Back to Journals » International Medical Case Reports Journal » Volume 16
Carbimazole-Resistant Grave’s Thyrotoxicosis is a Diagnostic and Therapeutic Dilemma, Case Report with Literature Review
Authors Ata F , Khan AA , Tahir S , Al Amer Z
Received 21 July 2023
Accepted for publication 3 October 2023
Published 28 November 2023 Volume 2023:16 Pages 783—790
DOI https://doi.org/10.2147/IMCRJ.S429561
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Fateen Ata,1 Adeel Ahmad Khan,1 Shuja Tahir,2 Zaina Al Amer1
1Department of Endocrinology, Hamad Medical Corporation, Doha, Qatar; 2Department of Cardiothoracic Surgery, St James’s Hospital, Dublin, Ireland
Correspondence: Fateen Ata
Department of Endocrinology, Hamad General Hospital, Hamad Medical Corporation, Doha, 3050, Qatar
, Tel +974 66055176
, Email [email protected]; [email protected]
Background: Carbimazole (CBZ) (or methimazole) is the most used drug inducing and maintaining remission in thyrotoxicosis, especially Grave’s disease (GD). Rarely, situations arise when patients do not respond to recommended or even supratherapeutic doses of CBZ. It poses a challenge to diagnose drug resistance and ultimately manage hyperthyroidism, which can otherwise be fatal if left untreated. Propylthiouracil (PTU) has been used as an alternative in such patients amid increased side effect risks. Additionally, definitive therapy has been recommended with ablation or surgery. However, the best modality of inducing euthyroidism in drug-resistant patients is yet to be established. On literature search, twenty similar cases were found in the literature search. This study summarizes the past literature with addition of a new case of anti-thyroid drug resistant (ATDR) GD.
Case Presentation: A 34-year-old female presented with a 5-day history of progressively worsening fatigue, heat intolerance, sweating, and palpitations. She was diagnosed with GD based on her thyroid function tests (TFTs) and started on CBZ and propranolol. Despite being compliant with CBZ 20 mg once daily and then twice daily, her TFTs remained unchanged for 4 months. However, patient revisited the emergency with continued thyrotoxicosis and unchanged TFTs. Her dose was eventually increased to 20 mg thrice daily, and administration under supervision did not improve her TFTs. The patient was shifted to PTU 150 mg thrice daily with steroids, with minimal improvement. The patient eventually underwent thyroidectomy to avoid long-term PTU use.
Conclusion: ATDR GD is rare and remains a diagnostic and therapeutic challenge. Optimal management should focus on carefully excluding other possibilities and shared decision-making in its management. Most patients may require definitive therapy; hence, arrangements should be made timely with simultaneous attempts to reduce the thyrotoxic state, which otherwise poses a continued threat to patients’ life with potentially serious complications.
Keywords: carbimazole, propylthiouracil, drug resistance, thyrotoxicosis, Grave’s disease
Introduction
Drug resistance is not an uncommon challenge in managing infectious diseases.1 However, rarely can it complicate the management of non-communicable diseases. Thyrotoxicosis is a state of abnormally high thyroid hormone circulating in the blood, which can result in serious complications, including heart failure, arrhythmia, and pulmonary hypertension among others.2,3 Literature on the antithyroid drug (ATD) resistant thyrotoxicosis is limited to case reports.4–19 Although rare, multiple reports do argue in favor of its existence, albeit with many unanswered questions. The most significant and practical knowledge gaps exist in the mechanism of ATD resistance, accurate methods of diagnosing drug resistance, and best management modality. Proposed etiologies for drug resistance in patients with thyrotoxicosis include malabsorption, higher than-normal metabolism, antibodies to the drugs, and abnormalities in the intrathyroidal accumulation or action of the drugs.13 Whether alternative medicines such as propylthiouracil (PTU), steroids, lithium, and cholestyramine should be tried, or patients should be referred for definitive management remains uncertain. This case report and literature review discuss the clinicodemographic details of patients with drug-resistant thyrotoxicosis with insights from the current literature.
Case Presentation
A 34-year-old female with no past medical or surgical history was presented to the emergency department (ED) with a five-day history of progressively worsening fatigue, heat intolerance, sweating, and palpitations. Her symptoms did not have a diurnal variation. The patient described fatigue and palpitations present with milder intensity for the last two years but was never bothersome enough to seek medical attention. Upon further questioning, the patient reported five kilograms of unintentional weight loss over the past five months. She did not report diarrhea, respiratory symptoms, or chest pain. Her menstruation was regular. The patient quit smoking three months before her presentation and never consumed alcohol. The patient denied any excessive use of caffeine in her diet.
A physical exam revealed euthermia (36.6 °C), tachycardia with heart rate (HR) up to 120 beats per minute (BPM), raised blood pressure (140/100 mmHg), a respiratory rate of 20 breaths per minute, oxygen saturation at 99% on room air and a body mass index (BMI) of 23.5 kg/m2. Further examination revealed a diffuse goiter, fine bilateral tremors on outstretched hands, bilateral proptosis, bilateral lid lag and retraction, more on the right side. An electrocardiogram showed sinus tachycardia (ST) without ischemic changes or signs of chronic hypertension. A chest X-ray and bedside transthoracic echocardiogram did not reveal of any pathology. A complete blood profile and kidney and liver function tests were unremarkable.
Further blood investigations revealed thyrotoxicosis with fully suppressed thyroid stimulating hormone (TSH) (<0.01 mIU/L, normal range: 0.3–4.2), with raised T3 (17.8 pmol/L, normal range: 3.7–6.4) and T4 (52.5 pmol/L, normal range: 11–23.3). Her TSH receptor antibody (TRAB) was positive (16.5 IU/L, cut off 1.75 IU/L), hence confirming a diagnosis of Grave’s disease (GD). The patient was educated about GD and the effects and side effects (especially agranulocytosis) of CBZ. She was then started on CBZ 20 mg once daily (OD) and Propranolol 80 mg sustained release (SR) OD with an outpatient follow-up for repeat TFT and further dose adjustment. However, after four weeks, the patient revisited the ED with a three-day history of palpitations associated with chest discomfort and dizziness. The physical exam revealed ST (HR 137 BPM) and a thyroid exam similar to her initial visit. A repeat TFT panel showed TSH <0.01, T3: 17.5, and T4: 64.1. She denied non-compliance with the medication. CBZ was increased to 20 mg twice daily (BD), and Propranolol 80 mg SR was continued. The patient was seen in the endocrine clinic after two weeks. She still complained of palpitations and heat intolerance; however, ST had settled.
Two weeks post-clinic visit, the patient visited the ED for the third time with a 1-day history of bothersome palpitations. Examination revealed ST (HR140 BPM), and her BMI had increased to 24.5 kg/m2. TFTs showed persistent hyperthyroidism (TSH <0.01, T3: 12.1, T4: 54.1). The patient was admitted and given CBZ 20 mg thrice daily (TD) and propranolol 160 mg SR OD under supervision. Her TFTs were repeated after five days, showing persistent hyperthyroidism with only a marginal improvement in T4 (39 pmol/L). Medication non-adherence as a possibility was discussed in a detailed interview with the patient. The patient insisted on strict compliance to the treatment. She was bothered by persistent symptoms, with no underlying stress or depression, and wanted to get the symptoms treated. She had gained some weight and had a reduction in her absolute neutrophil count (Figure 1), hinting toward compliance with the medications.
 |
Figure 1 Absolute neutrophil count of the patient since the diagnosis of Grave’s disease and initiation of antithyroid drugs. |
Additionally, her TRAB reduced from 16.5 to 6.9. She was discharged (on CBZ 20 mg TD and propranolol 160 mg SR OD) with a close follow-up plan.
At the follow-up visit after four weeks, she remained hyperthyroid (TSH: 0.01, T3: 11.1, T4: 43.9) with a normal heart rate. The patient was asked to bring empty bottles of her medicine to check her compliance, which strengthened the possibility of taking the medicines as prescribed. Malabsorption was ruled out with a negative celiac screen (negative anti-tissue transglutaminase antibodies) and normal cyanocobalamin and albumin levels. CBZ resistant GD was considered at this point and was shifted to PTU 100 mg TD with propranolol 160 mg SR OD and prednisolone 20 mg daily for two months with a tapering regimen. At the four-week follow-up, she had no improvement in her TFTs (TSH: <0.01, T3: 11.3, T4: 40.1). After another four-week follow-up, her steroids were stopped, and PTU was increased to 150 mg TD due to persistently deranged TFTs (TSH: <0.01, T3: 12, T4: 40.4). Patient had a partial response to the increased dose of PTU with repeat TFTs after one month showing TSH <0.01, T3: 7, T4: 25.5. The TFT trend of the patient from diagnosis to the last follow-up is graphed (Figure 2). Figure 2A shows the trend of T3 level, Figure 2B shows the trend of T4 level through the patient timeline.
Given only a partial response to increasing doses of PTU, patient was referred to surgical team to get definitive treatment via thyroidectomy, avoiding possible hepatotoxic and hematologic complications of long-term higher doses of PTU. The patient tolerated total thyroidectomy well without any complications. Her last thyroid profile was normal (TSH 3.42) on replacement levothyroxine 75 mcg daily.
Discussion
Thyrotoxicosis is a state of excess thyroid hormones in the body. This can be secondary to excess thyroid hormone production or release from the thyroid gland itself (most commonly secondary to GD) or exogenous/ectopic origin of thyroid hormone. Excess production or release of thyroid hormone from the thyroid gland itself is defined as hyperthyroidism.20 Antithyroid drugs (ATD) are usually the first-line treatment in patients with GD. Among the ATDs, CBZ (or methimazole) is the drug of choice for remission induction and maintenance of euthyroidism. Propylthiouracil (PTU) has limited indications due to its increased hepatotoxicity risk. It is mainly used in the first trimester of pregnancy (owing to the teratogenic potential of CBZ) and in thyroid storms.21 Resistance to CBZ is not an established indication to shift patients to PTU, and it is uncertain whether the patients will have similar resistance to PTU or will respond to it.
Definitive treatment of GD is achieved through radio-active iodine ablation (RAIA) or surgery. Situations where either surgery or RAIA can be opted include patients who do not respond to ATDs or have contraindications to ATDs, patients with thyrotoxic periodic paralysis, and those with inactive Grave’s ophthalmopathy.21 Situations where RAIA is preferred modality include females with GD who plan pregnancy in near future (more than 6 months ahead), patients who are at higher surgical risk should be managed via RAIA, and those who have a lack of access to a high volume thyroid surgery center.21
On the other hand, female patients who desire pregnancy in the next 6 months should undergo surgery to avoid fetal adverse effects of RAIA. Other situations where surgery is preferred include patients with pulmonary hypertension or heart failure, active Grave’s ophthalmopathy, large goiters with compressive symptoms, patients who previously had neck surgeries, co-occurrence of hyperparathyroidism requiring surgery and those with suspected or confirmed thyroid malignancy.21
ATD resistance is an uncommon yet potentially challenging situation. What causes ATD resistance is unclear due to limited data available. Proposed etiologies for drug resistance in patients with thyrotoxicosis include malabsorption, higher than-normal metabolism, antibodies to the drugs, and abnormalities in the intrathyroidal accumulation or action of the drugs.13 Figure 3 shows the proposed mechanisms of ATD-resistance in the thyroid gland.
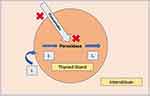 |
Figure 3 Mechanism of thyroid hormone production with mechanism of action of antithyroid drugs. Red Crosses denote the potential causes of resistance (inadequate uptake of drug, inability of action of the drug despite adequate uptake). Figure concept taken from Katzung and Trevor’s Pharmacology Examination and Board Review.22 |
The most integral part of its diagnosis is ruling out non-compliance and malabsorption, which can mimic the clinical presentation. Unlike hypothyroidism, where the levothyroxine absorption test plays a vital role in ruling out absorption and non-compliance, no such tests are validated in hyperthyroidism.23 There are no guidelines on measuring drug levels in the blood to identify absorption or adherence issues. In one case reported by Li et al in 1995, to elucidate the reasons for resistance to methimazole (MMI), the patient’s serum and intrathyroidal MMI concentrations were determined by high-pressure liquid chromatography (HPLC). The authors found similar concentrations, which ruled out compliance or absorption issues. After her serum containing MMI was incubated with Protein G, the MMI concentration of the fraction not bound to Protein G did not change significantly from that of untreated serum, ruling out the possibility of anti-MMI IgG antibody production. The intrathyroidal concentration of MMI was 3 microgram/gram of thyroid tissue, which is comparable to other reported tissue concentrations. However, given the fact that this patient was on a higher-than-normal dose (150 mg), her intrathyroidal MMI concentration was deemed relatively low, suggesting possible impairment of intrathyroidal MMI accumulation.4 Although it reliably diagnosed ATD resistance, limitations of this method include its interventional nature and cost and the fact that this can mainly be done post-surgical removal of the thyroid gland, where its role is mainly academic rather than practical. Medication non-compliance is a major hurdle in ideal patient care, especially in diseases where medications are to be taken for an extended duration in multiple daily dosing.24 Despite being a major obstacle in the treatment of many diseases, there is no consensus on how non-compliance can be effectively diagnosed.25 Methods that have been tried with variable pitfalls include manual pill counting, electronic pill counters, patient questionnaires, and patient self-reports, among others.26 Ruling out malabsorption syndromes in similar clinical scenarios is another essential aspect of correct diagnosis.27 A detailed history and relevant examination shall lead the treating physician to order tests that may help rule out malabsorption such as vitamins and albumin levels, celiac screening, and endoscopies in cases where suspicion is high. An accurate diagnosis is impractical because of the absence of practical objective methods of identifying ATD resistance and the presence of more common mimickers, such as non-adherence and absorption defects. It hence remains a diagnosis of careful exclusion of other possibilities.
Treatment of ATDR GD is controversial, mainly due to its rare occurrence. The limited available literature reports variable methodologies adopted. This encompasses a shift to PTU from CBZ use of other drugs in an attempt to control thyrotoxicoses such as steroids, lithium, and Lugol’s iodine. We reviewed 16 previously published articles where patients with ATD resistance were diagnosed and managed (Table 1).4–19
 |
Table 1 Previously Published Reports on Patients with Antithyroid Drug Resistant Thyrotoxicosis |
A total of 20 such cases were found in these reports, dating back to 1995.4 The mean age of patients with CBZ-resistant thyrotoxicosis was 33 ±12.5 years, among which 90% were females. Whether this female preponderance is specific to ATDR or just a result of the fact that GD itself is more common in females remains uncertain and would need exploration in larger cohorts. The influence of gender on drug resistance has been studied in infectious diseases, with conflicting results.28,29 The factors behind drug resistance in non-communicable diseases might differ; hence, reliable conclusions cannot be made as of now in the absence of extensive studies.
The most common etiology of thyrotoxicosis in ATDR patients was GD (60%). It also remains unclear whether ATDR is associated with GD more than other causes of thyrotoxicosis or an increased occurrence of GD itself (the most common etiology behind thyrotoxicosis). At least four cases also reported a co-resistance to PTU; hence, TFTs did not improve after a shift to PTU.7,9,15,16 PTU was tried in five more patients where it did not show resistance.4,7,11,13 These results show that although shifting to PTU in CBZ resistance is not a guideline-based indication, patients might respond to it, eliminating or prolonging the need for definitive management. This can be particularly helpful in patients with contraindications to definitive management or who refuse RAIA or surgery. However, a careful case-by-case decision has to be made with a detailed education of the patient regarding the adverse effects of PTU. Out of 20 patients, 18 patients required definitive management. Five patients underwent RAIA, whereas 13 underwent thyroidectomy. Other treatments tried included steroids in 11 patients, Lugol’s iodine in 3 patients, and lithium and intravenous thiamazole in 1 patient each. One case published in 2018 reported the successful use of a herbal alternative (Anemarrhena Bunge) in a patient with CBZ-resistant GD.14 The use of herbal products in GD has a weak scientific basis and highlights the lack of clear guidelines in managing ATDR Grave’s thyrotoxicosis.30 A predominant use of steroids in ATDR GD takes its basis from prior studies documenting the efficacy of steroids in thyrotoxicosis secondary to GD via anti-inflammatory effects on the thyroid gland as well as the decreased peripheral conversion of T4 to T3.30 Our patient, however, did not respond to steroids. The dose and duration of steroid use in ATDR GD are other areas of research that can help strengthen guidelines for the safe use of medicine in ATDR patients. Nevertheless, the limited literature shows that despite various treatments being used with variable efficacy, definitive treatment is required in most cases, and the safest and probably the most efficacious approach entails attempt to achieve euthyroidism and simultaneous arrangements of RAIA or surgery.
Conclusion
Antithyroid drug-resistant Grave’s disease is uncommon, though it exists and has been reported multiple times. It remains a diagnostic and therapeutic challenge, with accurate methods of diagnosis and the safest management approach yet to be established. Optimal management should focus on carefully excluding other differential diagnoses and shared decision-making in the best treatment strategy. Most patients may require definitive therapy; hence, arrangements should be made timely with simultaneous attempts to reduce the thyrotoxic state, which otherwise poses a continued threat to patients’ life with potentially serious complications.
Abbreviations
ATDR, Antithyroid drug resistant; GD, Grave’s disease; ED, Emergency department; HR, Heart rate; CBZ, Carbimazole; PTU, Propylthiouracil; TFTs, Thyroid function tests; SR, Sustained release; BPM, Beats per minute; TSH, Thyroid stimulating hormone; TRAB, TSH receptor antibody; OD, Once daily; TD, Thrice daily; BD, Twice daily; ST, Sinus tachycardia; HPLC, High-pressure liquid chromatography; RAIA, Radio-active iodine ablation.
Ethics Approval and Consent to Participate
This work is original, has not been, and is not under consideration for publication in any other Journal. The study was conducted in full compliance with the principles of the “Declaration of Helsinki”, Good Clinical Practice (GCP) and other relevant guidelines. The Case report was approved by the ethics committee of Medical Research Center (MRC) Qatar. Written informed consent was taken from the patient prior to conducting this work.
Consent to Publication
Written informed consent was taken from the patient prior to publishing this work.
Acknowledgments
The publication of this article was funded by the Qatar National Library.
Author Contributions
Fateen Ata conceptualized and supervised the study. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was not funded. However, Article processing charges are covered by Qatar National Library.
Disclosure
None of the authors has any conflict of interest in the publication of this manuscript.
References
1. Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. P T. 2015;40(4):277–283.
2. Ata F, Khan AA, Yousaf Z, et al. The clinical characteristics and outcomes of patients with pulmonary hypertension in association with hyperthyroid state: a systematic review. Medicine. 2022;101(26):e29832. doi:10.1097/MD.0000000000029832
3. Klein I, Danzi S. Thyroid disease and the heart. Circulation. 2007;116(15):1725–1735. doi:10.1161/CIRCULATIONAHA.106.678326
4. Li H, Okuda J, Akamizu T, Mori T. A hyperthyroid patient with Graves’ disease who was strongly resistant to methimazole: investigation on possible mechanisms of the resistance. Endocr J. 1995;42(5):697–704. doi:10.1507/endocrj.42.697
5. Jude EB, Dale J, Kumar S, Dodson PM. Treatment of thyrotoxicosis resistant to carbimazole with corticosteroids. Postgrad Med J. 1996;72(850):489–491.
6. Corvilain B, Schinohoritis P. Carbimazole--resistant thyrotoxicosis. Postgrad Med J. 1997;73(864):686. doi:10.1136/pgmj.73.864.686
7. Lee S, Kapoor D, Thomas W, Jones T. Thionamide resistant thyrotoxicosis - three illustrative cases. In:
8. Sebastián-Ochoa A, Quesada-Charneco M, Fernández-García D, Reyes-García R, Rozas-Moreno P, Escobar-Jiménez F. Dramatic response to cholestyramine in a patient with Graves’ disease resistant to conventional therapy. Thyroid. 2008;18(10):1115–1117. doi:10.1089/thy.2008.0094
9. Saleem T, Sheikh A, Masood Q. Resistant thyrotoxicosis in a patient with Graves disease: a case report. J Thyroid Res. 2011;2011(649084):1–4. doi:10.4061/2011/649084
10. Yang Y, Hwang S, Kim M, et al. Refractory Graves’ disease successfully cured by adjunctive cholestyramine and subsequent total thyroidectomy. Endocrinol Metab. 2015;30(4):620–625. doi:10.3803/EnM.2015.30.4.620
11. Chae SB, Kim ES, Lee YI, Min BR. A case of methimazole-resistant severe Graves’ disease: dramatic response to cholestyramine. IJT. 2016;9(2):190–194.
12. Nagi D, Holems S, Jenkins R. Thyrotoxicosis resistant to treatment: graves’ disease or Factitious thyrotoxicosis: a puzzle. In:
13. Ramtahal R, Dhanoo A. A case of Graves’ disease resistant to carbimazole. West Indian Med J. 2016;2016:266.
14. Kim J, Kim TH. A methimazole resistant patient with Graves’ disease (GD): a case report of mid-term management with herbal decoctions mainly composed of Anemarrhena Bunge. Complement Ther Med. 2018;39:109–113. doi:10.1016/j.ctim.2018.05.015
15. Linardi A, Michou E, Ilias I, et al. Resistant thyrotoxicosis due to Graves’ disease in pregnancy: case report and review of the literature. Cureus. 2018;10(8):e3232. doi:10.7759/cureus.3232
16. Diack N, Ndiaye N, Sene M, et al. Resistance to anti-thyroid drugs in Graves’ disease: clinical-biological characteristics and alternative therapy in tropical area. Open J Endocr Metab Dis. 2020;10(11):147–153. doi:10.4236/ojemd.2020.1011014
17. Lewandowski K, Dabrowska K, Gluchowska M, Lewinski A. Pulse methylprednisolone as preparation for thyroidectomy for drug-resistant amiodarone-induced thyrotoxicosis. In:
18. Mori Y, Hiromura M, Terasaki M, et al. Very rare case of Graves’ disease with resistance to methimazole: a case report and literature review. J Int Med Res. 2021;49(3):300060521996192. doi:10.1177/0300060521996192
19. Kamrul-Hasan AB, Mondal E. Carbimazole-resistant Graves’ disease responding to oral prednisolone: a case report. Mymensingh Med J. 2022;31(2):553–555.
20. De Leo S, Lee SY, Braverman LE. Hyperthyroidism. Lancet. 2016;388(10047):906–918. doi:10.1016/S0140-6736(16)00278-6
21. Ross DS, Burch HB, Cooper DS. 2016 American thyroid association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016;26(10):1343–1421. doi:10.1089/thy.2016.0229
22. Trevor A, Katzung B, Masters S. Katzung & Trevor’s Pharmacology Examination and Board Review.
23. Balla M, Jhingan RM, Rubin DJ. Rapid levothyroxine absorption testing: a case series of nonadherent patients. Int J Endocrinol Metab. 2015;13(4):e31051.
24. Addo B, Sencherey S, Babayara MNK. Medication noncompliance among patients with chronic diseases attending a primary health facility in a Periurban District in Ghana. Int J Chronic Dis. 2018;2018:7187284. doi:10.1155/2018/7187284
25. Ye S, Krupka DJ, Davidson KW. Diagnosing medication non-adherence in a patient with myocardial infarction. Front Psychol. 2012;3:267. doi:10.3389/fpsyg.2012.00267
26. Jimmy B, Jose J. Patient medication adherence: measures in daily practice. Oman Med J. 2011;26(3):155–159. doi:10.5001/omj.2011.38
27. Butov DO. Malabsorption syndromes in patients with tuberculosis as a cause of ineffective treatment: how to diagnose and overcome? Infusion Chemother. 2020;3.2:24–25.
28. McQuaid CF, Horton KC, Dean AS, Knight GM, White RG. The risk of multidrug- or rifampicin-resistance in males versus females with tuberculosis. Eur Respir J. 2020;56(3):2000626. doi:10.1183/13993003.00626-2020
29. Al Hamdan AS, Alghamdi AA, Alyousif GF, et al. Evaluating the prevalence and the risk factors of gram-negative multi-drug resistant bacteria in Eastern Saudi Arabia. Infect Drug Resist. 2022;15:475–490. doi:10.2147/IDR.S350048
30. He Q, Dong H, Gong M, et al. New therapeutic horizon of Graves’ hyperthyroidism: treatment regimens based on immunology and ingredients from traditional Chinese medicine. Front Pharmacol. 2022;13:1.
 © 2023 The Author(s). This work is published by Dove Medical Press Limited, and licensed under a Creative Commons Attribution License.
The full terms of the License are available at http://creativecommons.org/licenses/by/4.0/.
The license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
© 2023 The Author(s). This work is published by Dove Medical Press Limited, and licensed under a Creative Commons Attribution License.
The full terms of the License are available at http://creativecommons.org/licenses/by/4.0/.
The license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

