Back to Journals » Clinical Interventions in Aging » Volume 18
Carbapenem-Resistant Klebsiella pneumoniae Infection and Its Risk Factors in Older Adult Patients
Authors Çölkesen F, Tarakçı A, Eroğlu E, Kacar F, Özdemir Armağan Ş, Can S, Erol B, Aksay O, Çölkesen F
Received 28 January 2023
Accepted for publication 8 May 2023
Published 6 July 2023 Volume 2023:18 Pages 1037—1045
DOI https://doi.org/10.2147/CIA.S406214
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Maddalena Illario
Fatma Çölkesen,1 Arzu Tarakçı,2 Esma Eroğlu,3 Fatma Kacar,4 Şule Özdemir Armağan,2 Selver Can,5 Bağdat Erol,6 Oyagül Aksay,6 Fatih Çölkesen7
1Department of Infectious Diseases and Clinical Microbiology, Konya Numune Hospital, Konya, Turkey; 2Department of Infectious Diseases and Clinical Microbiology, University of Health Sciences, Konya City Hospital, Konya, Turkey; 3Department of Infectious Diseases and Clinical Microbiology, Meram State Hospital, Konya, Turkey; 4Department of Infectious Diseases and Clinical Microbiology, Medova Hospital, Konya, Turkey; 5Department of Infectious Diseases and Clinical Microbiology, University of Health Sciences, Beyhekim Training and Research Hospital, Konya, Turkey; 6Infection Control Committee, University of Health Sciences, Konya City Hospital, Konya, Turkey; 7Division of Clinical Immunology and Allergy, Department of Internal Medicine, Necmettin Erbakan University Meram Faculty of Medicine, Konya, Turkey
Correspondence: Fatih Çölkesen, Division of Clinical Immunology and Allergy, Department of Internal Medicine, Necmettin Erbakan University Meram Faculty of Medicine, Konya, Turkey, Tel +903322354500, Fax +903322356786, Email [email protected]
Introduction: Carbapenem-resistant Klebsiella pneumoniae (CRKP) infection has recently gained worldwide interest due to limited treatment options and high morbidity and mortality rates. The aim of this study was to determine the risk factors of carbapenem-resistant K. pneumoniae (CRKP) infection in older adult patients.
Material and Methods: This retrospective, single-center study included 132 patients with healthcare-associated CRKP infection (case group) and 150 patients with healthcare-associated carbapenem-susceptible K. pneumoniae (CSKP) infection (control group), aged > 65 years.
Results: In the CRKP and CSKP groups, 79 (59.8%) and 80 (53.3%) patients were males, and the mean ages were 77.8 ± 7.8 and 76.6 ± 7.7 years, respectively. Diabetes mellitus (DM), malignancy, cardiovascular diseases (CVDs), surgical intervention, invasive mechanical ventilation, central venous catheter insertion, parenteral nutrition, hospitalization in the previous 6 months, antibiotic use in the previous 3 months, and exposure to cephalosporins, fluoroquinolones, and carbapenems were significantly more common in the CRKP than the CSKP group (all p < 0.05). The multivariate logistic regression analysis identified malignancy, CVDs, DM, invasive mechanical ventilation, hospitalization in the previous 6 months, ICU admission, and exposure to cephalosporins, quinolones, and carbapenems as independent risk factors for CRKP infection in older adult patients.
Conclusion: DM, malignancy, CVDs, ICU admission, invasive mechanical ventilation, and exposure to ceftriaxone, fluoroquinolones, and carbapenems were independent risk factors for CRKP infection in older adult patients. The identification of risk factors for CRKP infection can help to prevent and treat CRKP infection.
Keywords: older adult patients, infection, carbapenem, Klebsiella pneumoniae, resistance
Introduction
Carbapenem-resistant Gram-negative bacteria, mainly Klebsiella pneumoniae, are an emerging cause of healthcare-associated infections (HAI) that pose a important threat to public health.1,2 The percentage of K. pneumoniae infections resistant to carbapenems continues to rise, with proportions exceeding 50% in parts of the Europe and Eastern Mediterranean.3–5 CRKP infection is difficult to treat, as carbapenems are generally considered antibiotics of last resort for serious K. pneumoniae infections. Genes that cause carbapenem resistance are present in K. pneumoniae, rendering nearly all available treatment options ineffective. Mortality rates reach 33–50% in patients infected with CRKP in different parts of the world, which is significantly higher than the death rate caused by CSKP infection.2–4 Therefore, prevention of CRKP infection is important not only to avoid poor prognosis and even death, but also to prevent widespread transmission of carbapenem resistance via transferable genetic factors.6,7
The most common risk factors for CRKP are hospitalization and broad-spectrum antibiotic use, especially carbapenem. Identification of the risk factors for CRKP and isolation of patients with these risk factors are essential for preventing the spread of CRKP. Furthermore, the identification of CRKP carriers with active surveillance cultures can be used to control CRKP spread.8 The antimicrobial resistance patterns can guide empirical antibiotic treatment to prevent inappropriate antibiotic use and decrease the development of resistance.
Advancements in the medical sciences (eg, organ transplant and cancer and stem cell treatments) have led to an increase in life expectancy. Older patients are prone to infections due to the effects of environmental factors and chronic changes to the genetic structure. The classical signs and symptoms of infection are absent or indistinct in older adults, which leads to delayed diagnosis and frequent and inappropriate antibiotic use. Inappropriate antibiotic use leads to resistance of pathogens in older patients, which causes prolonged hospitalization and increased mortality rates and treatment costs.9
There have been many studies investigating CRKP risk factors. Some of these have compared CRKP infections with CSKP infections, while some studies have compared patients without CRKP infections. There are studies investigating the risk factors for CRKP infection in special patient groups such as neonatal intensive care units, COVID-19 intensive care units, hematological malignancies, transplant patients, and pediatric patients.10–15 However, despite all these increasing numbers of studies, no study is investigating the risk factors for CRKP infection in the elderly population. This article is the first study to investigate risk factors for CRKP infection in the elderly population. The aim of this study was to examine CRKP HAI rates and risk factors in older patients admitted to the Konya Training and Research Hospital, Turkey.
Materials and Methods
Study Design
This retrospective case-control study was conducted at Konya Training and Research Hospital, a large tertiary hospital. The study was approved by the Local Ethics Committee of Konya Training and Research Hospital of University of Health Sciences (no: 08/01/2019/28-20) and conducted in accordance with the Declaration of Helsinki (1964). A total of 462 patients were enrolled based on the inclusion criteria and 180 were excluded for reasons shown in Figure 1. Consequently, 282 patients with confirmed K. pneumoniae infection were finally enrolled in the study.
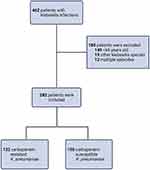 |
Figure 1 Flow chart of screening of patients. Inclusion and exclusion criteria were strictly applied during screening. |
Patients
We included patients with HAI who were aged > 65 years and hospitalized in the ICU or medical or surgical clinics between January 1, 2015, and September 30, 2019, and followed up for at least 48 h after CRKP culture growth. Patients with multiple CRKP infections were only included once.
Control Group
The control group included patients with HAI who were aged > 65 years and hospitalized for at least 48 h after CSKP culture growth.
Microbiological Examination
The isolated microorganisms were identified using conventional methods and the VITEK2 Compact® (bioMérieux, Marcy l’Etoile, France) automated system. Antibiotic susceptibilities were tested using the disc diffusion method based on the 2017 Clinical and Laboratory Standards Institute criteria.16 Strains with carbapenem (imipenem and meropenem) resistance on the disc diffusion test and reduced sensitivity were included in the study.
Data Collection
The infection control committee carried out daily surveillance visits of the hospital. Data from the National Hospital Infections Surveillance and Control Unit and microbiology laboratory were used to identify the cases. In accordance with the Centers for Disease Control and Prevention guidelines,17 the National Healthcare Safety Network criteria were used to diagnose region-specific infections in the case and control groups. Age, gender, clinical or ICU follow-up data, hospitalization and infection-related diagnoses, causative agent and antibiotic susceptibility thereof, and risk factors for infection were recorded. The risk factors for infection included DM, CVDs, chronic renal failure (CRF), chronic pulmonary disease, malignancy, neurological disease, history of ICU admission or hospitalization in the previous 6 months, antibiotic use in the previous 3 months, antibiotics used before the infection, presence of a central venous or urinary catheter, hemodialysis, parenteral nutrition, and surgical or invasive intervention. Tracheostomy, percutaneous endoscopic gastrostomy, and invasive mechanical ventilation were considered invasive interventions. Antibiotic use during the pre-infection period was defined as the use of antibiotics for at least 3 days during the current hospitalization.
Statistical Analysis
Data were analyzed using SPSS software (version 22.0; IBM Corp., Armonk, NY, USA). Continuous and categorical variables are presented as mean ± standard deviation (SD) and number (n) with percentage (%), respectively. Independent Samples t-test and chi-square test were used to evaluate continuous and categorical data, respectively. Univariate and multivariate logistic regression analyses were used to identify risk factors for CRKP. Variables with p-value < 0.1 in the univariate analysis were included in the forward, stepwise multivariate logistic regression analysis. p < 0.05 was considered statistically significant.
Results
General Characteristics of Patients
The study included 132 and 150 patients with CRKP and CSKP, respectively. In total, 79 (59.8%) and 80 (53.3%) participants were males, and the mean ages were 77.8 ± 7.8 and 76.6 ± 7.7 years, in the case and control groups, respectively. The durations of hospital stay before infection were 43.2 and 39.2 days in the case and control groups, respectively. In the CRKP group, 75.7% (n = 100), 16.7% (n = 22), and 7.6% (n = 10) patients were admitted to the ICU, medical clinics, and surgical clinics, respectively. In the CSKP group, 80% (n = 120), 10% (n = 15), and 10% (n = 15) of patients were admitted to the ICU, medical clinics, and surgical clinics, respectively. The age, gender, and mean duration of hospital stay before infection were not significantly different between the case and control groups (p = 0.288, p = 0.362, and p = 0.412, respectively). No significant difference was observed in the proportion of patients with CRF, chronic lung disease, or neurological disease between the groups. DM (p = 0.019), malignancy (p < 0.001), CVD (p = 0.016), surgical intervention (p < 0.001), invasive mechanical ventilation (p = 0.034), central venous catheter insertion (p = 0.036), parenteral nutrition (p = 0.024), history of hospitalization in the previous 6 months (p = 0.021), ICU stay (p = 0.001), history of antibiotic use in the previous 3 months (p = 0.025), and exposure to cephalosporins, fluoroquinolones, and carbapenems (p < 0.001 for each) were significantly more common in the CRKP group than the CSKP group. The demographic and clinical characteristics of patients are summarized in Table 1.
 |
Table 1 Demographics and Clinical Characteristics of Patients in the CRKP and CSKP Groups |
Bacterial growth was mainly observed in the respiratory tract samples of both the CRKP and CSKP groups (Table 2).
 |
Table 2 Sources of Culture Samples |
Risk Factors for CRKP Infection
The results of the univariate and multivariate logistic regression analyses are shown in Table 3. In univariate regression analysis, malignancy, CVDs, DM, central venous catheter insertion, invasive mechanical ventilation, parenteral nutrition, surgical intervention, history of hospitalization in the previous 6 months, ICU admission, history of antibiotic use in the previous 3 months, and exposure to cephalosporins, quinolones, and carbapenems were risk factors for CRKP infection. Variables with p-value < 0.1 in the univariate analysis were included in the forward, stepwise multivariate logistic regression analysis. Multivariate logistic regression analysis identified malignancy (odds ratio [OR] = 2.547, 95% confidence interval [CI] = 1.345–7.620, p = 0.019), CVDs (OR = 1.977, 95% CI = 1.126–3.795, p = 0.032), DM (OR = 2.311, 95% CI = 1.279–5.088, p = 0.021), invasive mechanical ventilation (OR = 2.492, 95% CI = 1.114–4.768, p = 0.038), history of hospitalization in the previous 6 months (OR = 2.168, 95% CI = 1.213–3.977, p = 0.033), ICU admission (OR = 2.168, 95% CI = 1.213–3.977, p = 0.033), cephalosporin exposure (OR = 3.048, 95% CI = 1.412–9.126, p = 0.008), quinolone exposure (OR = 4.112, 95% CI = 1.588–14.746, p = 0.005), and carbapenem exposure (OR = 2.646, 95% CI = 1.204–8.312, p = 0.014) as risk factors for CRKP infection (Table 3).
 |
Table 3 Logistic Regression Analysis of Risk Factors for Carbapenem-Resistant K. pneumoniae Infection (n = 282) |
Discussion
In the CRKP group, DM, malignancy, CVDs, surgical intervention, invasive mechanical ventilation, central venous catheter insertion, parenteral nutrition, history of hospitalization in the previous 6 months, antibiotic use in the previous 3 months, and exposure to cephalosporins, fluoroquinolones, and carbapenems were significantly more common than in the CSKP group. In multivariate logistic regression analysis, malignancy, CVDs, DM, invasive mechanical ventilation, history of hospitalization in the previous 6 months, ICU admission, and exposure to cephalosporins, quinolones, and carbapenems were independent risk factors for CRKP infection in older patients.
Older adult patients are susceptible to infections due to age-related decline in immune function, age-related structural and functional changes in the organs, malnutrition, and comorbidities.18 Infection is the primary cause of death in one-third of individuals aged 65 years or older and contributes to the death of many others. Infection has a significant impact on morbidity in older adults, causing functional decline and exacerbating underlying diseases.19 A high degree of clinical suspicion is required to identify infections in older patients because they may not have fever and may present with atypical symptoms (eg, delirium).
The relationship between antibiotic exposure and resistance is well documented. In the present study, exposure to cephalosporins, fluoroquinolones, and carbapenems was a risk factor for CRKP infection. In previous studies, the use of cephalosporins, fluoroquinolones, antipseudomonal penicillin, and carbapenems was a risk factor for CRKP infection.20–23 The development and spread of antibiotic resistance are related to the antibiotic pressure applied to the microbial environment and exposure to different antibiotic concentrations. Antibiotic pressure leads to the selection of resistant strains, spontaneous genetic variation, and bacterial survival. The presence of more than one antibiotic in the bacterial domain creates pressure, which results in the selection of bacteria with multiple resistance mechanisms. Therefore, bacteria regulate the resistance mechanisms to survive in varying environmental conditions and increase the mutation rate during stressful situations.24 Fluoroquinolone exposure leads to selective pressure that causes excessive proliferation of insensitive strains or facilitates the activation of internal mechanisms that confer resistance to more than one antibiotic class.21 Long-term use of carbapenems disrupts the micro-ecological balance of human body, which inhibits or kills a large number of CSKP, thereby increasing CRKP growth. In addition, K. pneumoniae carbapenemase production leads to carbapenem resistance. Long-term use of carbapenems triggers the production of acquired K. pneumoniae carbapenemase, which hydrolyzes penicillin, cephalosporins, and carbapenems, thereby reducing their antimicrobial effects.25 Unnecessary antibiotics in the older population are most commonly advised for urinary tract infections (asymptomatic bacteriuria), acute gastroenteritis, and upper respiratory tract infections.26 Antimicrobial exposure in older patients can be reduced by avoiding antibiotics for asymptomatic bacteriuria and during end-of-life treatment, as well as prescribing antibiotics for the shortest effective duration.27,28
In the present study, DM, CVDs, and malignancy were independent risk factors for CRKP infection in the older patients. The incidence of these diseases increases with age. Previous studies have shown that DM, immunosuppression, solid tumors, and hematological malignancy increase the risk of CRKP infection.23,25,29,30 Patients with malignancy are at a significantly increased risk of CRKP infection due to immunosuppression and a high incidence of antibiotic use. In contrast to the current study, a previous study reported that malignancy was protective against CRKP infection, especially in patients hospitalized under isolation conditions.31
Diabetic patients are more prone to developing infections due to abnormal vascularity, autonomic function, cell-mediated immunity, and phagocytic function.32 Patients with CVDs may be at an increased risk of CRKP infection due to frequent hospitalizations and invasive interventions. Older patients with chronic diseases (eg, chronic obstructive pulmonary disease, DM, and heart failure) have immune dysfunction, which results in susceptibility to common infections.33
The average age and life expectancy of the population are increasing in many countries. Therefore, an increasing number of older patients are being admitted to the ICU. Previous studies suggest that age is a restrictive factor for ICU admission and determines treatment intensity.34 In the present study, a history of ICU admission and hospitalization in the previous 6 months were independent risk factors for CRKP infection. Many previous studies have also reported ICU admission to be a risk factor for CRKP infection.20,22,35 In patients admitted to the ICU, the risk of infection with resistant microorganisms increases because of comorbidities, critical condition, frequent use of antimicrobials, and invasive procedures. In addition, the empirical use of carbapenem in ICU patients increases the risk of CRKP.36
The current and previous studies have reported a relationship between invasive mechanical ventilation and CRKP.23 Disrupted mucosal barrier due to invasive procedures causes colonization and allows entry of microorganisms into the body, thereby increasing the risk of CRKP infection.37
The present study had several limitations. Molecular analysis for resistance was not performed; therefore, information regarding carbapenemase type was not available. In addition, CRKP colonization was not investigated. Because the study was conducted at a single hospital, the results have limited generalizability. Finally, the study was conducted retrospectively; therefore, disease severity indexes and mortality could not be assessed.
Conclusion
Due to the increasing life expectancy, advanced life support, and widespread use of invasive interventions, older patients are more frequently admitted to the hospital. This study investigated the risk factors for CRKP infection in older patients, and found that DM, malignancy, CVDs, ICU admission, invasive mechanical ventilation, and exposure to ceftriaxone, fluoroquinolones, and carbapenems were independent risk factors for CRKP infection. The identification of risk factors for CRKP infection can help to prevent and treat CRKP infection.
Data Sharing Statement
The data that support the findings are available from the corresponding author (Fatma Çölkesen) upon reasonable request.
Ethics Approval
The study was approved by the Local Ethics Committee of Konya Training and Research Hospital of University of Health Sciences (no: 08/01/2019/28-20) and conducted in accordance with the Declaration of Helsinki (2013). Patients’ demographic characteristics, medical history, and laboratory values were retrospectively collected from electronic medical records. The ethics committee does not require patient consent for data from the electronic registry system retrospectively. Data, photographs, etc. that may reveal the identity of the patients were not included in the article.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sector.
Disclosure
The authors declare that they do not have a conflict of interest.
References
1. Logan LK, Weinstein RA. The epidemiology of carbapenem-resistant Enterobacteriaceae: the impact and evolution of a global menace. J Infect Dis. 2017;215(supp–1):S28–S36. doi:10.1093/infdis/jiw282
2. Zhu WM, Yuan Z, Zhou HY. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection relative to two types of control patients: a systematic review and meta-analysis. Antimicrob Resist Infect Control. 2020;9(1):1–13.
3. World Health Organization. Antimicrobial resistance global report on surveillance. Available from: https://apps.who.int/iris/bitstream/handle/10665/112642/9789241564748_eng.pdf;jsessionid=7CD2D037F35036393D8BC456B03B1991?sequence=1.
4. Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Pathog Glob Health. 2015;109(7):309–318. doi:10.1179/2047773215Y.0000000030
5. European Centre for Disease Prevention and Control. Surveillance of antimicrobial resistance in Europe - annual report of the European Antimicrobial Resistance Surveillance Network (EARS-Net) 2017. Available from: https://ecdc.europa.eu/sites/portal/files/.
6. Gupta N, Limbago BM, Patel JB, et al. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin Infect Dis. 2011;53(1):60–67. doi:10.1093/cid/cir202
7. Yigit H, Queenan AM, Anderson GJ, et al. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother. 2001;45(4):1151–1161. doi:10.1128/AAC.45.4.1151-1161.2001
8. French CE, Coope C, Conway L, et al. Control of carbapenemase-producing Enterobacteriaceae outbreaks in acute settings: an evidence review. J Hosp Infect. 2017;95(1):3–45. doi:10.1016/j.jhin.2016.10.006
9. Clinical and Laboratuary Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: 20th Informational Supplement Document M100-S20. Wayne PA: Clinical and Laboratory Standards Institute; 2010.
10. Zhang Y, Guo LY, Song WQ, et al. Risk factors for carbapenem-resistant K. pneumoniae bloodstream infection and predictors of mortality in Chinese paediatric patients. BMC Infect Dis. 2018;18(1):1–10. doi:10.1186/s12879-018-3160-3
11. Kang JS, Yi J, Ko MK, et al. Prevalence and risk factors of carbapenem-resistant enterobacteriaceae acquisition in an emergency intensive care unit in a tertiary hospital in Korea: a case-control study. J Korean Med Sci. 2019;34(18). doi:10.3346/jkms.2019.34.e140
12. Micozzi A, Gentile G, Minotti C, et al. Carbapenem-resistant Klebsiella pneumoniae in high-risk haematological patients: factors favouring spread, risk factors and outcome of carbapenem-resistant Klebsiella pneumoniae bacteremias. BMC Infect Dis. 2017;17(1):1–12. doi:10.1186/s12879-017-2297-9
13. Wu D, Cai J, Liu J. Risk factors for the acquisition of nosocomial infection with carbapenem-resistant Klebsiella pneumoniae. South Med J. 2011;104(2):106–110. doi:10.1097/SMJ.0b013e318206063d
14. Zhang F, Zhong J, Ding H, et al. Analysis of risk factors for carbapenem-resistant Klebsiella pneumoniae infection and its effect on the outcome of early infection after kidney transplantation. Front Cell Infect Microbiol. 2021;11:989. doi:10.3389/fcimb.2021.726282
15. Mędrzycka-Dąbrowska W, Lange S, Zorena K, et al. Carbapenem-resistant Klebsiella pneumoniae infections in ICU COVID-19 patients—a scoping review. J Clin Med. 2021;10(10):2067. doi:10.3390/jcm10102067
16. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; 27th Informational Supplement. M100-S27. Wayne, PA: Clinical and Laboratory Standards Institute; 2017.
17. Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6(1):149–163. doi:10.2217/fon.09.136
18. Mody L, Riddell J, Kaye K, et al. Common infections In: Williams BA, Chang A, Ahalt C, et al. editors. Current Diagnosis&Treatment: Geriatrics.
19. Candevir Ulu A, Kurtaran B, Inal AS, et al. Risk factors of carbapenem-resistant Klebsiella pneumoniae infection: a seriousthreat in ICUs. Med Sci Monit. 2015;21:219–224. doi:10.12659/MSM.892516
20. Falagas ME, Rafailidis PI, Kofteridis D, et al. Klebsiella pneumoniae Risk factors of carbapenem-resistant infections: a matched case control study. J Antimicrob Chemother. 2007;60(5):1124–1130. doi:10.1093/jac/dkm356
21. Gómez Rueda V, Zuleta Tobón JJ. Klebsiella pneumoniae Risk factors for infection with carbapenem-resistant: a case–case–control study. Colomb Med. 2014;45:54–60. doi:10.25100/cm.v45i2.1417
22. Mills JP, Talati NJ, Alby K, et al. The epidemiology of carbapenem-resistant Klebsiella pneumoniae colonization and infection among long-term acute care hospital residents. Infect Control Hosp Epidemiol. 2016;37(1):55–60. doi:10.1017/ice.2015.254
23. Orsi G, Bencardino A, Vena A, et al. Patient risk factors for outer membrane permeability and KPC-producing carbapenem-resistant Klebsiella pneumoniae isolation: results of a double case–control study. Infection. 2013;41(1):61–67. doi:10.1007/s15010-012-0354-2
24. Huang PH, Cheng YH, Chen WY, et al. Risk factors and mechanisms of in vivo emergence of colistin resistance in carbapenem-resistant Klebsiella pneumoniae. Int J Antimicrob Agents. 2021;57(6):106342. doi:10.1016/j.ijantimicag.2021.106342
25. Li J, Li Y, Song N, et al. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection: a meta-analysis. J Glob Antimicrob Resist. 2020;21:306–313. doi:10.1016/j.jgar.2019.09.006
26. Peron EP, Hirsch AA, Jury LA, et al. Another setting for stewardship: high rate of unnecessary antimicrobial use in a veterans affairs long-term care facility. J Am Geriatr Soc. 2013;61(2):289. doi:10.1111/jgs.12099
27. Crnich CJ, Jump R, Trautner B, et al. Optimizing antibiotic stewardship in nursing homes: a narrative review and recommendations for improvement. Drugs Aging. 2015;32(9):699. doi:10.1007/s40266-015-0292-7
28. Borer A, Saidel-Odes L, Eskira S, et al. Klebsiella pneumoniae risk factors for developing clinical infection with carbapenem-resistant in hospital patients initially only colonized with carbapenem-resistant K pneumoniae. Am J Infect Control. 2012;40(5):421–425. doi:10.1016/j.ajic.2011.05.022
29. Zhang Y, Guo LY, Song WQ, et al. Pneumoniae risk factors for carbapenem-resistant bloodstream infection and predictors of mortality in Chinese paediatric patients. BMC Infect Dis. 2018;18(1):248.
30. Schwaber MJ, Klarfeld-Lidji S, Navon-Venezia S, et al. Predictors of carbapenem-resistant Klebsiella pneumoniae acquisition among hospitalized adults and effect of acquisition on mortality. Antimicrob Agents Chemother. 2008;52(3):1028–1033. doi:10.1128/AAC.01020-07
31. Singh SK, Sridhar GR. Infections and diabetes. Int J Diabetes Dev Ctries. 2015;35(2):59–62. doi:10.1007/s13410-015-0417-x
32. Castle SC, Uyemura K, Fulop T, et al. Host resistance and immune responses in advanced age. Clin Geriatr Med. 2007;23(3):463. doi:10.1016/j.cger.2007.03.005
33. Kofteridis DP, Valachis A, Dimopoulou D, et al. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection/colonization: a case–case-control study. J Infect Chemother. 2014;20(5):293–297. doi:10.1016/j.jiac.2013.11.007
34. Tian L, Tan R, Chen Y, et al. Klebsiella pneumoniae Epidemiology of bloodstream infections in a teaching hospital: factors related to the carbapenem resistance and patient mortality. Antimicrob Resist Infect Control. 2016;5(1):48. doi:10.1186/s13756-016-0145-0
35. Correa L, Martino MD, Siqueira I, et al. A hospital-based matched case–control study to identify clinical outcome andrisk factors associated with carbapenem-resistant Klebsiella pneumoniae infection. BMC Infect Dis. 2013;13(1):80. doi:10.1186/1471-2334-13-80
36. da Silva KE, Maciel WG, Sacchi FPC, et al. Risk factors for KPC-producing Klebsiella pneumoniae: watch out for surgery. J Med Microbiol. 2016;65(65):547–553. doi:10.1099/jmm.0.000254
37. Girmenia C, Rossolini GM, Piciocchi A, et al. Infections by carbapenem-resistant Klebsiella pneumoniae in SCT recipients: a nationwide retrospective survey from Italy. Bone Marrow Translant. 2015;50(2):282–288. doi:10.1038/bmt.2014.231
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
