182–9204. doi:10.3390/ijms14059182
86. Vandrovcova M, Bacakova L. Adhesion, growth and differentiation of osteoblasts on surface-modified materials developed for bone implants. Physiol Res. 2011;403–417. doi:10.33549/physiolres.932045
87. Kopova I, Lavrentiev V, Vacik J, Bacakova L. Growth and potential damage of human bone-derived cells cultured on fresh and aged C60/Ti films. PLoS One. 2015;10(4):e0123680. doi:10.1371/journal.pone.0123680
88. Xiao L, Aoshima H, Saitoh Y, Miwa N. Highly hydroxylated fullerene localizes at the cytoskeleton and inhibits oxidative stress in adipocytes and a subcutaneous adipose-tissue equivalent. Free Radic Biol Med. 2011;51(7):1376–1389. doi:10.1016/j.freeradbiomed.2011.05.026
89. Wang J, Jiang J, Li F, et al. Emerging carbon-based quantum dots for sustainable photocatalysis. Green Chem. 2023;25(1):32–58. doi:10.1039/D2GC03160D
90. Li H, Kang Z, Liu Y, Lee S-T. Carbon nanodots: synthesis, properties and applications. J Mater Chem. 2012;22(46):24230. doi:10.1039/c2jm34690g
91. Ponomarenko LA, Schedin F, Katsnelson MI, et al. Chaotic Dirac Billiard in graphene quantum dots. Science. 2008;320(5874):356–358. doi:10.1126/science.1154663
92. Yan X, Cui X, Li B, Li L. Large, solution-processable graphene quantum dots as light absorbers for photovoltaics. Nano Lett. 2010;10(5):1869–1873. doi:10.1021/nl101060h
93. Lim SY, Shen W, Gao Z. Carbon quantum dots and their applications. Chem Soc Rev. 2015;44(1):362–381. doi:10.1039/C4CS00269E
94. Xu X, Ray R, Gu Y, et al. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J Am Chem Soc. 2004;126(40):12736–12737. doi:10.1021/ja040082h
95. Li H, He X, Kang Z, et al. Water-soluble fluorescent carbon quantum dots and photocatalyst design. Angew Chemie Int Ed. 2010;49(26):4430–4434. doi:10.1002/anie.200906154
96. Li Y, Hu Y, Zhao Y, et al. An electrochemical avenue to green-luminescent graphene quantum dots as potential electron-acceptors for photovoltaics. Adv Mater. 2011;23(6):776–780. doi:10.1002/adma.201003819
97. Liu H, Ye T, Mao C. Fluorescent carbon nanoparticles derived from candle soot. Angew Chemie Int Ed. 2007;46(34):6473–6475. doi:10.1002/anie.200701271
98. Zong J, Zhu Y, Yang X, Shen J, Li C. Synthesis of photoluminescent carbogenic dots using mesoporous silica spheres as nanoreactors. Chem Commun. 2011;47(2):764–766. doi:10.1039/C0CC03092A
99. Dong Y, Lin J, Chen Y, Fu F, Chi Y, Chen G. Graphene quantum dots, graphene oxide, carbon quantum dots and graphite nanocrystals in coals. Nanoscale. 2014;6(13):7410–7415. doi:10.1039/C4NR01482K
100. Costa RS, de Castro MO, da Silva GH, et al. Carbon-dots from babassu coconut (Orbignya speciosa) biomass: synthesis, characterization, and toxicity to Daphnia magna. Carbon Trends. 2021;5:100133. doi:10.1016/j.cartre.2021.100133
101. Wang X, Qu K, Xu B, Ren J, Qu X. Microwave assisted one-step green synthesis of cell-permeable multicolor photoluminescent carbon dots without surface passivation reagents. J Mater Chem. 2011;21(8):2445. doi:10.1039/c0jm02963g
102. Lu J, Yeo PSE, Gan CK, Wu P, Loh KP. Transforming C60 molecules into graphene quantum dots. Nat Nanotechnol. 2011;6(4):247–252. doi:10.1038/nnano.2011.30
103. Miao P, Han K, Tang Y, Wang B, Lin T, Cheng W. Recent advances in carbon nanodots: synthesis, properties and biomedical applications. Nanoscale. 2015;7(5):1586–1595. doi:10.1039/C4NR05712K
104. Shan D, Hsieh J-T, Bai X, Yang J. Citrate-based fluorescent biomaterials. Adv Healthc Mater. 2018;7(18):1800532. doi:10.1002/adhm.201800532
105. Lin L, Rong M, Luo F, Chen D, Wang Y, Chen X. Luminescent graphene quantum dots as new fluorescent materials for environmental and biological applications. TrAC Trends Anal Chem. 2014;54:83–102. doi:10.1016/j.trac.2013.11.001
106. Hoshino A, Fujioka K, Oku T, et al. Physicochemical properties and cellular toxicity of nanocrystal quantum dots depend on their surface modification. Nano Lett. 2004;4(11):2163–2169. doi:10.1021/nl048715d
107. Srivastava I, Misra SK, Ostadhossein F, Daza E, Singh J, Pan D. Surface chemistry of carbon nanoparticles functionally select their uptake in various stages of cancer cells. Nano Res. 2017;10(10):3269–3284. doi:10.1007/s12274-017-1518-2
108. Li M, Wu W, Ren W, et al. Synthesis and upconversion luminescence of N-doped graphene quantum dots. Appl Phys Lett. 2012;101(10):103107. doi:10.1063/1.4750065
109. Feng Q, Cao Q, Li M, Liu F, Tang N, Du Y. Synthesis and photoluminescence of fluorinated graphene quantum dots. Appl Phys Lett. 2013;102(1):013111. doi:10.1063/1.4774264
110. Janus Ł, Piątkowski M, Radwan-Pragłowska J, Bogdał D, Matysek D. Chitosan-based carbon quantum dots for biomedical applications: synthesis and characterization. Nanomaterials. 2019;9(2):274. doi:10.3390/nano9020274
111. Bhati A, Anand SR, Saini D, Khare P, Dubey P, Sonkar SK. Self-doped nontoxic red-emitting Mg–N-embedded carbon dots for imaging, Cu(ii) sensing and fluorescent ink. New J Chem. 2018;42(24):19548–19556. doi:10.1039/C8NJ04754E
112. Sun Y-P, Zhou B, Lin Y, et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J Am Chem Soc. 2006;128(24):7756–7757. doi:10.1021/ja062677d
113. Sun Y-P, Wang X, Lu F, et al. Doped carbon nanoparticles as a new platform for highly photoluminescent dots. J Phys Chem C. 2008;112(47):18295–18298. doi:10.1021/jp8076485
114. Hamilton IP, Li B, Yan X, Li L. Alignment of colloidal graphene quantum dots on polar surfaces. Nano Lett. 2011;11(4):1524–1529. doi:10.1021/nl200298c
115. Ming H, Ma Z, Liu Y, et al. Large scale electrochemical synthesis of high quality carbon nanodots and their photocatalytic property. Dalt Trans. 2012;41(31):9526. doi:10.1039/c2dt30985h
116. Zhao H, Chang Y, Liu M, Gao S, Yu H, Quan X. A universal immunosensing strategy based on regulation of the interaction between graphene and graphene quantum dots. Chem Commun. 2013;49(3):234–236. doi:10.1039/C2CC35503E
117. Luo P, Li C, Shi G. Synthesis of gold@carbon dots composite nanoparticles for surface enhanced Raman scattering. Phys Chem Chem Phys. 2012;14(20):7360. doi:10.1039/c2cp40767a
118. Cao L, Wang X, Meziani MJ, et al. Carbon dots for multiphoton bioimaging. J Am Chem Soc. 2007;129(37):11318–11319. doi:10.1021/ja073527l
119. Tao H, Yang K, Ma Z, et al. In vivo NIR fluorescence imaging, biodistribution, and toxicology of photoluminescent carbon dots produced from carbon nanotubes and graphite. Small. 2012;8(2):281–290. doi:10.1002/smll.201101706
120. Soumya K, More N, Choppadandi M, Aishwarya DA, Singh G, Kapusetti G. A comprehensive review on carbon quantum dots as an effective photosensitizer and drug delivery system for cancer treatment. Biomed Technol. 2023;4:11–20. doi:10.1016/j.bmt.2023.01.005
121. Hsu P-C, Chen P-C, Ou C-M, Chang H-Y, Chang H-T. Extremely high inhibition activity of photoluminescent carbon nanodots toward cancer cells. J Mater Chem B. 2013;1(13):1774. doi:10.1039/c3tb00545c
122. Singh RK, Patel KD, Mahapatra C, Kang MS, Kim H-W. C-Dot generated bioactive organosilica nanospheres in theranostics: multicolor luminescent and photothermal properties combined with drug delivery capacity. ACS Appl Mater Interfaces. 2016;8(37):24433–24444. doi:10.1021/acsami.6b07494
123. Jing Y, Zhu Y, Yang X, Shen J, Li C. Ultrasound-triggered smart drug release from multifunctional core−shell capsules one-step fabricated by coaxial electrospray method. Langmuir. 2011;27(3):1175–1180. doi:10.1021/la1042734
124. Peng Z, Zhao T, Zhou Y, Li S, Li J, Leblanc RM. Bone tissue engineering via carbon-based nanomaterials. Adv Healthc Mater. 2020;9(5):1901495. doi:10.1002/adhm.201901495
125. Eivazzadeh-Keihan R, Maleki A, de la Guardia M, et al. Carbon based nanomaterials for tissue engineering of bone: building new bone on small black scaffolds: a review. J Adv Res. 2019;18:185–201. doi:10.1016/j.jare.2019.03.011
126. Khajuria DK, Kumar VB, Gigi D, Gedanken A, Karasik D. Accelerated bone regeneration by nitrogen-doped carbon dots functionalized with hydroxyapatite nanoparticles. ACS Appl Mater Interfaces. 2018;10(23):19373–19385. doi:10.1021/acsami.8b02792
127. Gogoi S, Kumar M, Mandal BB, Karak N. A renewable resource based carbon dot decorated hydroxyapatite nanohybrid and its fabrication with waterborne hyperbranched polyurethane for bone tissue engineering. RSC Adv. 2016;6(31):26066–26076. doi:10.1039/C6RA02341J
128. Lu Y, Li L, Li M, et al. Zero-dimensional carbon dots enhance bone regeneration, osteosarcoma ablation, and clinical bacterial eradication. Bioconjug Chem. 2018;29(9):2982–2993. doi:10.1021/acs.bioconjchem.8b00400
129. Kumar R, Kumar VB, Gedanken A. Sonochemical synthesis of carbon dots, mechanism, effect of parameters, and catalytic, energy, biomedical and tissue engineering applications. Ultrason Sonochem. 2020;64:105009. doi:10.1016/j.ultsonch.2020.105009
130. Kumar VB, Kumar R, Gedanken A, Shefi O. Fluorescent metal-doped carbon dots for neuronal manipulations. Ultrason Sonochem. 2019;52:205–213. doi:10.1016/j.ultsonch.2018.11.017
131. Nissan I, Kumar VB, Porat Z, Makovec D, Shefi O, Gedanken A. Sonochemically-fabricated Ga@C-dots@Ga nanoparticle-aided neural growth. J Mater Chem B. 2017;5(7):1371–1379. doi:10.1039/C6TB02508K
132. Kumar VB, Marcus M, Porat Z, et al. Ultrafine highly magnetic fluorescent γ-Fe 2 O 3 /NCD nanocomposites for neuronal manipulations. ACS Omega. 2018;3(2):1897–1903. doi:10.1021/acsomega.7b01666
133. Bahrampour Juybari K, Rizwan K, Faramarz S, et al. Carbon quantum dots as multi-purpose nanomaterial in stem cell therapy. Chem Biodivers. 2023;20(4). doi:10.1002/cbdv.202200721
134. Farshidfar N, Fooladi S, Nematollahi MH, Iravani S. Carbon dots with tissue engineering and regenerative medicine applications. RSC Adv. 2023;13(21):14517–14529. doi:10.1039/D3RA02336B
135. Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354(6348):56–58. doi:10.1038/354056a0
136. Charlier J-C, Blase X, Roche S. Electronic and transport properties of nanotubes. Rev Mod Phys. 2007;79(2):677–732. doi:10.1103/RevModPhys.79.677
137. Avouris P, Chen Z, Perebeinos V. Carbon-based electronics. Nat Nanotechnol. 2007;2(10):605–615. doi:10.1038/nnano.2007.300
138. Ismail RA, Mohsin MH, Ali AK, Hassoon KI, Erten-Ela S. Preparation and characterization of carbon nanotubes by pulsed laser ablation in water for optoelectronic application. Phys E Low-Dimensional Syst Nanostructures. 2020;119:113997. doi:10.1016/j.physe.2020.113997
139. Ribeiro H, Schnitzler MC, da Silva WM, Santos AP. Purification of carbon nanotubes produced by the electric arc-discharge method. Surfaces Interfaces. 2021;26:101389. doi:10.1016/j.surfin.2021.101389
140. Shi Z, Lian Y, Zhou X, et al. Mass-production of single-wall carbon nanotubes by arc discharge method This work was supported by the National Natural Science Foundation of China, No. 29671030. Carbon NY. 1999;37(9):1449–1453. doi:10.1016/S0008-6223(99)00007-X
141. Szabó A, Perri C, Csató A, Giordano G, Vuono D, Nagy JB. Synthesis methods of carbon nanotubes and related materials. Materials (Basel). 2010;3(5):3092–3140. doi:10.3390/ma3053092
142. Zhang Y, Gu H, Iijima S. Single-wall carbon nanotubes synthesized by laser ablation in a nitrogen atmosphere. Appl Phys Lett. 1998;73(26):3827–3829. doi:10.1063/1.122907
143. Jin H, Shu H, Bai G, Chen D, Zeng Q. In situ synthesis of CNTs in HfB2 powders by chemical vapor deposition of methane to fabricate reinforced HfB2 composites. J Alloys Compd. 2018;745:1–7. doi:10.1016/j.jallcom.2018.02.194
144. Hoyos-Palacio LM, García AG, Pérez-Robles JF, González J, Martínez-Tejada HV. Catalytic effect of Fe, Ni, Co and Mo on the CNTs production. IOP Conf Ser Mater Sci Eng. 2014;59:012005. doi:10.1088/1757-899X/59/1/012005
145. Mohammadian N, Ghoreishi S, Hafeziyeh S, Saeidi S, Dionysiou D. Optimization of synthesis conditions of carbon nanotubes via ultrasonic-assisted floating catalyst deposition using response surface methodology. Nanomaterials. 2018;8(5):316. doi:10.3390/nano8050316
146. Flahaut E, Bacsa R, Peigney A, Laurent C. Gram-scale CCVD synthesis of double-walled carbon nanotubes. Chem Commun. 2003;(12):1442. doi:10.1039/b301514a
147. Zhang R, Zhang Y, Zhang Q, Xie H, Qian W, Wei F. Growth of half-meter long carbon nanotubes based on Schulz–Flory distribution. ACS Nano. 2013;7(7):6156–6161. doi:10.1021/nn401995z
148. Tans SJ, Verschueren ARM, Dekker C. Room-temperature transistor based on a single carbon nanotube. Nature. 1998;393(6680):49–52. doi:10.1038/29954
149. Shulaker MM, Hills G, Patil N, et al. Carbon nanotube computer. Nature. 2013;501(7468):526–530. doi:10.1038/nature12502
150. Coleman JN, Khan U, Blau WJ, Gun’ko YK. Small but strong: a review of the mechanical properties of carbon nanotube–polymer composites. Carbon NY. 2006;44(9):1624–1652. doi:10.1016/j.carbon.2006.02.038
151. Cui F, Li T, Wang D, Yi S, Li J, Li X. Recent advances in carbon-based nanomaterials for combating bacterial biofilm-associated infections. J Hazard Mater. 2022;431:128597. doi:10.1016/j.jhazmat.2022.128597
152. Madannejad R, Shoaie N, Jahanpeyma F, Darvishi MH, Azimzadeh M, Javadi H. Toxicity of carbon-based nanomaterials: reviewing recent reports in medical and biological systems. Chem Biol Interact. 2019;307:206–222. doi:10.1016/j.cbi.2019.04.036
153. Vajtai R, ed. Springer Handbook of Nanomaterials. Berlin Heidelberg: Springer; 2013. doi:10.1007/978-3-642-20595-8
154. Endo M, Kim Y, Hayashi T, et al. Vapor-grown carbon fibers (VGCFs). Carbon NY. 2001;39(9):1287–1297. doi:10.1016/S0008-6223(00)00295-5
155. Kim C, Yang KS, Kojima M, et al. Fabrication of electrospinning-derived carbon nanofiber webs for the anode material of lithium-ion secondary batteries. Adv Funct Mater. 2006;16(18):2393–2397. doi:10.1002/adfm.200500911
156. Tran PA, Zhang L, Webster TJ. Carbon nanofibers and carbon nanotubes in regenerative medicine. Adv Drug Deliv Rev. 2009;61(12):1097–1114. doi:10.1016/j.addr.2009.07.010
157. Lee C, Wei X, Kysar JW, Hone J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science. 2008;321(5887):385–388. doi:10.1126/science.1157996
158. Marcano DC, Kosynkin DV, Berlin JM, et al. Improved synthesis of graphene oxide. ACS Nano. 2010;4(8):4806–4814. doi:10.1021/nn1006368
159. Manikandan V, Lee NY. Reduced graphene oxide: biofabrication and environmental applications. Chemosphere. 2023;311:136934. doi:10.1016/j.chemosphere.2022.136934
160. MacInnes MM, Hlynchuk S, Acharya S, Lehnert N, Maldonado S. Reduction of graphene oxide thin films by cobaltocene and decamethylcobaltocene. ACS Appl Mater Interfaces. 2018;10(2):2004–2015. doi:10.1021/acsami.7b15599
161. Bourlinos AB, Gournis D, Petridis D, Szabó T, Szeri A, Dékány I. Graphite oxide: chemical reduction to graphite and surface modification with primary aliphatic amines and amino acids. Langmuir. 2003;19(15):6050–6055. doi:10.1021/la026525h
162. Liu Z, Robinson JT, Sun X, Dai H. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J Am Chem Soc. 2008;130(33):10876–10877. doi:10.1021/ja803688x
163. Sahoo NG, Bao H, Pan Y, et al. Functionalized carbon nanomaterials as nanocarriers for loading and delivery of a poorly water-soluble anticancer drug: a comparative study. Chem Commun. 2011;47(18):5235. doi:10.1039/c1cc00075f
164. Erol O, Uyan I, Hatip M, Yilmaz C, Tekinay AB, Guler MO. Recent advances in bioactive 1D and 2D carbon nanomaterials for biomedical applications. Nanomed Nanotechnol Biol Med. 2018;14(7):2433–2454. doi:10.1016/j.nano.2017.03.021
165. Volkov Y, McIntyre J, Prina-Mello A. Graphene toxicity as a double-edged sword of risks and exploitable opportunities: a critical analysis of the most recent trends and developments. 2D Mater. 2017;4(2):022001. doi:10.1088/2053-1583/aa5476
166. Kim J, Jeon J-H, Kim H-J, Lim H, Oh I-K. Durable and water-floatable ionic polymer actuator with hydrophobic and asymmetrically laser-scribed reduced graphene oxide paper electrodes. ACS Nano. 2014;8(3):2986–2997. doi:10.1021/nn500283q
167. Gao W, ed. Graphene Oxide. Springer International Publishing; 2015. doi:10.1007/978-3-319-15500-5
168. Maleki M, Zarezadeh R, Nouri M, et al. Graphene oxide: a promising material for regenerative medicine and tissue engineering. Biomol Concepts. 2020;11(1):182–200. doi:10.1515/bmc-2020-0017
169. Lee WC, Lim CHYX, Shi H, et al. Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide. ACS Nano. 2011;5(9):7334–7341. doi:10.1021/nn202190c
170. Zhou M, Lozano N, Wychowaniec JK, et al. Graphene oxide: a growth factor delivery carrier to enhance chondrogenic differentiation of human mesenchymal stem cells in 3D hydrogels. Acta Biomater. 2019;96:271–280. doi:10.1016/j.actbio.2019.07.027
171. Stobinski L, Lesiak B, Malolepszy A, et al. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J Electron Spectros Relat Phenomena. 2014;195:145–154. doi:10.1016/j.elspec.2014.07.003
172. Chen X, Hai X, Wang J. Graphene/graphene oxide and their derivatives in the separation/isolation and preconcentration of protein species: a review. Anal Chim Acta. 2016;922:1–10. doi:10.1016/j.aca.2016.03.050
173. Dhandayuthapani B, Yoshida Y, Maekawa T, Kumar DS. Polymeric scaffolds in tissue engineering application: a review. Int J Polym Sci. 2011;2011:1–19. doi:10.1155/2011/290602
174. Danie Kingsley J, Ranjan S, Dasgupta N, Saha P. Nanotechnology for tissue engineering: need, techniques and applications. J Pharm Res. 2013;7(2):200–204. doi:10.1016/j.jopr.2013.02.021
175. Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat Mater. 2009;8(6):457–470. doi:10.1038/nmat2441
176. Qu H, Fu H, Han Z, Sun Y. Biomaterials for bone tissue engineering scaffolds: a review. RSC Adv. 2019;9(45):26252–26262. doi:10.1039/C9RA05214C
177. Amini AR, Laurencin CT, Nukavarapu SP. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng. 2012;40(5):363–408. doi:10.1615/CritRevBiomedEng.v40.i5.10
178. Huey DJ, Hu JC, Athanasiou K. Unlike bone, cartilage regeneration remains elusive. Science. 2012;338(6109):917–921. doi:10.1126/science.1222454
179. Campana V, Milano G, Pagano E, et al. Bone substitutes in orthopaedic surgery: from basic science to clinical practice. J Mater Sci Mater Med. 2014;25(10):2445–2461. doi:10.1007/s10856-014-5240-2
180. Gao C, Peng S, Feng P, Shuai C. Bone biomaterials and interactions with stem cells. Bone Res. 2017;5(1):17059. doi:10.1038/boneres.2017.59
181. Oryan A, Alidadi S, Moshiri A, Maffulli N. Bone regenerative medicine: classic options, novel strategies, and future directions. J Orthop Surg Res. 2014;9(1):18. doi:10.1186/1749-799X-9-18
182. Aryaei A, Jayatissa AH, Jayasuriya AC. The effect of graphene substrate on osteoblast cell adhesion and proliferation. J Biomed Mater Res Part A. 2014;102(9):3282–3290. doi:10.1002/jbm.a.34993
183. Crowder SW, Prasai D, Rath R, et al. Three-dimensional graphene foams promote osteogenic differentiation of human mesenchymal stem cells. Nanoscale. 2013;5(10):4171. doi:10.1039/c3nr00803g
184. Gao C, Liu T, Shuai C, Peng S. Enhancement mechanisms of graphene in nano-58S bioactive glass scaffold: mechanical and biological performance. Sci Rep. 2015;4(1):4712. doi:10.1038/srep04712
185. Jakus AE, Shah RN. Multi and mixed 3D-printing of graphene-hydroxyapatite hybrid materials for complex tissue engineering. J Biomed Mater Res Part A. 2017;105(1):274–283. doi:10.1002/jbm.a.35684
186. Luo J, Zhang X, Ong’achwa Machuki J, et al. Three-dimensionally N-doped graphene–hydroxyapatite/agarose as an osteoinductive scaffold for enhancing bone regeneration. ACS Appl Bio Mater. 2019;2(1):299–310. doi:10.1021/acsabm.8b00599
187. Elkhenany H, Amelse L, Lafont A, et al. Graphene supports in vitro proliferation and osteogenic differentiation of goat adult mesenchymal stem cells: potential for bone tissue engineering. J Appl Toxicol. 2015;35(4):367–374. doi:10.1002/jat.3024
188. Olad A, Bakht Khosh Hagh H, Mirmohseni A, Farshi Azhar F. Graphene oxide and montmorillonite enriched natural polymeric scaffold for bone tissue engineering. Ceram Int. 2019;45(12):15609–15619. doi:10.1016/j.ceramint.2019.05.071
189. Natarajan J, Madras G, Chatterjee K. Development of graphene oxide-/galactitol polyester-based biodegradable composites for biomedical applications. ACS Omega. 2017;2(9):5545–5556. doi:10.1021/acsomega.7b01139
190. Zhou T, Li G, Lin S, et al. Electrospun Poly(3-hydroxybutyrate- co −4-hydroxybutyrate)/Graphene oxide scaffold: enhanced properties and promoted in vivo bone repair in rats. ACS Appl Mater Interfaces. 2017;9(49):42589–42600. doi:10.1021/acsami.7b14267
191. Díez-Pascual AM, Díez-Vicente AL. Poly(propylene fumarate)/Polyethylene Glycol-modified graphene oxide nanocomposites for tissue engineering. ACS Appl Mater Interfaces. 2016;8(28):17902–17914. doi:10.1021/acsami.6b05635
192. Zhang Y, Wang C, Fu L. Fabrication and application of novel porous scaffold in situ-loaded graphene oxide and osteogenic peptide by cryogenic 3D printing for repairing critical-sized bone defect. Molecules. 2019;24(9):1669. doi:10.3390/molecules24091669
193. Liang C, Luo Y, Yang G, et al. Graphene oxide hybridized nHAC/PLGA scaffolds facilitate the proliferation of MC3T3-E1 cells. Nanoscale Res Lett. 2018;13(1):15. doi:10.1186/s11671-018-2432-6
194. Yu Z, Xiao C, Huang Y, et al. Enhanced bioactivity and osteoinductivity of carboxymethyl chitosan/nanohydroxyapatite/graphene oxide nanocomposites. RSC Adv. 2018;8(32):17860–17877. doi:10.1039/C8RA00383A
195. Nosrati H, Mamoory RS, Le DQS, Bünger CE. Preparation of reduced graphene oxide/hydroxyapatite nanocomposite and evaluation of graphene sheets/hydroxyapatite interface. Diam Relat Mater. 2019;100:107561. doi:10.1016/j.diamond.2019.107561
196. Nie W, Peng C, Zhou X, et al. Three-dimensional porous scaffold by self-assembly of reduced graphene oxide and nano-hydroxyapatite composites for bone tissue engineering. Carbon NY. 2017;116:325–337. doi:10.1016/j.carbon.2017.02.013
197. Lee JH, Shin YC, Jin OS, et al. Reduced graphene oxide-coated hydroxyapatite composites stimulate spontaneous osteogenic differentiation of human mesenchymal stem cells. Nanoscale. 2015;7(27):11642–11651. doi:10.1039/C5NR01580D
198. Kim J, Kim Y-R, Kim Y, et al. Graphene-incorporated chitosan substrata for adhesion and differentiation of human mesenchymal stem cells. J Mater Chem B. 2013;1(7):933. doi:10.1039/c2tb00274d
199. Wang Q, Chu Y, He J, et al. A graded graphene oxide-hydroxyapatite/silk fibroin biomimetic scaffold for bone tissue engineering. Mater Sci Eng C. 2017;80:232–242. doi:10.1016/j.msec.2017.05.133
200. Fu C, Bai H, Zhu J, et al. Enhanced cell proliferation and osteogenic differentiation in electrospun PLGA/hydroxyapatite nanofibre scaffolds incorporated with graphene oxide. PLoS One. 2017;12(11):e0188352. doi:10.1371/journal.pone.0188352
201. Boga JC, Miguel SP, de Melo-Diogo D, Mendonça AG, Louro RO, Correia IJ. In vitro characterization of 3D printed scaffolds aimed at bone tissue regeneration. Colloids Surfaces B Biointerfaces. 2018;165:207–218. doi:10.1016/j.colsurfb.2018.02.038
202. Xue D, Chen E, Zhong H, et al. Immunomodulatory properties of graphene oxide for osteogenesis and angiogenesis. Int J Nanomedicine. 2018;13:5799–5810. doi:10.2147/IJN.S170305
203. Wang Q, Li M, Cui T, et al. A novel Zwitterionic hydrogel incorporated with graphene oxide for bone tissue engineering: synthesis, characterization, and promotion of osteogenic differentiation of bone mesenchymal stem cells. Int J Mol Sci. 2023;24(3):2691. doi:10.3390/ijms24032691
204. Narita N, Kobayashi Y, Nakamura H, et al. Multiwalled carbon nanotubes specifically inhibit osteoclast differentiation and function. Nano Lett. 2009;9(4):1406–1413. doi:10.1021/nl8030746
205. Usui Y, Aoki K, Narita N, et al. Carbon nanotubes with high bone-tissue compatibility and bone-formation acceleration effects. Small. 2008;4(2):240–246. doi:10.1002/smll.200700670
206. Pei B, Wang W, Dunne N, Li X. Applications of carbon nanotubes in bone tissue regeneration and engineering: superiority, concerns, current advancements, and prospects. Nanomaterials. 2019;9(10):1501. doi:10.3390/nano9101501
207. Rodrigues BVM, Silva AS, Melo GFS, Vasconscellos LMR, Marciano FR, Lobo AO. Influence of low contents of superhydrophilic MWCNT on the properties and cell viability of electrospun poly (butylene adipate-co-terephthalate) fibers. Mater Sci Eng C. 2016;59:782–791. doi:10.1016/j.msec.2015.10.075
208. da Cunha MR, Alves MC, Calegari ARA, et al. In vivo study of the osteoregenerative potential of polymer membranes consisting of chitosan and carbon nanotubes. Mater Res. 2017;20(3):819–825. doi:10.1590/1980-5373-mr-2016-1112
209. Khalid P, Hussain M, Rekha P, Arun A. Carbon nanotube-reinforced hydroxyapatite composite and their interaction with human osteoblast in vitro. Hum Exp Toxicol. 2015;34(5):548–556. doi:10.1177/0960327114550883
210. Li H, Zhao Q, Li B, et al. Fabrication and properties of carbon nanotube-reinforced hydroxyapatite composites by a double in situ synthesis process. Carbon NY. 2016;101:159–167. doi:10.1016/j.carbon.2016.01.086
211. Türk S, Altınsoy I, Çelebi Efe G, Ipek M, Özacar M, Bindal C. 3D porous collagen/functionalized multiwalled carbon nanotube/chitosan/hydroxyapatite composite scaffolds for bone tissue engineering. Mater Sci Eng C. 2018;92:757–768. doi:10.1016/j.msec.2018.07.020
212. Rajesh R, Dominic Ravichandran Y, Jeevan Kumar Reddy M, Ryu SH, Shanmugharaj AM. Development of functionalized multi-walled carbon nanotube-based polysaccharide–hydroxyapatite scaffolds for bone tissue engineering. RSC Adv. 2016;6(85):82385–82393. doi:10.1039/C6RA16709H
213. Yamakoshi Y, Umezawa N, Ryu A, et al. Active oxygen species generated from photoexcited fullerene (C 60) as potential medicines: o 2 - • versus 1 O 2. J Am Chem Soc. 2003;125(42):12803–12809. doi:10.1021/ja0355574
214. Krishnan V, Kasuya Y, Ji Q, et al. Vortex-aligned fullerene nanowhiskers as a scaffold for orienting cell growth. ACS Appl Mater Interfaces. 2015;7(28):15667–15673. doi:10.1021/acsami.5b04811
215. Yudoh K. Water-soluble fullerene (C60) inhibits the osteoclast differentiation and bone destruction in arthritis. Int J Nanomedicine. 2009;233. doi:10.2147/IJN.S7505
216. Lee NK, Choi YG, Baik JY, et al. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood. 2005;106(3):852–859. doi:10.1182/blood-2004-09-3662
217. Liu H, Yang X, Zhang Y, Dighe A, Li X, Cui Q. Fullerol antagonizes dexamethasone-induced oxidative stress and adipogenesis while enhancing osteogenesis in a cloned bone marrow mesenchymal stem cell. J Orthop Res. 2012;30(7):1051–1057. doi:10.1002/jor.22054
218. Zhang Q, Mochalin VN, Neitzel I, et al. Fluorescent PLLA-nanodiamond composites for bone tissue engineering. Biomaterials. 2011;32(1):87–94. doi:10.1016/j.biomaterials.2010.08.090
219. Zhang Q, Mochalin VN, Neitzel I, et al. Mechanical properties and biomineralization of multifunctional nanodiamond-PLLA composites for bone tissue engineering. Biomaterials. 2012;33(20):5067–5075. doi:10.1016/j.biomaterials.2012.03.063
220. Parizek M, Douglas TE, Novotna K, et al.. Nanofibrous poly(lactide-co-glycolide) membranes loaded with diamond nanoparticles as promising substrates for bone tissue engineering. Int J Nanomedicine. 2012:1931. doi:10.2147/IJN.S26665
221. Ostadhossein F, Benig L, Tripathi I, Misra SK, Pan D. Fluorescence detection of bone microcracks using monophosphonated carbon dots. ACS Appl Mater Interfaces. 2018;10(23):19408–19415. doi:10.1021/acsami.8b03727
222. Peng Z, Miyanji EH, Zhou Y, et al. Carbon dots: promising biomaterials for bone-specific imaging and drug delivery. Nanoscale. 2017;9(44):17533–17543. doi:10.1039/C7NR05731H
223. Lee KK, Lee J-G, Park CS, et al. Bone-targeting carbon dots: effect of nitrogen-doping on binding affinity. RSC Adv. 2019;9(5):2708–2717. doi:10.1039/C8RA09729A
224. Gogoi S, Maji S, Mishra D, Devi KSP, Maiti TK, Karak N. Nano-bio engineered carbon dot-peptide functionalized water dispersible hyperbranched polyurethane for bone tissue regeneration. Macromol Biosci. 2017;17(3):1600271. doi:10.1002/mabi.201600271
225. Shao D, Lu M, Xu D, et al. Carbon dots for tracking and promoting the osteogenic differentiation of mesenchymal stem cells. Biomater Sci. 2017;5(9):1820–1827. doi:10.1039/C7BM00358G
226. Sarkar C, Chowdhuri AR, Kumar A, et al. One pot synthesis of carbon dots decorated carboxymethyl cellulose- hydroxyapatite nanocomposite for drug delivery, tissue engineering and Fe3+ ion sensing. Carbohydr Polym. 2018;181:710–718. doi:10.1016/j.carbpol.2017.11.091
227. Camarero-Espinosa S, Rothen-Rutishauser B, Foster EJ, Weder C. Articular cartilage: from formation to tissue engineering. Biomater Sci. 2016;4(5):734–767. doi:10.1039/c6bm00068a
228. Palazzo C, Nguyen C, Lefevre-Colau -M-M, Rannou F, Poiraudeau S. Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med. 2016;59(3):134–138. doi:10.1016/j.rehab.2016.01.006
229. Hunter DJ, Schofield D, Callander E. The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol. 2014;10(7):437–441. doi:10.1038/nrrheum.2014.44
230. Martín AR, Patel JM, Zlotnick HM, Carey JL, Mauck RL. Emerging therapies for cartilage regeneration in currently excluded ‘red knee’ populations. npj Regen Med. 2019;4(1):12. doi:10.1038/s41536-019-0074-7
231. Liu Y, Zhou G, Cao Y. Recent progress in cartilage tissue engineering—our experience and future directions. Engineering. 2017;3(1):28–35. doi:10.1016/J.ENG.2017.01.010
232. Antonioli E, Lobo AO, Ferretti M, et al. An evaluation of chondrocyte morphology and gene expression on superhydrophilic vertically-aligned multi-walled carbon nanotube films. Mater Sci Eng C. 2013;33(2):641–647. doi:10.1016/j.msec.2012.10.010
233. King AAK, Matta-Domjan B, Large MJ, et al. Pristine carbon nanotube scaffolds for the growth of chondrocytes. J Mater Chem B. 2017;5(41):8178–8182. doi:10.1039/C7TB02065A
234. Trzeciak T, Rybka JD, Akinoglu EM, Richter M, Kaczmarczyk J, Giersig M. In vitro evaluation of carbon nanotube-based scaffolds for cartilage tissue engineering. J Nanosci Nanotechnol. 2016;16(9):9022–9025. doi:10.1166/jnn.2016.12733
235. Markowski J, Magiera A, Lesiak M, Sieron AL, Pilch J, Blazewicz S. Preparation and characterization of nanofibrous polymer scaffolds for cartilage tissue engineering. J Nanomater. 2015;2015:1–9. doi:10.1155/2015/564087
236. Ghassemi T, Saghatolslami N, Matin MM, Gheshlaghi R, Moradi A. CNT-decellularized cartilage hybrids for tissue engineering applications. Biomed Mater. 2017;12(6):065008. doi:10.1088/1748-605X/aa8435
237. Mirmusavi MH, Zadehnajar P, Semnani D, Karbasi S, Fekrat F, Heidari F. Evaluation of physical, mechanical and biological properties of poly 3-hydroxybutyrate-chitosan-multiwalled carbon nanotube/silk nano-micro composite scaffold for cartilage tissue engineering applications. Int J Biol Macromol. 2019;132:822–835. doi:10.1016/j.ijbiomac.2019.03.227
238. Chuang E-Y, Chiang C-W, Wong P-C, Chen C-H. Hydrogels for the application of articular cartilage tissue engineering: a review of hydrogels. Adv Mater Sci Eng. 2018;2018:1–13. doi:10.1155/2018/4368910
239. Liao J, Qu Y, Chu B, Zhang X, Qian Z. Biodegradable CSMA/PECA/Graphene porous hybrid scaffold for cartilage tissue engineering. Sci Rep. 2015;5(1):9879. doi:10.1038/srep09879
240. Shamekhi MA, Mirzadeh H, Mahdavi H, Rabiee A, Mohebbi-Kalhori D, Baghaban Eslaminejad M. Graphene oxide containing chitosan scaffolds for cartilage tissue engineering. Int J Biol Macromol. 2019;127:396–405. doi:10.1016/j.ijbiomac.2019.01.020
241. Deliormanlı AM. Direct write assembly of Graphene/Poly(ε-Caprolactone) composite scaffolds and evaluation of their biological performance using mouse bone marrow mesenchymal stem cells. Appl Biochem Biotechnol. 2019;188(4):1117–1133. doi:10.1007/s12010-019-02976-5
242. Zhang B, Wei P, Zhou Z, Wei T. Interactions of graphene with mammalian cells: molecular mechanisms and biomedical insights. Adv Drug Deliv Rev. 2016;105:145–162. doi:10.1016/j.addr.2016.08.009
243. Gopinathan J, Pillai MM, Sahanand KS, Rai BKD, Selvakumar R, Bhattacharyya A. Synergistic effect of electrical conductivity and biomolecules on human meniscal cell attachment, growth, and proliferation in poly- ε -caprolactone nanocomposite scaffolds. Biomed Mater. 2017;12(6):065001. doi:10.1088/1748-605X/aa7f7b
244. Wang L, Cao W, Wang X, et al. Biodegradable silver-loaded polycation modified nanodiamonds/polyurethane scaffold with improved antibacterial and mechanical properties for cartilage tissue repairing. J Mater Sci Mater Med. 2019;30(4):41. doi:10.1007/s10856-019-6244-8
245. Wu Y-C, Wang Y-C, Wang W-T, et al. Fluorescent nanodiamonds enable long-term detection of human adipose-derived stem/stromal cells in an in vivo chondrogenesis model using decellularized extracellular matrices and fibrin glue polymer. Polymers (Basel). 2019;11(9):1391. doi:10.3390/polym11091391
246. Su L-J, Wu M-S, Hui YY, et al. Fluorescent nanodiamonds enable quantitative tracking of human mesenchymal stem cells in miniature pigs. Sci Rep. 2017;7(1):45607. doi:10.1038/srep45607
247. Das B, Dadhich P, Pal P, et al. Doping of carbon quantum dots (CDs) in calcium phosphate nanorods for inducing ectopic chondrogenesis via activation of the HIF-α/SOX-9 pathway. ACS Omega. 2019;4(1):374–386. doi:10.1021/acsomega.8b01763
248. Yudoh K. Water-soluble fullerene (c60) inhibits the development of arthritis in the rat model of arthritis. Int J Nanomedicine. 2009;217. doi:10.2147/IJN.S7653
249. Yudoh K, Shishido K, Murayama H, et al. Water-soluble C60 fullerene prevents degeneration of articular cartilage in osteoarthritis via down-regulation of chondrocyte catabolic activity and inhibition of cartilage degeneration during disease development. Arthritis Rheum. 2007;56(10):3307–3318. doi:10.1002/art.22917
250. Dellinger AL, Cunin P, Lee D, et al. Inhibition of inflammatory arthritis using fullerene nanomaterials. PLoS One. 2015;10(4):e0126290. doi:10.1371/journal.pone.0126290
251. Pei Y, Cui F, Du X, et al. Antioxidative nanofullerol inhibits macrophage activation and development of osteoarthritis in rats. Int J Nanomedicine. 2019;14:4145–4155. doi:10.2147/IJN.S202466
252. Song J, Jia X, Minami K, et al. Large-area aligned fullerene nanocrystal scaffolds as culture substrates for enhancing mesenchymal stem cell self-renewal and multipotency. ACS Appl Nano Mater. 2020;3(7):6497–6506. doi:10.1021/acsanm.0c00973
253. van Bergen CJ, Kox LS, Maas M, Sierevelt IN, Kerkhoffs GMMJ, van Dijk CN. Arthroscopic treatment of osteochondral defects of the talus. J Bone Jt Surg. 2013;95(6):519–525. doi:10.2106/JBJS.L.00675
254. Li X, Ding J, Wang J, Zhuang X, Chen X. Biomimetic biphasic scaffolds for osteochondral defect repair. Regen Biomater. 2015;2(3):221–228. doi:10.1093/rb/rbv015
255. Shimomura K, Moriguchi Y, Murawski CD, Yoshikawa H, Nakamura N. Osteochondral tissue engineering with biphasic scaffold: current strategies and techniques. Tissue Eng Part B Rev. 2014;20(5):468–476. doi:10.1089/ten.teb.2013.0543
256. Parisi C, Salvatore L, Veschini L, et al. Biomimetic gradient scaffold of collagen–hydroxyapatite for osteochondral regeneration. J Tissue Eng. 2020;11:204173141989606. doi:10.1177/2041731419896068
257. Hsieh Y-H, Shen B-Y, Wang Y-H, Lin B, Lee H-M, Hsieh M-F. Healing of osteochondral defects implanted with biomimetic scaffolds of Poly(ε-Caprolactone)/Hydroxyapatite and glycidyl-methacrylate-modified hyaluronic acid in a Minipig. Int J Mol Sci. 2018;19(4):1125. doi:10.3390/ijms19041125
258. Li X, Li Y, Zuo Y, et al. Osteogenesis and chondrogenesis of biomimetic integrated porous PVA/gel/V-n-HA/pa6 scaffolds and BMSCs construct in repair of articular osteochondral defect. J Biomed Mater Res Part A. 2015;103(10):3226–3236. doi:10.1002/jbm.a.35452
259. Yan LP, Silva-Correia J, Oliveira MB, et al. Bilayered silk/silk-nanoCaP scaffolds for osteochondral tissue engineering: in vitro and in vivo assessment of biological performance. Acta Biomater. 2015;12(1):227–241. doi:10.1016/j.actbio.2014.10.021
260. Deliormanlı AM, Atmaca H. Biological response of osteoblastic and chondrogenic cells to graphene-containing PCL/Bioactive glass bilayered scaffolds for osteochondral tissue engineering applications. Appl Biochem Biotechnol. 2018;186(4):972–989. doi:10.1007/s12010-018-2758-7
261. Cao L, Zhang F, Wang Q, Wu X. Fabrication of chitosan/graphene oxide polymer nanofiber and its biocompatibility for cartilage tissue engineering. Mater Sci Eng C. 2017;79:697–701. doi:10.1016/j.msec.2017.05.056
262. Zhang Y, Zhang M, Jiang H, et al. Bio-inspired layered chitosan/graphene oxide nanocomposite hydrogels with high strength and pH-driven shape memory effect. Carbohydr Polym. 2017;177:116–125. doi:10.1016/j.carbpol.2017.08.106
263. Siqueira IAWB, Corat MAF, Das Cavalcanti BN, et al. In vitro and in vivo studies of novel Poly(d, l -lactic acid), superhydrophilic carbon nanotubes, and nanohydroxyapatite scaffolds for bone regeneration. ACS Appl Mater Interfaces. 2015;7(18):9385–9398. doi:10.1021/acsami.5b01066
264. Stocco T, Antonioli E, Elias C, et al. Cell viability of porous Poly(d,l-lactic acid)/vertically aligned carbon nanotubes/nanohydroxyapatite scaffolds for osteochondral tissue engineering. Materials (Basel). 2019;12(6):849. doi:10.3390/ma12060849
265. Liu M, Zeng X, Ma C, et al. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017;5(1):17014. doi:10.1038/boneres.2017.14
266. Li J, Chen G, Xu X, et al. Advances of injectable hydrogel-based scaffolds for cartilage regeneration. Regen Biomater. 2019;6(3):129–140. doi:10.1093/rb/rbz022
267. Grasman JM, Zayas MJ, Page RL, Pins GD. Biomimetic scaffolds for regeneration of volumetric muscle loss in skeletal muscle injuries. Acta Biomater. 2015;25:2–15. doi:10.1016/j.actbio.2015.07.038
268. Guo B, Ma PX. Conducting polymers for tissue engineering. Biomacromolecules. 2018;19(6):1764–1782. doi:10.1021/acs.biomac.8b00276
269. Dong R, Ma PX, Guo B. Conductive biomaterials for muscle tissue engineering. Biomaterials. 2020;229:119584. doi:10.1016/j.biomaterials.2019.119584
270. Ku SH, Park CB. Myoblast differentiation on graphene oxide. Biomaterials. 2013;34(8):2017–2023. doi:10.1016/j.biomaterials.2012.11.052
271. Chaudhuri B, Bhadra D, Moroni L, Pramanik K. Myoblast differentiation of human mesenchymal stem cells on graphene oxide and electrospun graphene oxide–polymer composite fibrous meshes: importance of graphene oxide conductivity and dielectric constant on their biocompatibility. Biofabrication. 2015;7(1):015009. doi:10.1088/1758-5090/7/1/015009
272. Thrivikraman G, Mallik PK, Basu B. Substrate conductivity dependent modulation of cell proliferation and differentiation in vitro. Biomaterials. 2013;34(29):7073–7085. doi:10.1016/j.biomaterials.2013.05.076
273. Patel A, Xue Y, Mukundan S, et al. Cell-instructive graphene-containing nanocomposites induce multinucleated Myotube formation. Ann Biomed Eng. 2016;44(6):2036–2048. doi:10.1007/s10439-016-1586-6
274. Bajaj P, Rivera JA, Marchwiany D, Solovyeva V, Bashir R. Graphene-based patterning and differentiation of C2C12 myoblasts. Adv Healthc Mater. 2014;3(7):995–1000. doi:10.1002/adhm.201300550
275. Ahadian S, Ramón-Azcón J, Chang H, et al. Electrically regulated differentiation of skeletal muscle cells on ultrathin graphene-based films. RSC Adv. 2014;4(19):9534. doi:10.1039/c3ra46218h
276. Krueger E, Chang AN, Brown D, et al. Graphene foam as a three-dimensional platform for Myotube growth. ACS Biomater Sci Eng. 2016;2(8):1234–1241. doi:10.1021/acsbiomaterials.6b00139
277. Shin YC, Kang SH, Lee JH, Kim B, Hong SW, Han D-W. Three-dimensional graphene oxide-coated polyurethane foams beneficial to myogenesis. J Biomater Sci Polym Ed. 2018;29(7–9):762–774. doi:10.1080/09205063.2017.1348738
278. Segers VFM, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451(7181):937–942. doi:10.1038/nature06800
279. Rodrigues ICP, Kaasi A, Maciel Filho R, Jardini AL, Gabriel LP. Cardiac tissue engineering: current state-of-the-art materials, cells and tissue formation. Einstein (São Paulo). 2018;16(3). doi:10.1590/s1679-45082018rb4538
280. Mooney E, Mackle JN, Blond DJ-P, et al. The electrical stimulation of carbon nanotubes to provide a cardiomimetic cue to MSCs. Biomaterials. 2012;33(26):6132–6139. doi:10.1016/j.biomaterials.2012.05.032
281. Namgung S, Baik KY, Park J, Hong S. Controlling the growth and differentiation of human mesenchymal stem cells by the arrangement of individual carbon nanotubes. ACS Nano. 2011;5(9):7383–7390. doi:10.1021/nn2023057
282. Ren J, Xu Q, Chen X, et al. Superaligned carbon nanotubes guide oriented cell growth and promote electrophysiological homogeneity for synthetic cardiac tissues. Adv Mater. 2017;29(44):1702713. doi:10.1002/adma.201702713
283. Zhou J, Chen J, Sun H, et al. Engineering the heart: evaluation of conductive nanomaterials for improving implant integration and cardiac function. Sci Rep. 2015;4(1):3733. doi:10.1038/srep03733
284. Wu Y, Shi X, Li Y, et al. Carbon nanohorns promote maturation of neonatal rat ventricular myocytes and inhibit proliferation of cardiac fibroblasts: a promising scaffold for cardiac tissue engineering. Nanoscale Res Lett. 2016;11(1):284. doi:10.1186/s11671-016-1464-z
285. Martinelli V, Bosi S, Peña B, et al. 3D carbon-nanotube-based composites for cardiac tissue engineering. ACS Appl Bio Mater. 2018;1(5):1530–1537. doi:10.1021/acsabm.8b00440
286. Zhou J, Yang X, Liu W, et al. Injectable OPF/graphene oxide hydrogels provide mechanical support and enhance cell electrical signaling after implantation into myocardial infarct. Theranostics. 2018;8(12):3317–3330. doi:10.7150/thno.25504
287. Han B, Zhang Y, Zhu L, et al. Plasmonic-assisted graphene oxide artificial muscles. Adv Mater. 2018:1806386. doi:10.1002/adma.201806386
288. El-Sherbiny IM, Yacoub MH. Hydrogel scaffolds for tissue engineering: progress and challenges. Glob Cardiol Sci Pract. 2013;2013(3):38. doi:10.5339/gcsp.2013.38
289. Spicer CD. Hydrogel scaffolds for tissue engineering: the importance of polymer choice. Polym Chem. 2020;11(2):184–219. doi:10.1039/C9PY01021A
290. Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliv Rev. 2012;64:18–23. doi:10.1016/j.addr.2012.09.010
291. Huang Q, Zou Y, Arno MC, et al. Hydrogel scaffolds for differentiation of adipose-derived stem cells. Chem Soc Rev. 2017;46(20):6255–6275. doi:10.1039/c6cs00052e
292. Cui X, Lee JJL, Chen WN. Eco-friendly and biodegradable cellulose hydrogels produced from low cost okara: towards non-toxic flexible electronics. Sci Rep. 2019;9(1):18166. doi:10.1038/s41598-019-54638-5
293. Catoira MC, Fusaro L, Di Francesco D, Ramella M, Boccafoschi F. Overview of natural hydrogels for regenerative medicine applications. J Mater Sci Mater Med. 2019;30(10):115. doi:10.1007/s10856-019-6318-7
294. Gaharwar AK, Peppas NA, Khademhosseini A. Nanocomposite hydrogels for biomedical applications. Biotechnol Bioeng. 2014;111(3):441–453. doi:10.1002/bit.25160
295. Rehman SR, Augustine R, Zahid AA, Ahmed R, Tariq M, Hasan A. Reduced graphene oxide incorporated gelma hydrogel promotes angiogenesis for wound healing applications. Int J Nanomedicine. 2019;14:9603–9617. doi:10.2147/IJN.S218120
296. Im K, Nguyen DN, Kim S, et al. Graphene-embedded hydrogel nanofibers for detection and removal of aqueous-phase dyes. ACS Appl Mater Interfaces. 2017;9(12):10768–10776. doi:10.1021/acsami.7b01163
297. Wang Z, Li J, Jiang L, Xiao S, Liu Y, Luo J. Zwitterionic hydrogel incorporated graphene oxide nanosheets with improved strength and lubricity. Langmuir. 2019;35(35):11452–11462. doi:10.1021/acs.langmuir.9b01640
298. Saravanan S, Vimalraj S, Anuradha D. Chitosan based thermoresponsive hydrogel containing graphene oxide for bone tissue repair. Biomed Pharmacother. 2018;107:908–917. doi:10.1016/j.biopha.2018.08.072
299. Vashist A, Kaushik A, Vashist A, et al. Advances in carbon nanotubes-hydrogel hybrids in nanomedicine for therapeutics. Adv Healthc Mater. 2018;7(9):1701213. doi:10.1002/adhm.201701213
300. Cui H, Yu Y, Li X, et al. Direct 3D printing of a tough hydrogel incorporated with carbon nanotubes for bone regeneration. J Mater Chem B. 2019;7(45):7207–7217. doi:10.1039/C9TB01494B
301. Anjali J, Jose VK, Lee J-M. Carbon-based hydrogels: synthesis and their recent energy applications. J Mater Chem A. 2019;7(26):15491–15518. doi:10.1039/C9TA02525A
302. Hu W, Zhang P, Liu X, et al. An amphiphobic graphene-based hydrogel as oil-water separator and oil fence material. Chem Eng J. 2018;353:708–716. doi:10.1016/j.cej.2018.07.147
303. Dong C, Lu J, Qiu B, Shen B, Xing M, Zhang J. Developing stretchable and graphene-oxide-based hydrogel for the removal of organic pollutants and metal ions. Appl Catal B Environ. 2018;222:146–156. doi:10.1016/j.apcatb.2017.10.011
304. Wang R, Xu C, Lee J-M. High performance asymmetric supercapacitors: new NiOOH nanosheet/graphene hydrogels and pure graphene hydrogels. Nano Energy. 2016;19:210–221. doi:10.1016/j.nanoen.2015.10.030
305. Tan Y, Wu D, Wang T, Liu P, Guo J, Jia D. Facile synthesis of functionalized graphene hydrogel for high performance supercapacitor with high volumetric capacitance and ultralong cycling stability. Appl Surf Sci. 2018;455:683–695. doi:10.1016/j.apsusc.2018.05.161
306. Lai E, Yue X, Ning W, Huang J, Ling X, Lin H. Three-dimensional graphene-based composite hydrogel materials for flexible supercapacitor electrodes. Front Chem. 2019;7. doi:10.3389/fchem.2019.00660
307. Liao Y, Liang K, Ren Y, Huang X. Fabrication of SiOx-G/PAA-PANi/Graphene composite with special cross-doped conductive hydrogels as anode materials for lithium ion batteries. Front Chem. 2020;8. doi:10.3389/fchem.2020.00096
308. Lv J, Kong C, Yang C, et al. Wearable, stable, highly sensitive hydrogel–graphene strain sensors. Beilstein J Nanotechnol. 2019;10:475–480. doi:10.3762/bjnano.10.47
309. Pinto AM, Moreira S, Gonçalves IC, Gama FM, Mendes AM, Magalhães FD. Biocompatibility of poly(lactic acid) with incorporated graphene-based materials. Colloids Surfaces B Biointerfaces. 2013;104:229–238. doi:10.1016/j.colsurfb.2012.12.006
310. Lim HN, Huang NM, Lim SS, Harrison I, Chia CH. Fabrication and characterization of graphene hydrogel via hydrothermal approach as a scaffold for preliminary study of cell growth. Int J Nanomedicine. 2011;1817. doi:10.2147/IJN.S23392
311. Li W, Wang J, Ren J, Qu X. 3D graphene oxide-polymer hydrogel: near-infrared light-triggered active scaffold for reversible cell capture and on-demand release. Adv Mater. 2013;25(46):6737–6743. doi:10.1002/adma.201302810
312. Liao G, Hu J, Chen Z, Zhang R, Wang G, Kuang T. Preparation, properties, and applications of graphene-based hydrogels. Front Chem. 2018;6. doi:10.3389/fchem.2018.00450
313. Fan Z, Liu B, Wang J, et al. A novel wound dressing based on Ag/Graphene polymer hydrogel: effectively kill bacteria and accelerate wound healing. Adv Funct Mater. 2014;24(25):3933–3943. doi:10.1002/adfm.201304202
314. Jing X, Mi H-Y, Napiwocki BN, Peng X-F, Turng L-S. Mussel-inspired electroactive chitosan/graphene oxide composite hydrogel with rapid self-healing and recovery behavior for tissue engineering. Carbon NY. 2017;125:557–570. doi:10.1016/j.carbon.2017.09.071
315. Leganés Bayón J, Sánchez-Migallón AM, Díaz-Ortiz Á, et al. On-demand hydrophobic drug release based on microwave-responsivegraphene hydrogel scaffolds. Chem A Eur J. 2020:
316. Wang J, Wang D, Yan H, et al. An injectable ionic hydrogel inducing high temperature hyperthermia for microwave tumor ablation. J Mater Chem B. 2017;5(22):4110–4120. doi:10.1039/C7TB00556C
317. Rivero RE, Molina MA, Rivarola CR, Barbero CA. Pressure and microwave sensors/actuators based on smart hydrogel/conductive polymer nanocomposite. Sensors Actuators B Chem. 2014;190:270–278. doi:10.1016/j.snb.2013.08.054
318. Elías AL, Carrero-Sánchez JC, Terrones H, Endo M, Laclette JP, Terrones M. Viability studies of pure carbon- and nitrogen-doped nanotubes with Entamoeba histolytica: from amoebicidal to biocompatible structures. Small. 2007;3(10):1723–1729. doi:10.1002/smll.200700331
319. Carrero-Sánchez JC, Elías AL, Mancilla R, et al. Biocompatibility and toxicological studies of carbon nanotubes doped with nitrogen. Nano Lett. 2006;6(8):1609–1616. doi:10.1021/nl060548p
320. Yeh Y-T, Gulino K, Zhang Y, et al. A rapid and label-free platform for virus capture and identification from clinical samples. Proc Natl Acad Sci. 2020;117(2):895–901. doi:10.1073/pnas.1910113117
321. Yeh Y-T, Zhou Y, Zou D, et al. Rapid size-based isolation of extracellular vesicles by three-dimensional carbon nanotube arrays. ACS Appl Mater Interfaces. 2020;12(11):13134–13139. doi:10.1021/acsami.9b20990
322. Yeh Y-T, Tang Y, Sebastian A, et al. Tunable and label-free virus enrichment for ultrasensitive virus detection using carbon nanotube arrays. Sci Adv. 2016;2(10). doi:10.1126/sciadv.1601026
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


