Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Bullous Grover’s Disease in a Chinese Tibetan Adolescent: A Case Report
Authors Wang Q , Luo N , Lei M, Chen X, Li C, Hao P
Received 3 May 2022
Accepted for publication 6 July 2022
Published 17 July 2022 Volume 2022:15 Pages 1371—1376
DOI https://doi.org/10.2147/CCID.S373228
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Qiuyue Wang,1,2,* Nana Luo,1,2,* Min Lei,1,2 Xian Chen,3 Chunxiao Li,2 Pingsheng Hao2
1School of Clinical Medicine, Chengdu University of Traditional Chinese Medicine, Chengdu, People’s Republic of China; 2Department of Dermatology, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, People’s Republic of China; 3Department of Pathology, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Chunxiao Li; Pingsheng Hao, Department of Dermatology, Hospital of Chengdu University of Traditional Chinese Medicine, No. 39 Shi-er-qiao Road, Chengdu 610075, Sichuan, People’s Republic of China, Tel +86-18981937829 ; +86-13881965024, Fax +86-028-87732407, Email [email protected]; [email protected]
Abstract: Grover’s disease (GD), also known as Transient acantholytic dermatosis, has no typical clinical rash features. It usually occurs in elderly white men but very rarely in China. This is a disease of acantholysis and dyskeratosis, which is usually considered to be spontaneous remission. The skin lesions of the disease are diverse, and the main symptom is severe itching. We have reported a case of GD in a 14-year-old Chinese Tibetan male whose clinical manifestations were pruritic red papule, generalized red papules, papulo vesicles and blisters ranging from millet rice to soybean size. Skin lesions change rapidly and variously. In order to confirm the diagnosis, we have done skin biopsies, immunofluorescence, dermoscopy, microscopy and other examinations. Pathological skin biopsy showed acantholysis. Intraepidermal blisters and the presence of blisters on the basal cells as well as under the stratum corneum can be observed on the same pathological section. Type IV collagen immunohistochemistry showed blisters in the epidermis. The diagnosis of GD depended on the exclusion of other diseases. After we performed whole exon sequencing (WES) on DNA from the patient’s blood, pathogenic gene mutations were not found. Pustular psoriasis, Subcorneal pustular dermatosis, Herpesvirus infections, Dermatitis herpetiformis, Pemphigus vulgaris, Norwegian scabies, Darier’s disease, and Hailey-Hailey disease were all excluded. We successfully treated adolescent GD with minocycline combined with methotrexate. The patient was followed up for 19 months without recurrence.
Keywords: Grover’s disease, Chinese, minocycline, methotrexate, adolescent, case report
Introduction
Grover’s disease (GD) is generally considered to be a self-limited, primary, non-familial, acantholytic skin disease, which mainly manifests as itching, dispersed, erythema papules or vesicular papules was first reported in 1970.1 The skin lesions of the disease are diverse, severe itching being the main symptom, with varying degrees of itching ranging from mild to severe. People with severe pruritus often have multiple disseminated lesions affecting the neck, shoulders, torso, arms, and legs.2 The lesions are characterized by erythematous or reddish-brown keratinizing papules on the anterior chest, upper back, and lower thorax, which are widely dispersed and have no tendency to concomitant, while the scalp, palms, as well as soles of the feet are usually unaffected.3 GD has been reported to present with acne-like, vesicular, pustular, and rare bullous lesions, as well as unusual distribution, including unilateral or herpes zoster rashes.4,5 Pigmentation may subside after inflammation, and there are no systemic symptoms associated with GD. The histopathological features of GD are four different histological patterns of acantholysis.6 Namely Darier-like, with focal acantholysis and dyskeratosis on the basal layer; pemphigus vulgaris, with discrete basal fissures There are a few acanthophyll cells on the upper part, and the overlying epidermis is basically intact; Hailey Hailey-like, in which there are a large number of acantholytic cells covering the fissures on the base; the last is spongy, with some spinous layers in the spongy lesion loosen cells, or adjacent to spongy lesions. Some cases are based on one pattern, but it is more common to find two or more patterns in a biopsy specimen.7 Topical corticosteroids are the most frequent treatment for GD, followed by systemic retinoids and corticosteroids, respectively.8 Oral methotrexate and minocycline therapy for adolescent with GD had not been reported.
Case Presentation
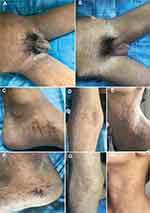
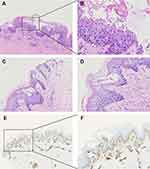
In August 2020, a 14-year-old Chinese Tibetan male was admitted to our department due to papules and pustules on the groin and scrotum accompanied by severe exudation. The patient’s chief complaint was generalized papules and pustules with exudate for 2 months. Initially, except for the arms, below the knees, and above the collarbone, the rest of the skin had densely or scattered red papules, some of which were pustules, incrustation, and pus-like exudate of the groin and scrotum (Figure 1A). Laboratory revealed that C-reactive protein (CRP) of 81.21mg/L, eosinophils of 1.20*109/L, percentage of eosinophils (EO%) of 13.5%, and Immunoglobulin E (IgE) of 201 IU/mL. Staphylococcus aureus was found in the secretions, so we considered that the patient had a skin infection. Physical examination and other laboratory results were normal. After one week of antibiotic treatment, CRP was 2.63 mg/L, eosinophils were 1.40*109/L, and EO% was 17.7%. Severe itching was the only symptom; after treatment for five days, the skin lesions quickly improved (Figure 1B). Thereafter the skin lesions changed rapidly. After that, red papules began to appear on both feet (Figure 1C). In a short time, skin lesions are all over the body, except the head, face, and soles of the feet (Figure 1D and E). Thus, we did a second biopsy (Figure 1F). The patient did not have any other underlying diseases, and there was not a similar medical record in his family. Pathological skin biopsy of right thigh showed acantholysis, as well as intraepidermal blisters and the presence of blisters on the basal cells as well as under the stratum corneum can be observed on the same pathological section (Figure 2A-D). The second histopathologic biopsy revealed only mild spongy edema, but no other abnormalities. In combination with the two histopathological results, we did not find viral inclusion bodies, subcorneal accumulation of neutrophils, and spongiosiform pustules of Kogoj, so we did not consider Herpesvirus infections, Subcorneal pustular dermatosis, and pustular psoriasis for diagnosis. Direct immunofluorescence was negative for Immuno-globulin A (IgA), IgM, IgG, and Complement 3, so Dermatitis herpetiformis and Pemphigus vulgaris were excluded. Type IV collagen immunohistochemistry suggested intra-epidermal blisters (Figure 2E and F). We did a dermoscopy of most of the patient’s lesions and rashes, as well as the crevices of the fingers, focusing on excluding Norwegian scabies; we did not find tunnels, and we did not see scabies and eggs under the microscope.
We performed whole exon sequencing (WES) on DNA from the patient’s blood, pathogenic gene mutations were not found. Also, we did not find mutations in the genes ATP2A2 and ATP2C1,9,10 completely ruling out Hailey-Hailey disease and Darier’s disease. Therefore, after excluding Pustular psoriasis, Subcorneal pustular dermatosis, Herpesvirus infections, Dermatitis herpetiformis, Pemphigus vulgaris, Norwegian scabies, Darier’s disease, and Hailey-Hailey disease, we finally diagnosed GD based on the histopathology and the clinical symptom of the patient.
For treatment, we initially used topical magnesium sulfate solution (7.5g magnesium sulfate and 500mL normal saline, once a day in the morning) and povidone-iodine solution (10mL 5% povidone-iodine solution and 100mL normal saline, once a day in the afternoon) for 6 days till the exudate disappeared. Local use of steroid ointment mixed with antibiotic ointment on dry skin lesions, oral antihistamines, and systemic intravenous antibiotics. Skin lesions change rapidly, and when we thought the skin lesions disappeared, a new wave of new skin lesions started on the feet. We did a second skin biopsy. At this time, the previous treatment had no effect. We chose to use minocycline combined with methotrexate based on the patient’s skin lesions and our experience. Oral minocycline hydrochloride (50 mg, twice a day) and methotrexate tablets (7.5 mg, once a week) were used. On the tenth day after use minocycline and methotrexate, the patient’s skin lesions subsided, improved, and was discharged (Figure 1D and G). There was no recurrence after 19 months of follow-up. Continued follow-up is significant.
Discussion
GD is easily misdiagnosed, and it is rarely reported in China. To our knowledge, this is the first reported case of GD in an adolescent successfully treated with minocycline and methotrexate.
The clinicopathological abnormalities of the 14-year-old boy include generalized red papules, papulo vesicles and blisters ranging from millet rice to soybean size. Skin lesions change rapidly, and scabs can be accompanied by exudation. Pathological skin biopsy revealed acantholysis, and the presence of blisters on the basal cells and under the stratum corneum can be simultaneously observed on a same pathological section. Type IV collagen immunohistochemistry suggested intra-epidermal blisters. In the first pathological biopsy, both Darier-like, Pemphigus Vulgaris-like, and Hailey Hailey-like patterns were observed. Although histologically similar, Hailey-Hailey disease and Darier’s disease were excluded in this case due to the absence of a history of Darier and Hailey-Hailey disease, absence of keratinoid verruca in the seborrheic area, hair follicle involvement, or abnormalities of nails and mucous membranes, and the absence of related gene ATP2A2 and ATP2C1 mutations in WES. These findings together supported a diagnosis of GD.
The aetiology and pathogenesis of GD are still unclear, which may be related to some physical and chemical factors,11–13 such as light and radiation damage, overheating, sweating, dry skin, ultraviolet radiation and Malassezia, and other infections. Recently, GD has also been reported in patients with COVID-19.14 The age of the patient we have reported is significantly lower than the average age of GD onset.2 Our patient lived in a high-altitude area for a long time and often took outdoor exercise. Ultraviolet exposure cannot be ruled out as the cause of the disease.
At the time of admission, the patient showed pustules, scabs and pus-like exudates in the groin and scrotum, and Staphylococcus aureus was detected in the skin lesions of the groin. This may be related to the relatively humid environment in the groin and perineum, which increases the colonization of microorganisms, and the massive proliferation of Staphylococcus aureus in the environment of local immune imbalance of the skin.15 It is well known that Staphylococcus aureus is the main pathogen causing skin and soft tissue infection.16 Staphylococcus aureus widely exists on the skin surface and upper respiratory tract, which can cause skin and soft tissue inflammation, osteoarthritis, pneumonia and other related infections.17 After one week of antibiotic treatment, the infection of this patient was significantly controlled, but then the patient’s condition deteriorated further. It is not clear whether infection is a predisposing factor or a direct cause. Skin lesions change rapidly, and then antibiotic treatment is ineffective. Combining the patient’s skin lesions and examination results, we used oral minocycline combined with methotrexate, and the skin lesions were controlled.
To our knowledge, there is no standardized treatment for GD. First-line therapy includes topical steroids and oral antihistamines.18 In more severe cases, systemic corticosteroids, retinoids, and phototherapy might be used.18 In the latest report, monoclonal antibody dupilumab was used to treat GD.19 It suggested the role of type2 helper T lymphocyte (TH2) cytokine signaling in GD as a possible manifestation of an aging immune system. In our case, after the initial failure of normal treatment, we chose to use minocycline combined with methotrexate. They may appear to provide an effective treatment option. Methotrexate is the most frequently used immunosuppressant in the treatment of autoimmune skin diseases, such as psoriasis.20 According to our clinical experience, we also often use methotrexate to treat complex and severe rashes. Minocycline is a tetracycline antibiotic. There is evidence that in addition to antibacterial effects, minocycline also has certain cytoprotective effects.21 The use of minocycline combined with methotrexate to treat autoimmune diseases or skin diseases has not been reported much. A recent study has shown good efficacy in the treatment of rheumatoid arthritis. Methotrexate and minocycline-loaded nanoparticles were developed to relieve inflammation and disease progression/joints stiffness and control the bacterial infections associated with rheumatoid arthritis.22 The pathogenesis of inflammatory skin diseases is complex and diverse. Whether the pathogenesis of GD may be related to immunity still needs a lot of data support and more in-depth research.
Conclusion
Adolescent GD is rarely reported, and generalized GD is also rare. This case raised awareness of GD among dermatologists. It provides an important reminder for dermatologists to include GD in the differential diagnosis of various skin diseases, to reduce the rate of misdiagnosis.
Consent Statement
Informed consent for publication of the case details and associated images was obtained from the patient and his parent were performed in accordance with the Helsinki Declaration. Institutional approval was not required to publish the case details.
Funding
This case report required no funding during its preparation. Hospital of Chengdu University of Traditional Chinese Medicine Scientific Research Capacity Enhancement “Hundred Talents Program”, (20B04) will support the study and the journal’s rapid service fee.
Disclosure
Qiuyue Wang and Nana Luo are co-first authors for this study. Chunxiao Li and Pingsheng Hao are co-correspondence authors for this study. The authors have no conflicts of interest to declare in this work.
References
1. Grover RW. Transient acantholytic dermatosis. Arch Dermatol. 1970;101(4):426–434. doi:10.1001/archderm.1970.04000040048010
2. Gantz M, Butler D, Goldberg M, Ryu J, McCalmont T, Shinkai K. Atypical features and systemic associations in extensive cases of Grover disease: a systematic review. J Am Acad Dermatol. 2017;77(5):952–957.e1. doi:10.1016/j.jaad.2017.06.041
3. Fantini F, Kovacs E, Scarabello A. Unilateral transient acantholytic dermatosis (Grover’s disease) along Blaschko lines. J Am Acad Dermatol. 2002;47(2):319–320. doi:10.1067/mjd.2002.120596
4. Liss WA, Norins AL. Zosteriform transient acantholytic dermatosis. J Am Acad Dermatol. 1993;29(5 Pt 1):797–798. doi:10.1016/s0190-9622(08
5. Brown-Joel ZO, Chung J, Stone MS. Pityriasis rubra pilaris-like eruption in the setting of transient acantholytic dermatosis. JAAD Case Rep. 2019;5(8):733–735. doi:10.1016/j.jdcr.2019.06.025
6. Fernández-Figueras MT, Puig L, Cannata P, et al. Grover disease: a reappraisal of histopathological diagnostic criteria in 120 cases. Am J Dermatopathol. 2010;32(6):541–549. doi:10.1097/DAD.0b013e3181c80cf9
7. Chalet M, Grover R, Ackerman AB. Transient acantholytic dermatosis: a reevaluation. Arch Dermatol. 1977;113(4):431–435. doi:10.1001/archderm.1977.01640040039004
8. Bellinato F, Maurelli M, Gisondi P, Girolomoni G. Clinical features and treatments of transient acantholytic dermatosis (Grover’s disease): a systematic review. J Dtsch Dermatol Ges. 2020;18(8):826–833. doi:10.1111/ddg.14202
9. Dhitavat J, Fairclough RJ, Hovnanian A, Burge SM. Calcium pumps and keratinocytes: lessons from Darier’s disease and Hailey-Hailey disease. Br J Dermatol. 2004;150(5):821–828. doi:10.1111/j.1365-2133.2004.05904.x
10. Wang Z, Wang Z, Sun L, et al. Whole exome sequencing improves mutation detection in Hailey-Hailey disease. J Dermatol. 2021;48(7):989–992. doi:10.1111/1346-8138.15828
11. Kaddu S, Müllegger RR, Kerl H. Grover’s disease associated with Sarcoptes scabiei. Dermatology. 2001;202(3):252–254. doi:10.1159/000051647
12. Grover RW, Rosenbaum R. The association of transient acantholytic dermatosis with other skin diseases. J Am Acad Dermatol. 1984;11(2 Pt 1):253–256. doi:10.1016/s0190-9622(84
13. Guana AL, Cohen PR. Transient acantholytic dermatosis in oncology patients. J Clin Oncol. 1994;12(8):1703–1709. doi:10.1200/jco.1994.12.8.1703
14. Matar S, Oulès B, Sohier P, et al. Cutaneous manifestations in SARS-CoV-2 infection (COVID-19): a French experience and a systematic review of the literature. J Eur Acad Dermatol Venereol. 2020;34(11):e686–e689. doi:10.1111/jdv.16775
15. Beele H, Smet S, Van Damme N, Beeckman D. Incontinence-Associated Dermatitis: pathogenesis, Contributing Factors, Prevention and Management Options. Drugs Aging. 2018;35(1):1–10. doi:10.1007/s40266-017-0507-1
16. Humphries R, Bobenchik AM, Hindler JA, Schuetz AN. Overview of Changes to the Clinical and Laboratory Standards Institute Performance Standards for Antimicrobial Susceptibility Testing, M100. J Clin Microbiol. 2021;59(12):e0021321. doi:10.1128/jcm.00213-21
17. Oliveira D, Borges A, Simões M. Staphylococcus aureus Toxins and Their Molecular Activity in Infectious Diseases. Toxins. 2018;10(6):548. doi:10.3390/toxins10060252
18. Aldana PC, Khachemoune A. Grover disease: review of subtypes with a focus on management options. Int J Dermatol. 2020;59(5):543–550. doi:10.1111/ijd.14700
19. Vivehanantha S, Carr RA, McGrath JA, Taibjee SM, Madhogaria S, Ilchyshyn A. Epidermolysis Bullosa Pruriginosa: a Case With Prominent Histopathologic Inflammation. JAMA Dermatology. 2013;149(6):727–731. doi:10.1001/jamadermatol.2013.155
20. Kalb RE, Strober B, Weinstein G, Lebwohl M. Methotrexate and psoriasis: 2009 National Psoriasis Foundation Consensus Conference. J Am Acad Dermatol. 2009;60(5):824–837. doi:10.1016/j.jaad.2008.11.906
21. Haghi-Aminjan H, Asghari MH, Goharbari MH, Abdollahi M. A systematic review on potential mechanisms of minocycline in kidney diseases. Pharmacol Rep. 2017;69(4):602–609. doi:10.1016/j.pharep.2017.02.001
22. Janakiraman K, Krishnaswami V, Sethuraman V, Natesan S, Rajendran V, Kandasamy R. Development of Methotrexate and Minocycline-Loaded Nanoparticles for the Effective Treatment of Rheumatoid Arthritis. AAPS PharmSciTech. 2020;21(2):34. doi:10.1208/s12249-019-1581-y
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.