Back to Journals » OncoTargets and Therapy » Volume 15
Capmatinib in MET Exon 14 Skipping Mutation-Positive Lung Adenocarcinoma with Extensive Central Nervous System Metastasis
Authors Kim TW , Lee KM, Lee SH
Received 29 July 2022
Accepted for publication 25 August 2022
Published 31 August 2022 Volume 2022:15 Pages 941—946
DOI https://doi.org/10.2147/OTT.S382722
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Gaetano Romano
Tae Woo Kim,1 Kyung Mi Lee,2 Seung Hyeun Lee1
1Division of Pulmonary, Allergy and Critical Care Medicine, Department of Internal Medicine, Kyung Hee University College of Medicine, Kyung Hee University Hospital, Seoul, South Korea; 2Department of Radiology, Kyung Hee University College of Medicine, Kyung Hee University Hospital, Seoul, South Korea
Correspondence: Seung Hyeun Lee, Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Internal Medicine, Kyung Hee University College of Medicine, Kyung Hee University Hospital, Kyungheedae-ro 23, Dongdaemun-gu, Seoul, 02447, South Korea, Tel +82 2 958 8511, Fax +82 2 968 1848, Email [email protected]
Abstract: Several selective mesenchymal–epithelial transition (MET) inhibitors have recently demonstrated favorable systemic efficacy in MET exon 14 skipping mutation-positive non-small cell lung cancer. However, there are limited data on their efficacy against central nervous system (CNS) metastasis, especially leptomeningeal seeding. Recently, we encountered a case of a 65-year-old woman who was diagnosed with metastatic lung adenocarcinoma. As routine molecular testing showed no genomic alterations, including epidermal growth factor receptor mutation and anaplastic lymphoma kinase translocation, the patient received a frontline platinum-doublet followed by paclitaxel. However, the tumor did not respond to these therapies, and her condition became deleterious owing to extensive brain and leptomeningeal metastases. Plasma genotyping revealed that the tumor harbored a MET exon 14 skipping mutation, and we started capmatinib, a selective MET inhibitor. The CNS lesions markedly decreased and the performance status of the patient dramatically improved. Our report highlights the significant CNS activity of capmatinib, even in cases of leptomeningeal metastasis. In addition, this report emphasizes the importance of the active utilization of molecular profiling to detect rare but druggable genetic alterations for the better management of patients with lung cancer.
Keywords: lung cancer, capmatinib, MET exon 14 skipping mutation, leptomeningeal metastasis
Introduction
Mesenchymal–epithelial transition (MET) receptor tyrosine kinase, whose ligand is hepatocyte growth factor, activates several downstream signaling pathways involved in cell proliferation, differentiation, and migration.1 Genetic alterations of this receptor including amplification, fusion, and mutations can result in aberrant activation of the pathways in many cancers.2 MET exon 14 skipping mutations are one such alteration, and those are found in approximately 3% to 4% of patients with non-small cell lung cancer (NSCLC).3,4 The mutations were associated with poor prognosis and several multi-target kinase inhibitors such as crizotinib, and cabozantinib had shown only limited clinical efficacy.5–7 Capmatinib is a selective, highly potent MET inhibitor with activity in vitro and in mouse xenograft models of MET-activated cancer.8,9 The clinical efficacy of capmatinib has recently been proved in a multiple-cohort, Phase 2 study, and it was approved in several countries for the treatment of advanced NSCLC harboring MET exon 14 skipping mutations.10
Central nervous system (CNS) metastases, especially leptomeningeal metastases (LM), are critical complications of lung cancer, especially in patients with driver oncogenes.11 Certain newer-generation tyrosine kinase inhibitors (TKIs) targeting epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) translocation, such as osimertinib and alectinib, have shown efficacy in LM.12,13 However, the optimal treatment strategy for MET-positive NSCLC with CNS metastases is largely unknown. Here, we present a case of lung adenocarcinoma with extensive CNS metastasis and MET exon 14 skipping mutation, which responded dramatically to capmatinib.
Case Presentation
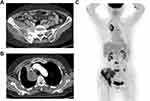
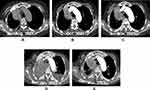
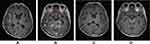
A 65-year-old female presented to our institution in March 2021 complaining of right leg pain and edema that had lasted for 2 weeks. She had never smoked and was not taking any medication. Computed tomography (CT) angiography of the lower extremities revealed diffuse deep vein thrombosis (DVT) in the right common iliac, external iliac, common femoral, popliteal, peroneal vein, and calf muscular branches. In addition, osteolytic and sclerotic lesions with extraosseous mass formation at the right iliac bone and ischium, suggestive of bone metastasis of malignancy, were noted (Figure 1A). As the chest x-ray incidentally showed a mass-like lesion in the right lung, a chest CT scan was performed. Scan results revealed a 6.2×4.3 cm mass in the right upper lobe abutting the right brachiocephalic vein (Figure 1B). A percutaneous needle biopsy was performed, and histopathology revealed moderately differentiated lung adenocarcinoma positive for thyroid transcription factor 1 (TTF-1). Brain magnetic resonance imaging (MRI) was negative for CNS metastasis, while positron emission tomography (PET) revealed a hypermetabolic mass in the right upper lobe with a maximum standard uptake value of 5.1, and multiple metastases at the lumbar spines (L3 and L5), left iliac bone, right sacrum, right iliac, and ischial bone, suggesting that the lung cancer was at stage IVB (Figure 1C). Routine mutational studies excluded several genetic alterations, including EGFR mutations, ALK fusions, and ROS proto-oncogene 1 fusions. The tumor proportion score for programmed death ligand 1 using the 22C3 pharmDx assay was 0%. Pemetrexed and cisplatin were initiated as first-line treatments while maintaining rivaroxaban for DVT. During treatment, the patient’s leg pain was not well controlled, requiring high-dose opioids, and Eastern Cooperative Oncology Group (ECOG) performance status (PS) remained at 3. Follow-up chest CT scan conducted after the fourth cycle of pemetrexed maintenance showed enlargement of the primary lung mass (Figure 2A). The carcinoembryonic antigen level was also elevated at 145.6 ng/mL, from 83.4 ng/mL at baseline. The treatment was stopped and the patient was switched to paclitaxel monotherapy. At the same time, next-generation sequencing (NGS) was performed using FoundationOne Liquid CDx, which revealed a MET exon 14 skipping mutation (c.2888–47_2888-24del24 in intron 13) with an allele fraction of 3% without other concurrent mutations. Although chest CT after two cycles of paclitaxel revealed partial response (Figure 2B), the follow-up scan showed progressive disease (Figure 2C). As no MET inhibitor was approved in South Korea at that time, the patient was admitted to the Managed Access Program for capmatinib by Novartis after obtaining signed informed consent, and at the same time switched to atezolizumab monotherapy to control the disease until the drug became available. After two cycles of atezolizumab, she visited the emergency department because of melena and a stuporous mentality. Her blood pressure was 85/60 mmHg and hemoglobin level was 6.5 g/dL. Digital rectal examination was positive, which suggested hypovolemic shock due to rivaroxaban-related gastrointestinal bleeding. Moreover, chest images showed total atelectasis of the right lung due to an increased right lung mass obstructing the right main bronchus (Figure 2D). Her mental status did not recover even after transfusion and blood pressure normalization. Brain MRI showed interval development of numerous and variable-sized masses and nodules in both the cerebral and cerebellar hemispheres and the spinal cord (Figure 3A), and enhancement along both the optic chiasm-optic tract, right trigeminal nerve, and both the seventh/eighth nerve complex, which were compatible with brain metastasis and LM (Figure 3B). On hospital day (HD) 4, capmatinib finally arrived and was immediately administered at 400 mg twice daily through a nasogastric tube. On HD 10, she could open her eyes without stimulation and became cooperative with verbal order. The mentality was fully recovered on HD 14, she could eat by herself, and ECOG PS recovered from 4 to 2. A follow-up chest CT scan taken 2 months after capmatinib was administered showed a markedly decreased right lung mass and improved atelectasis (Figure 2E). Surprisingly, extensive CNS metastases nearly completely disappeared on brain MRI (Figure 3C and D). As of May 2022, she maintained the response without any significant drug-related adverse events, except the Common Terminology Criteria for Adverse Events grade 1 peripheral edema.
Discussion
CNS metastasis is one of the challenging complications of NSCLC and is associated with poor prognosis despite aggressive local and systemic treatment.14–16 Tumors harboring driver oncogenes, such as EGFR mutations and ALK translocations, have shown to be associated with more frequent brain metastasis at the time of diagnosis as well as during the clinical course.17,18 LM is a rare but devastating type of CNS metastasis. A previous study reported that LM occurred in 3.8% of patients with NSCLC, mainly adenocarcinoma, with almost a third of them exhibiting concurrent brain metastases.11 Unfortunately, there is no clear consensus on the optimal management of LM. Although intrathecal therapy and radiotherapy combined with systemic treatment are recommended in the guidelines, their effect on the clinical outcome is modest.19 However, accumulating data support the potential benefits of molecular-targeted therapies for EGFR-mutated or ALK-rearranged NSCLC with LM.12,13
Recently, two selective MET inhibitors, campatinib and tepotinib, have been approved for the treatment of MET exon 14 skipping mutation-positive NSCLC.10,20 In the VISION phase 2 trial, tepotinib showed promising clinical efficacy, a response rate (RR) of 46% and a median duration of response (DoR) of 11.1 months.20 In addition, intracranial RR was 55%, with a median intracranial DoR of 9.5 months.20 In GEOMETRY mono-1 phase 2 trial, capmatinib showed RR of 41% in previously treated patients and 68% in those who had not received treatment previously.10 The intracranial RR was 54% including 4 patients with complete response.10 A recent real-world study on the early access program of capmatinib also reported intracranial RR of 46% and disease control rate of 91%, comparable with the previous phase 2 trial results.21 These data suggest the possible CNS activity of the novel MET inhibitors. A current international guideline recommends capmatinib for MET exon 14-positive NSCLC patients with brain metastasis.22 However, we need to further validate these results as previous studies were based on small subsets of the study population (11, 13, and 11 patients with evaluable brain lesions, respectively). Moreover, little is known about the efficacy of these drugs in LM. To the best of our knowledge, there are only two case reports that demonstrated the activity of the MET inhibitors on the CNS metastasis including LM in NSCLC.23,24 In one study, they reported a leptomeningeal response to capmatinib after progression on crizotinib and platinum-based doublet in MET-positive lung adenocarcinoma.23 Similar to our case, MRI taken 2 months after the capmatinib revealed a marked decrease of the parenchymal mass and leptomeningeal enhancement of the brain and spinal cords.23 The other reported a remarkable improvement of LM and poor PS after administration of tepotinib in a heavily treated, MET-positive patient.24 Patients in both cases received whole-brain radiotherapy (WBRT) before MET-directed treatment; however, it was not effective both clinically and radiologically, which is in accordance with previous study reporting limited value of WBRT in LM of NSCLC.25 The aforementioned clinical trials of MET-TKIs showed possible efficacy against brain metastases only in patients with favorable PS. At present, how reproducible CNS activity is in patients with poor PS is unclear. Since the landmark study by Inoue et al which demonstrated a clinical benefit of gefitinib in EGFR-positive patients with extremely poor PS, accumulating evidence consistently supports the validity of targeted therapies for the so-called “vulnerable” population, which includes the elderly and/or those with unfavorable PS.26–29 Taken together, existing data indicate that selective MET-TKIs alone can be beneficial even in MET-driven NSCLC patients with LM and poor PS. Treatment strategies for these patients need further research.
In summary, we describe a case of rapid radiological and clinical response to capmatinib in a patient with MET exon 14 mutation-positive lung adenocarcinoma and extensive CNS metastases. Although further evidence is needed, our report suggests that campatinib is a feasible option for MET-positive NSCLC patients with LM and a poor PS. In addition, our case emphasizes the importance of active molecular profiling, including blood genotyping, to select the optimal treatment strategy because clinically available treatment options are rapidly emerging for previously considered “challenging” targets.
Data Sharing Statement
All data generated or analyzed during this study are available in this manuscript.
Ethics Statements
This case report was approved by the Institutional Review Board of Kyung Hee University Hospital (KHUH 2022-06-010).
Consent for Publication
Written informed consent was obtained from the patient for publication of both the case report and accompanying images.
Acknowledgments
The authors thank the patient and her family for their permission to publish this case report.
Funding
This work was supported by grants from the Korea Institute of Oriental Medicine (grant number KSN2022240) of the Republic of Korea and the Basic Research Program through the National Research Foundation funded by the Ministry of Science and ICT (2019R1F1A1041812) of the Republic of Korea.
Disclosure
The authors have no conflicts of interest to declare in relation to this work.
References
1. Comoglio PM, Trusolino L, Boccaccio C. Known and novel roles of the MET oncogene in cancer: a coherent approach to targeted therapy. Nat Rev Cancer. 2018;18(6):341–358. doi:10.1038/s41568-018-0002-y
2. Onozato R, Kosaka T, Kuwano H, Sekido Y, Yatabe Y, Mitsudomi T. Activation of MET by gene amplification or by splice mutations deleting the juxtamembrane domain in primary resected lung cancers. J Thorac Oncol. 2009;4(1):5–11. doi:10.1097/JTO.0b013e3181913e0e
3. Awad MM, Oxnard GR, Jackman DM, et al. MET exon 14 mutations in non-small-cell lung cancer are associated with advanced age and stage-dependent MET genomic amplification and c-Met overexpression. J Clin Oncol. 2016;34(7):721–730. doi:10.1200/JCO.2015.63.4600
4. Frampton GM, Ali SM, Rosenzweig M, et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov. 2015;5(8):850–859. doi:10.1158/2159-8290.CD-15-0285
5. Tanizaki J, Okamoto I, Okamoto K, et al. MET tyrosine kinase inhibitor crizotinib (PF-02341066) shows differential antitumor effects in non-small cell lung cancer according to MET alterations. J Thorac Oncol. 2011;6(10):1624–1631. doi:10.1097/JTO.0b013e31822591e9
6. Yeung SF, Tong JHM, Law PPW, et al. Profiling of oncogenic driver events in lung adenocarcinoma revealed MET mutation as independent prognostic factor. J Thorac Oncol. 2015;10(9):1292–1300. doi:10.1097/JTO.0000000000000620
7. Yakes FM, Chen J, Tan J, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther. 2011;10(12):2298–2308. doi:10.1158/1535-7163.MCT-11-0264
8. Baltschukat S, Engstler BS, Huang A, et al. Capmatinib (INC280) is active against models of non-small cell lung cancer and other cancer types with defined mechanisms of MET activation. Clin Cancer Res. 2019;25(10):3164–3175. doi:10.1158/1078-0432.CCR-18-2814
9. Liu X, Wang Q, Yang G, et al. A novel kinase inhibitor, INCB28060, blocks c-MET-dependent signaling, neoplastic activities, and cross-talk with EGFR and HER-3. Clin Cancer Res. 2011;17(22):7127–7138. doi:10.1158/1078-0432.CCR-11-1157
10. Wolf J, Seto T, Han JY, et al. Capmatinib in MET exon 14-Mutated or MET-amplified non-small-cell lung cancer. N Engl J Med. 2020;383(10):944–957. doi:10.1056/NEJMoa2002787
11. Remon J, Le Rhun E, Besse B. Leptomeningeal carcinomatosis in non-small cell lung cancer patients: a continuing challenge in the personalized treatment era. Cancer Treat Rev. 2017;53:128–137. doi:10.1016/j.ctrv.2016.12.006
12. Flippot R, Biondani P, Auclin E, et al. Activity of EGFR tyrosine kinase inhibitors in NSCLC with refractory leptomeningeal metastases. J Thorac Oncol. 2019;14(8):1400–1407. doi:10.1016/j.jtho.2019.05.007
13. Gainor JF, Sherman CA, Willoughby K, et al. Alectinib salvages CNS relapses in ALK-positive lung cancer patients previously treated with crizotinib and ceritinib. J Thorac Oncol. 2015;10(2):232–236. doi:10.1097/JTO.0000000000000455
14. Gibson AJW, Li H, D’Silva A, et al. Impact of number versus location of metastases on survival in stage IV M1b non-small cell lung cancer. Med Oncol. 2018;35(9):117. doi:10.1007/s12032-018-1182-8
15. Sacks P, Rahman M. Epidemiology of brain metastases. Neurosurg Clin N Am. 2020;31(4):481–488. doi:10.1016/j.nec.2020.06.001
16. Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(4):419–425. doi:10.1200/JCO.2011.38.0527
17. Stanic K, Zwitter M, Hitij NT, Kern I, Sadikov A, Cufer T. Brain metastases in lung adenocarcinoma: impact of EGFR mutation status on incidence and survival. Radiol Oncol. 2014;48(2):173–183. doi:10.2478/raon-2014-0016
18. Lee JS, Hong JH, Sun S, et al. The impact of systemic treatment on brain metastasis in patients with non-small-cell lung cancer: a retrospective nationwide population-based cohort study. Sci Rep. 2019;9(1):18689. doi:10.1038/s41598-019-55150-6
19. National comprehensive cancer network guideline for NSCLC; 2022. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1450.
20. Paik PK, Felip E, Veillon R, et al. Tepotinib in non-small-cell lung cancer with MET exon 14 skipping mutations. N Engl J Med. 2020;383(10):931–943. doi:10.1056/NEJMoa2004407
21. Illini O, Fabikan H, Swalduz A, et al. Real-world experience with capmatinib in MET exon 14-mutated non-small cell lung cancer (RECAP): a retrospective analysis from an early access program. Ther Adv Med Oncol. 2022;14:17588359221103206. doi:10.1177/17588359221103206
22. National comprehensive cancer network guideline for central nervous system cancers; 2022. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1425.
23. Cravero P, Vaz N, Ricciuti B, et al. Leptomeningeal response to capmatinib after progression on crizotinib in a patient with MET Exon 14-Mutant NSCLC. JTO Clin Res Rep. 2020;1(4):100072. doi:10.1016/j.jtocrr.2020.100072
24. Ninomaru T, Okada H, Fujishima M, Irie K, Fukushima S, Hata A. Lazarus response to tepotinib for leptomeningeal metastases in a patient with MET Exon 14 skipping mutation-positive lung adenocarcinoma: case report. JTO Clin Res Rep. 2021;2(3):100145. doi:10.1016/j.jtocrr.2021.100145
25. Morris PG, Reiner AS, Szenberg OR, et al. Leptomeningeal metastasis from non-small cell lung cancer: survival and the impact of whole brain radiotherapy. J Thorac Oncol. 2012;7(2):382–385. doi:10.1097/JTO.0b013e3182398e4f
26. Inoue A, Kobayashi K, Usui K, et al. First-line gefitinib for patients with advanced non-small-cell lung cancer harboring epidermal growth factor receptor mutations without indication for chemotherapy. J Clin Oncol. 2009;27(9):1394–1400. doi:10.1200/JCO.2008.18.7658
27. Tsubata Y, Watanabe K, Saito R, et al. Osimertinib in poor performance status patients with T790M-positive advanced non-small-cell lung cancer after progression of first- and second-generation EGFR-TKI treatments (NEJ032B). Int J Clin Oncol. 2022;27(1):112–120. doi:10.1007/s10147-021-02043-2
28. Chang CY, Chen CY, Chang SC, Lai YC, Wei YF. Efficacy and prognosis of first-line EGFR-tyrosine kinase inhibitor treatment in older adults including poor performance status patients with EGFR-mutated non-small-cell lung cancer. Cancer Manag Res. 2021;13:7187–7201. doi:10.2147/CMAR.S322967
29. Crvenkova S. Alectinib treatment of ALK positive non small cell lung cancer patients with brain metastases: our clinical experience. Pril. 2020;41(2):29–36. doi:10.2478/prilozi-2020-0030
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.