Back to Journals » Therapeutics and Clinical Risk Management » Volume 10
Building a diagnostic algorithm on localized neuropathic pain (LNP) and targeted topical treatment: focus on 5% lidocaine-medicated plaster
Received 7 December 2013
Accepted for publication 24 January 2014
Published 16 April 2014 Volume 2014:10 Pages 259—268
DOI https://doi.org/10.2147/TCRM.S58844
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Roberto Casale,1,2 Consalvo Mattia3
1Department of Clinical Neurophysiology and Pain Rehabilitation Unit, Foundation “Salvatore Maugeri”, Research and Care Institute, IRCCS, Pavia, Italy; 2EFIC Montescano Pain School, Montescano, Italy; 3Department of Medical-Surgical Sciences, Section of Anaesthesia, Intensive Care and Pain Medicine, Faculty of Medicine and Pharmacy, Sapienza University of Rome, Italy
Abstract: Within the broad definition of neuropathic pain, the refinement of clinical diagnostic procedures has led to the introduction of the concept of localized neuropathic pain (LNP). It is characterized by consistent and circumscribed area(s) of maximum pain, which are associated with negative or positive sensory signs and/or spontaneous symptoms typical of neuropathic pain. This description outlines the clinical features (currently lacking in guidelines and treatment recommendations) in patients for whom topical targeted treatment with 5% lidocaine-medicated plaster is suggested as first-line therapy. Few epidemiologic data are present in the literature but it is generally estimated that about 60% of neuropathic pain conditions are localized, and therefore identifiable as LNP. A mandatory clinical criterion for the diagnosis of LNP is that signs and symptoms must be present in a clearly identified and defined area(s). Cartographic recordings can help to define each area and to assess variations. The diagnosis of LNP relies on careful neurological examination more than on pain questionnaires, but it is recognized that they can be extremely useful for recording the symptom profiles and establishing a more targeted treatment. The most widely studied frequent/relevant clinical presentations of LNP are postherpetic neuralgia, diabetic neuropathy, and neuropathic postoperative pain. They successfully respond to treatment with 5% lidocaine-medicated plaster with equal if not better pain control but with fewer side effects versus conventional systemic treatments. Generally, the more localized the pain (ie, the area of an A4 sheet of paper) the better the results of topical treatment. This paper proposes an easy-to-understand algorithm to identify patients with LNP and to guide targeted topical treatments with 5% lidocaine medicated plaster.
Keywords: pain treatment, posterpethic neuralgia, diabetic polineuropathy, postsurgical neuropathic pain
Introduction
In 1994, the International Association for the Study of Pain (IASP) introduced the term neuropathic pain (NP) as part of the neurological disease spectrum as a recognition that there are two broad categories of pain of different origin: nociceptive and neuropathic, and that NP is related to a lesion or disease affecting the nervous system. Although a debate on the concept that chronic pain can be more or less of neuropathic origin is still ongoing,1 in the recent updating of IASP definitions, it has been reaffirmed that nociceptive pain originates when the nociceptors are stimulated, while NP has its origin from a lesion or a disease affecting the somatosensory system in both its two divisions of peripheral and central.2 The mechanisms of NP are complex and classically divided into peripheral and the less common central types, based largely on the gross location of pain origin. Peripheral NP is thus defined as pain arising as a direct consequence of a lesion or disease affecting the peripheral somatosensory system.2
Lidocaine topical formulations, from simple injections of the compound to local ointments and cream application with various base components, have been used for both acute and chronic pain treatments. Moreover, the lack of physiopatological knowledge on pain mechanisms mainly addressed its use for inflammatory pain.3 This perspective has changed with the definition of the importance of peripheral Na+ channels in the development of NP and by the parallel identification of a subset of neuropathic painful conditions in which the painful neuropathy is localized.4,5 Localized NP (LNP) has been defined as a type of NP that is “characterized by consistent and circumscribed area(s) of maximum pain associated with negative or positive sensory signs and/or spontaneous symptoms characteristic of neuropathic pain”.5 This paper is focused on how to build a flowchart to reach a correct diagnosis on LNP and to use a lidocaine-medicated plaster in this group of pathologies. The points that emerged from the discussion of a workshop on LNP held in Rome in March 2013 by an Italian Experts Panel* were the basis for this paper and are summarized in Table 1.
Prevalence and etiology of NP due to peripheral lesions or disease
The exact prevalence of NP, and particularly NP of peripheral origin, is not known. However, it is widely accepted that in the general population, a large number of subjects with heterogeneous lesions or diseases of the nervous system experience NP.6 A neuropathic component is thought to be present in approximately 25% of chronic pain patients.6,7 In the most recent European survey, it has been estimated that moderate-to-severe chronic pain occurs in 19% of adult Europeans.8 The European population has been estimated to be around 750 million, so from these data, it is possible to infer that approximately 35 million Europeans suffer from some form of NP.9 Peripheral NP has been reported in various clinical conditions (eg, diabetes, shingles, spinal cord injury, stroke, multiple sclerosis, cancer, and human immunodeficiency virus infection), as well as in more common conditions (eg, lumbar or cervical radiculopathies), and traumatic and postsurgical nerve injuries. Among these conditions, postherpetic neuralgia (PHN), and painful diabetic neuropathy have been extensively studied, partly because of the relatively higher incidence of pain in these conditions.1,10–12 Peripheral NP conditions such as pain arising from post-traumatic/surgery injuries have been studied less frequently, but interest in them is increasing. A European survey suggests that as many as 24% of NP sufferers are patients with post-traumatic/surgery peripheral NP.13 In an update to the UK General Practice Research Database (2006–2010), postoperative pain is included among the major groups of NP with a prevalence – as reported in more specific studies – of 13%–68%.14,15
Very few studies of the prevalence of LNP have been published in the literature. The only preliminary estimate of the prevalence was obtained in a survey by Mick et al in 2012.5 In this survey, both general practitioners and pain specialists were interviewed and asked how many of their patients with NP suffered from LNP. Despite the limitations of this methodology, the results clearly highlighted the presence of a group of pathologies in which a LNP component is highly represented: post-zoster pain/PHN (83.3%), neuropathic postoperative pain (71.1%), and diabetic neuropathy (62.9%). The prevalence across all NP conditions was approximately 60% (trigeminal neuralgia, painful neuropathy in human immunodeficiency virus infection, neuropathic cancer pain, neuropathic back pain, and other neuropathies were also ranked in this survey).
Diagnostic continuity from the NP grading system to the definition of LNP
It is worth noting that the most discouraging conclusion of the update of the UK General Practice Research Database, was that treatments with little evidence of efficacy in NP are still common.14 One major reason is the complexity of the nervous mechanisms involved.16 Although the current definition of NP is a step toward a more precise characterization and thus targeted treatment of pain due to lesions or diseases affecting the somatosensory system, several factors still limit the differentiation of NP from other conditions. In this context, a mechanism-based approach to diagnosis and selected therapy is still far from being reached.17 However, it is recognized that not only etiology and pathological mechanisms, but also localization, can be important factors in diagnosis and prescribing a more targeted treatment.18 Thus, although it has some limitations, the recently proposed grading system of “definite”, “probable”, and “possible” NP retains its practical validity.19 Within the broad definition of NP, the refinement of clinical diagnostic procedures has led to the introduction of the concept of LNP.5
This definition was recently proposed to describe the clinical features (currently lacking in guidelines and treatment recommendations) in patients for whom topical treatment is suggested as first-line therapy.20,21 From the grading system of “definite”, “possible”, and “probable” chronic pain of neuropathic origin, a diagnosis of LNP should only be considered for clinical presentations in which the diagnosis of pain of neuropathic origin is “definite” or at least “probable”.19 In these two subdivisions, it may be possible for the pain to be localized to an area corresponding to a lesion/disease affecting a topographically defined part of the central or peripheral nervous system. A provisional diagnosis of LNP should only be applied where the presence of a peripheral nervous lesion or disease has been ascertained. In other words, the pivotal starting point in making a correct diagnosis of LNP is a “definite”, or “probable”, diagnosis of NP, in which the pain has arisen as a direct consequence of a lesion or disease affecting the peripheral part of the somatosensory system.
Importance of topography of pain and related signs and symptoms in the diagnosis of LNP
A mandatory clinical criterion for the diagnosis of LNP is that signs and symptoms must be present in the defined area(s).5 This area should be clearly identified and demarcated. The diagnostic approach to LNP syndromes should therefore include a correct description of the topography of pain and its related signs and symptoms. One practical way to proceed is to ask the patient to draw the area(s) of his/her pain while in the waiting room before the consultation. This drawing should later be checked by the doctor during the physical examination to obtain a pain map and a description of the sensory profiles. Many, equally valid, examples of cartographic recordings (pain maps) are available in the literature.22 However, it is important to note that only a few report another fundamental point in the algorithm of LNP so far identified, ie, whether the pain is felt superficially or deep in the tissues. The McGill Pain Questionnaire includes a good example of cartography as it requires the patient under examination to mark painful areas as superficial (S) or deep (I).23 This information is essential as only superficial pain felt in a circumscribed area can be considered to fulfill a pivotal requirement of the diagnostic criteria for LNP.
Value of questionnaires in diagnosing LNP
Another important point is the extent to which pain questionnaires are useful in diagnosing LNP. Firstly, it is essential to comment on the value of questionnaires in diagnosing NP, or indeed, whether questionnaires are able to discriminate between neuropathic and nociceptive pain. Many widely accepted questionnaires are used in the diagnosis of NP.24–27 However, it must be stressed that no questionnaire can be used alone, as a single instrument, as there are no pathognomonic symptoms that distinguish pain of different etiologies: neuropathic, nociceptive, or even dysfunctional or maladaptive.28 Indeed, up to 20% of patients fulfilling the criteria for a painful condition of neuropathic origin are not identified by any of the most widely used questionnaires, and the prevalence of NP in epidemiological studies varies from 3.3% to 17.9%.29 One of the most frequently used questionnaires in LNP studies is the DN4, but some other questionnaires have almost the same utility.22,25
Symptom profiles in LNP
If a peripheral lesion or disease has been assessed as the cause of a superficial and localized area of sensory disturbance, then spontaneous and evoked painful symptoms can be investigated and recorded. Determining sensory profiles can be useful by aiding recognition of the underlying mechanisms sustaining the state of chronic pain.30 It has been reported that patients suffering from NP show quite distinct profiles of both spontaneous and evoked pain symptoms, such as burning, throbbing and shooting pain, hyperalgesia, and allodynia. These sensory profiles could help identify patients with LNP who might respond to topical treatments.21,31 Spontaneous, uncomfortable sensory symptoms such as paresthesia and dysesthesia should be considered additional, but less crucial, clinical features. It is important – but unfortunately, not always possible – to detect the concomitant presence of negative sensory symptoms; these are always overshadowed by allodynic reactions, making their identification quite difficult, if not impossible.
It is useful to comment on the sensory changes, other than the expected pain reduction, that can be induced by topical treatments, with particular reference to the 5% lidocaine-medicated plaster. Topically applied, this plaster has an analgesic, pain-relieving effect, but does not have anesthetic activity causing numbness. This is because it does not block nerve conduction and does not, therefore, affect mechanical sensory perception in normal subjects.32 However, a pivotal observation is that this treatment can reveal the presence of previously obscured areas of negative sensory abnormalities. It is reasonable to hypothesize that 5% lidocaine-medicated plaster does this by reducing allodynic and hyperalgesic reactions, so that negative sensory disturbances become evident at post-treatment clinical examination. If this is the case, the appearance of these negative symptoms should not be regarded as the result of an anesthetic blockade of afferents induced by the treatment. It is also recommended that the affected areas are assessed both clinically and with quantitative sensory testing.
Minimum set of clinical and instrumental investigations in a daily practice setting
It is universally recognized that the clinical examination is invaluable and that the sensory examination is the most important part, as it determines the choice of any quantitative and instrumental assessments. This is stated almost literally in the first EFNS (European Federation Neurological Societies) guidelines on NP, published in 2004.33
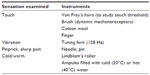
A thorough clinical examination is therefore crucial in the diagnosis of LNP. Identifying affected areas and the presence of any positive and negative sensory signs can be done at the patient’s bedside, using a few inexpensive instruments such as a hammer, tuning fork, Lindblom’s roller for hot and cold thermal profiles, and von Frey’s filaments (or their electronic version, or with a piece of cotton wool, or even with a finger). The use of validated semi-quantitative sensory testing methods is strongly recommended but not essential for the definitive diagnosis of NP.34,35 Sensory testing can give important information when used in pre- and post-treatment examinations. A list of inexpensive instruments that can easily be used for this purpose is given in Table 2.
No information is included here on history-taking and the clinical examination, since these should be standard practice for any pain specialist. However, for more details on the systematic search for neurological abnormalities, the reader is referred to the websites run by major international societies such as the IASP or the European Federation of IASP Chapters (EFIC), which both have excellent educational programs available (for example, on the “EFIC Montescano School for clinical and instrumental diagnosis in neuropathic pain” website).36
A practical algorithm to identify patients with LNP and guide treatments
A clear definition of LNP enables this type of pain to be treated with targeted therapies, in this case, with targeted topical treatments. Although the 5% lidocaine-medicated plaster has been used in pain conditions with potentially mixed nociceptive and NP, its main therapeutic use is for conditions of NP with a high probability of LNP.37
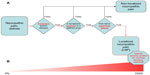
Precise placement of the plasters is critically important and clearly depends upon the accuracy of the clinical examination. The painful area should be localized and of a size that allows coverage of the area of maximum pain. A proposed algorithm for diagnosing and treating LNP is shown in Figure 1.
In this algorithm, the steps toward diagnosis follow a strict progression, to facilitate a practical approach to patients potentially affected by LNP. The first step is an accurate assessment of the presence of a “definite or probable pain of neuropathic origin”, ie, the presence of a lesion or disease of the somatosensory system. This is followed by verifying the presence of signs and symptoms in a defined, and most importantly, superficial area. Finally, sensory profiles are identified to help determine the potential suitability for localized treatment. The first three steps are mandatory for a diagnosis of LNP. The fourth step (identification of sensory profiles) is intended to help clinicians identify subgroups of patients affected by the same NP, but who, because of different sensory profiles, could be expected to respond differently to treatments; the ultimate aim is to obtain the highest percentage of positive results.21,31
Differences between topical and transdermal treatments
A fundamental distinction should be made between transdermal and targeted topical treatments. The transdermal route is used to obtain systemic effects and no local effects are expected, while targeted topical treatments exert their action mainly at the site of application. Opioids such as fentanyl or buprenorphine can be administered using patches; however, as they exert their analgesic action at a systemic level, they have the same adverse effects as when administered systemically.38 Administering analgesics through the skin and producing a clinically useful concentration of the drug only at the site of application – without systemic concentrations – is correctly defined as “topical”. This privileged route of administration should be considered as first-line treatment in the case of LNP.20,21,39 The 5% lidocaine-medicated plaster, being a topical treatment, has minimal systemic absorption of the active substance. Consequently, there is a low risk of toxicity and pharmacological interactions.40 However, as potential additive systemic effects cannot be completely excluded, it should be used with caution in patients receiving class 1 antiarrhythmic drugs or other local anesthetics, although absorption from the skin is normally extremely low.41
Adherence, compliance, and related “dosage” in targeted topical treatments
In chronic conditions requiring lengthy treatment, the complexity of therapy in terms of different compounds, and different routes and frequency of administration, are important factors influencing compliance and adherence to treatment.42,43
There are studies in the literature in which 5% lidocaine-medicated plaster was used for long periods of time.44 In NP, particularly, the therapeutic approach is often symptomatic, long-lasting, and involves polypharmacy.45 Also, many of the drugs used impose complex dosage schemes that further limit patient adherence to treatment, and can cause side effects and pharmacological interactions. These effects – especially sedation, nausea, dizziness, and constipation – can compromise the daily activities and social life of the patient even more, creating an unequal balance between analgesia, tolerability, functionality, and recovery. Topical treatment reduces the typical side effects of oral administration from 41% to 5.8% when compared with pregabalin.46 Side effects are mostly cutaneous reactions which occur in a limited number of patients and are mainly located at the site of application: erythema, rash, pruritus, and skin irritation.41,44,46 The efficacy and tolerability of treatment with the 5% lidocaine-medicated plaster has been confirmed in long-term studies, and in elderly patients with numerous comorbidities being treated with multiple medications.47 The proven efficacy and excellent short- to long-term tolerability of the 5% lidocaine-medicated plaster has led to its being proposed as the first-line drug of choice for treating PHN in most evidence-based guidelines.20,21 In polymedicated elderly patients, the simple treatment scheme associated with the use of patches, instead of oral treatment at regular intervals, is considered particularly useful.
The use of up to three plasters simultaneously has been proposed and is considered to constitute one dose. In clinical practice, patients apply 1.1 plasters on average.48 The use of up to four plasters was considered safe in clinical trials, but more than this number could lead to loss of compliance and adherence to the treatment.43
The most frequent and relevant clinical presentations of LNP
The most frequent presentations of LNP are PHN, diabetic neuropathy, and neuropathic postoperative pain. In 20%–30% of herpes zoster cases, the pain symptoms persist for many months, if not years.49 PHN is associated with a significant loss of the axons of primary sensory neurons in both the periphery and the central nervous system. The reason for persistence of the pain has not yet been completely clarified.
PHN pain is typically localized, unilateral, distributed along the dermatome or a branch of the trigeminal nerve, and felt “on the skin”. The pain, so intense as to interfere with sleep and other normal daily activities, is usually described as shooting, burning, or stabbing.30,31 It can be triggered by light tactile stimuli, such as dressing or taking a shower.50 Four weeks’ treatment with 5% lidocaine-medicated plaster produced a McGill Pain Questionnaire Short Form total scores improvement of −7.6±6.66 (mean ± standard deviation) versus −5.3±7.93 under pregabalin, as well as a faster onset and higher level of compliance.51 It is worth noting that a further 8 weeks’ combined treatment with both agents induced a notable additional reduction in pain.51
Generally, 10%–20% of patients with diabetes experience pain, but this proportion rises to 40%–50% among those with diabetic polyneuropathy.52 Diabetic neuropathy is characterized by the dysfunction of unmyelinated and small diameter myelinated, slow conduction, nerve fibers. Two forms can be differentiated, diffuse and focal. Sensory symptoms are more evident than motor involvement, and appear in the more distal portions of limbs, progressing proximally in a “glove” or “stocking” distribution, depending upon the type of nerve fibers involved. Typically, there are negative symptoms such as reduced sensitivity, and positive ones which can range from a spontaneous burning sensation (causalgia) associated with uncomfortable thermal (hot and cold) sensation, to thermal and mechanical dynamic allodynia in the foot. The pain may be continuous or intermittent, and is typically described as shooting or stabbing, with alterations in peripheral sensitivity that are difficult to control.30,31,53
If sufficiently localized, diabetic polyneuropathy can be treated topically with 5% lidocaine-medicated plaster. Up to four lidocaine plasters for a maximum of 12 hours per day have been compared with pregabalin (up to 600 mg/day).46 The effects on pain, particularly on allodynia, were the same with both treatments, but with a significant lower incidence of adverse reactions in favor of 5% lidocaine-medicated plaster. This resulted in an overall improvement in quality of life in the topical treatment group, with a mean change in an EQ-5D (European Quality Of Life-5 Dimensions) estimated health state score of 0.12 versus 0.04.46 When compared with other drugs used in managing painful diabetic polyneuropathy (ie, gabapentin, amitriptyline, and capsaicin), the efficacy of 5% lidocaine plaster in controlling pain was similar to that of the comparators but was accompanied by a lower incidence of side effects, resulting in a greater improvement in quality of life.52
Chronic postsurgical and post-traumatic pain is a significant clinical and economic problem. By definition, it develops after surgery and is not related to any preoperative pain at the same site.54,55 Risk types of surgery include thoracotomy, breast surgery, inguinal hernia surgery, and especially amputations.56 Only limited data related to the mechanisms and pathophysiology leading to chronic postsurgical pain are available. One of the most important mechanisms is spontaneous and evoked ectopic discharges from an injured nerve, leading to changes in the central nervous system.16,55 The efficacy and tolerability of 5% lidocaine-medicated plaster in treating postsurgical and post-traumatic NP have been assessed after 12 weeks of treatment with significant pain reduction and no side effects.57
Interestingly, in another clinical trial involving patients with NP arising from various conditions, satisfactory clinical results and an excellent safety profile allowed treatment to be simplified, as a consequence of a significant reduction in concomitant drugs, both in the general study group and in patients over 70 years of age.58 Even more interesting, in patients with NP following trauma to the upper or lower limbs, good control of pain intensity was associated with a reduction of the area of painful skin; this reduction was 50%–100% in 94.7% of subjects.59
Discussion
One of the most intuitive ways to control NP of peripheral origin is to interrupt the sensory input from the injured or diseased nerve, as with lidocaine during local and/or regional anesthesia.60,61 A single injection of anesthetic represents a simple, effective, and inexpensive way of providing good analgesia for a variety of pain conditions without major side effects. This property of injected lidocaine might suggest extending its use to chronic NP. However, the pain relief produced is usually short-lived. In the case of chronic LNP, it would have to be repeated, potentially leading to dose-related systemic side effects that would prohibit this route of administration for long periods of time.61 Furthermore, the infiltration blocks all nerve fibers, leading to both sensory and motor deficits.62 The introduction of the 5% lidocaine-medicated plaster is, therefore, of real benefit in the long-term treatment of localized chronic pain. It acts by blocking abnormally functioning (sensitized) Nav 1.7 and Nav 1.8 Na+ channels in dermal nociceptors.4 The blockade thereby reduces ectopic discharges and raises the peripheral ectopic discharge threshold. After repeated application, downregulation occurs, and as a direct consequence, influences central mechanisms. The 5% lidocaine-medicated plaster combines the efficacy of a local anesthetic with minimal systemic absorption and low risk of drug interactions, giving the formulation an excellent safety and tolerability profile. Furthermore, the way in which the active substance is released ensures that the concentration of lidocaine at the site of application is such that the drug binds to Na+ channels in damaged fibers, but not in undamaged ones, producing an analgesic and non-anesthetic action.63
The first key issue is recognizing that peripheral NP is reported in almost all neurological diseases affecting the peripheral nervous system. The exact prevalence of NP and particularly, of pain of peripheral origin is not known. However, in the general population, a large number of subjects with heterogeneous lesions or diseases of the nervous system experience NP.6,13,14 All over the world, millions of people have painful diabetic neuropathy, suffer from PHN, or have pain after surgery or trauma. Information on the prevalence of LNP is even scantier, even though data in the literature suggest that the presence of an LNP component is very common in post-zoster pain/PHN, neuropathic postoperative pain, and diabetic neuropathy.
From recognizing the social impact of this type of chronic pain, the starting point was the IASP definition of chronic pain, which is the most widely accepted guideline on NP, and their definition of LNP.2,5,20,21 Building on these elements, a flow chart was proposed (Figure 1A and B), linking the diagnosis of chronic NP to that of LNP. The most relevant concept is that LNP is a subset of NP and its diagnosis is, therefore, an improvement and refinement of the diagnosis of NP.
This second key point is that improved definition of LNP offers a rationale for the use of targeted topical treatments. When diagnosing LNP and deciding treatment, the clinician must take into account the presence of peripheral lesions or disease of the peripheral nervous system, the area of the symptoms, and the superficial localization of them. Recognizing that symptom profiles can be key factors in increasing the success rate of localized treatment is also important. Ideally, this rationalization will increase diagnostic and therapeutic success rates, especially when sensory profiles are taken into account (Figure 1B).
The third point is that, in specific clinical settings, a targeted topical treatment such as 5% lidocaine-medicated plaster could offer a higher success rate with fewer side effects. Moreover, targeted topical treatment can be successfully combined with systemic treatments in cases of refractory NP; the success rate is increased without a parallel increase in side effects.
A final consideration is the possibility that 5% lidocaine-medicated plasters can induce modification of the central sensitization that occurs concomitantly with peripheral changes in excitability and threshold, and also in common pathologies such as compression neuropathies.16,64,65 This hypothesis is supported by the reduction in the area of pain found in a recent study, which indicated that prolonged application of 5% lidocaine-medicated plasters can reduce the expanded receptor field and spinal cord excitability.66
Further epidemiological studies on specific LNPs are needed, together with more accurate definition of those sensory profiles that will respond better to a targeted topical treatment. Precise adherence to the LNP flowchart and recognition of the specific sensory profiles so far proposed can help doctors and patients in the management of these forms of NP due to peripheral lesions, achieving better results and improving the quality of life with fewer side effects.
Acknowledgments
The authors wish to thank the Italian Experts Panel* from the workshop on LNP held in Rome in March 2013. The points that emerged from that workshop were the basis for this paper.
The Italian Experts Panel includes: Massimo Allegri (Pavia), Francesco Amato (Cosenza), Caterina Aurilio (Naples), Laura Bertini (Rome), Pierluigi Canonico (Novara) Massimiliano Carassiti (Rome), Giancarlo Caruso (Bologna), Roberto Casale (Pavia), Amedeo Costantini (Chieti), Giorgio Cruccu (Rome), Guido Fanelli (Parma), Gabriele Finco (Cagliari), Alfredo Fogliardi (Fano), Diego Fornasari (Milan), Pierangelo Geppetti (Florence), Mariagrazia Grilli (Novara), Paolo Grossi (Milan), Pierangelo Lora Aprile (Brescia), Maria Lucia (Palermo), Sergio Mameli (Cagliari), Consalvo Mattia (Rome), Salvatore Palermo (Genoa), Francesco Paoletti (Perugia), Alfonso Papa (Naples), Enrico Polati (Verona), Leandro Provinciali (Ancona), William Raffaeli (Rimini), Alessandro Sabato (Rome), Angelo Schenone (Genoa), Gabriele Siciliano (Pisa), Valeria Tugnoli (Ferrara), and Renato Vellucci (Florence).
This paper was supported by an unrestricted grant from Grünenthal Italia SRL.
Disclosure
The authors report no conflicts of interest in this work.
References
Bennett MI, Smith BH, Torrance N, Lee AJ. Can pain can be more or less neuropathic? Comparison of symptom assessment tools with ratings of certainty by clinicians. Pain. 2006;122(3):289–294. | |
International Association for the Study of Pain. Classification of Chronic Pain, Second Edition (Revised). Available from: http://www.iasp-pain.org/PublicationsNews/Content.aspx?ItemNumber=1673. Accessed March 24, 2014. | |
Wildsmith JAW. Lidocaine: a more complex story than ‘simple’ chemistry suggests. Proc Hist Anaesth Soc. 2011;43:9–16. | |
Liu M, Wood JN. The roles of sodium channels in nociception: implications for mechanisms of neuropathic pain. Pain Med. 2011;12(Supp 3):S93–S99. | |
Mick G, Baron R, Brix Finnerup N, et al. What is localized neuropathic pain? A first proposal to characterize and define a widely used term. Pain Manage. 2012;2(1):71–77. | |
Torrance N, Smith BH, Bennett MI, Lee AJ. The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. J Pain. 2006;7(4):281–289. | |
Bowsher D. Neurogenic pain syndromes and their management. Br Med Bull. 1991;47(3):644–666. | |
Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287–333. | |
World Population Prospects: The 2006 Revision Population Database. UN – Department of Economic and Social Affairs. October 17, 2011. Available from: http://www.un.org/esa/population/publications/wpp2006/wpp2006.htm. Accessed February 27, 2014. | |
Ruocco V, Sangiuliano S, Brunetti G, Ruocco E. Beyond zoster: sensory and immune changes in zoster-affected dermatomes: a review. Acta Derm Venereol. 2012;92(4):378–382. | |
Collins SL, Moore RA, McQuay HJ, Wiffen P. Antidepressants and anticonvulsants for diabetic neuropathy and postherpetic neuralgia: a quantitative and systematic review. J Pain Symptom Manage. 2000;20(6):449–458. | |
Spallone V, Greco C. Painful and Painless diabetic neuropathy: one disease or two? Curr Diab Rep. 2013;13(4):533–549. | |
McDermott AM, Toelle TR, Rowbottom DJ, Schaefer CP, Dukes EM. The burden of neuropathic pain: results from a cross-sectional survey. Eur J Pain. 2006;10(2):127–135. | |
Hall GC, Morant S, Carroll D, Gabriel ZL, McQuay HJ. An observational descriptive study of the epidemiology and treatment of neuropathic pain in a UK general population. BMC Fam Pract. 2013;14(1):28. | |
Jung BF, Ahrendt GM, Oaklander AL, Dworkin RH. Neuropathic pain following breast cancer surgery: proposed classification and research update. Pain. 2003;104(1–2):1–13. | |
Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959–1964. | |
Finnerup NB, Scholz J, Attal N, et al. Neuropathic pain needs systematic classification. Eur J Pain. 2013;17(7):953–956. | |
Attal N, Fermanian C, Fermanian J, Lanteri-Minet M, Alchaar H, Bouhassira D. Neuropathic pain: are there distinct subtypes depending on the aetiology or anatomical lesion? Pain. 2008;138(2):343–353. | |
Treede RD, Jensen TS, Campbell JN, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70(18):1630–1635. | |
Attal N, Cruccu G, Baron R, et al; European Federation of Neurological Societies. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17:1113–1123. | |
Dworkin RH, O’Connor AB, Backonja M, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132(3):237–251. | |
Schott GD. The cartography of pain: the evolving contribution of pain maps. Eur J Pain. 2010;14(8):784–791. | |
Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1(3):277–299. | |
Bennett MI, Smith BH, Torrance N, Potter J. The S-LANSS score for identifying pain of predominantly neuropathic origin: validation for use in clinical and postal research. J Pain. 2005;6(3):149–158. | |
Bouhassira D, Attal N, Alchaar H, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain. 2005;114(1–2):29–36. | |
Portenoy R. Development and testing of a neuropathic pain screening questionnaire: ID Pain. Curr Med Res Opin. 2006;22(8):1555–1565. | |
Freynhagen R, Baron R, Gockel U, Tölle TR. PainDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22(10):1911–1920. | |
Hansson PT. Yet another questionnaire is born! Pain. 2010; 150(2):219. | |
Toth C, Lander J, Wiebe S. The prevalence and impact of chronic pain with neuropathic pain symptoms in the general population. Pain Med. 2009;10(5):918–929. | |
Baron R, Förster M, Binder A. Subgrouping of patients with neuropathic pain according to pain-related sensory abnormalities: a first step to a stratified treatment approach. Lancet Neurol. 2012;11(11):999–1005. | |
Baron R, Tölle TR, Gockel U, Brosz M, Freynhagen R. A cross-sectional cohort survey in 2100 patients with painful diabetic neuropathy and postherpetic neuralgia: differences in demographic data and sensory symptoms. Pain. 2009;146(1–2):34–40. | |
Gammaitoni AR, Alvarez NA, Galer BS. Safety and tolerability of the lidocaine patch 5%, a targeted peripheral analgesic: a review of the literature. J Clin Pharmacol. 2003;43(2):111–117. | |
Cruccu G, Anand P, Attal N, et al. EFNS guidelines on neuropathic pain assessment. Eur J Neurol. 2004;11(3):153–162. | |
Haanpää M, Attal N, Backonja M, et al. NeuPSIG guidelines on neuropathic pain assessment. Pain. 2011;152(1):14–27. | |
Backonja M, Attal N, Baron R, et al. Value of quantitative sensory testing in neurological and pain disorders: NeuPSIGconsensus. Pain. 2013;154(9):1807–1819. | |
Montescano Pain School [homepage on the Internet]. Available from: http://www.montescanoschool.eu. Accessed February 27, 2014. | |
Burch F, Codding C, Patel N, Sheldon E. Lidocaine patch 5% improves pain, stiffness, and physical function in osteoarthritis pain patients. A prospective, multicenter, open-label effectiveness trial. Osteoarthritis Cartilage. 2004;12:253–255. | |
Mattia C, Coluzzi F, Sonnino D, Anker-Møller E. Efficacy and safety of fentanyl HCl iontophoretic transdermal system compared with morphine intravenous patient-controlled analgesia for postoperative pain management for patient subgroups. Eur J Anaesthesiol. 2010;27(5):433–440. | |
Jorge LL, Feres CC, Teles VE. Topical preparations for pain relief: efficacy and patient adherence. J Pain Res. 2010;4:11–24. | |
Mick G, Correa-Illanes G. Topical pain management with the 5% lidocaine medicated plaster – a review. Curr Med Res Opin. 2012;28(6):937–951. | |
Grunenthal. Versatis 5% Medicated Plaster [webpage on the Internet]. Available from: http://www.medicines.org.uk/emc/medicine/19291. Accessed February 27, 2014. | |
Wolf MS, Curtis LM, Waite K, et al. Helping patients simplify and safely use complex prescription regimens. Arch Intern Med. 2011;171(4):300–305. | |
Mutasingwa DR, Ge H, Upshur REG. How applicable are clinical practice guidelines to elderly patients with comorbidities? Can Fam Physician. 2011;57(7):e253–e262. | |
Sabatowski R, Hans G, Tacken I, Kapanadze S, Buchheister B, Baron R. Safety and efficacy outcomes of long-term treatment up to 4 years with 5% lidocaine medicated plaster in patients with post-herpetic neuralgia. Curr Med Res Opin. 2012;28(8):1337–1346. | |
AIFA – Agenzia Italiana del Farmaco. Dialogo sui Farmaci – Nota 4 Update 15–02–2012 [Dialogue on Drugs - Note 4 Update 15–02–2012]. Availlable from: http://www.dialogosuifarmaci.it/documents/Note%20AIFA%2039_17%20edizio. Accessed February 27, 2014. Italian. | |
Baron R, Mayoral V, Leijon G, Binder A, Steigerwald I, Serpell M. 5% lidocaine medicated plaster versus pregabalin in post-herpetic neuralgia and diabetic polyneuropathy: an open-label, non-inferiority two-stage RCT study. Curr Med Res Opin. 2009;25:1663–1676. | |
Clère F, Delorme-Morin C, George B, et al. 5% lidocaine medicated plaster in elderly patients with postherpetic neuralgia. Result of a compassionate use program in France. Drugs Aging. 2011;28(9):693–702. | |
Ritchie M, Liedgens H, Nuijten M. Cost effectiveness of a lidocaine 5% medicated plaster compared with pregabalin for the treatment of postherpetic neuralgia in the UK: a Markov model analysis. Clin Drug Investig. 2010;30(2):71–87. | |
Jung BF, Johnson RW, Griffin DR, Dworkin RH. Risk factors for post herpetic neuralgia in patients with herpes zoster. Neurology. 2004;62(9):1545–1551. | |
Nalamachu S, Morley-Forster P. Diagnosing and managing postherpetic neuralgia. Drugs Aging. 2012;29(11):863–869. | |
Rehm S, Binder A, Baron R. Post-herpetic neuralgia: 5% lidocaine medicated plaster, pregabalin, or a combination of both? A randomized, open, clinical effectiveness study. Curr Med Res Opin. 2010;26(7):1607–1619. | |
Wolff RF, Bala MM, Westwood M, Kessels AG, Kleijnen J. 5% lidocaine medicated plaster in painful diabetic peripheral neuropathy (DPN): a systematic review. Swiss Med Weekly. 2010;140(21–22):297–306. | |
Veves A, Backonja M, Malik RA. Painful diabetic neuropathy: epidemiology, natural history, early diagnosis, and treatment options. Pain Med. 2008;9(6):660–674. | |
Gerbershagen H, Özgür E, Dagtekin O, et al. Preoperative pain as a risk factor for chronic post-surgical pain – six month follow-up after radical prostatectomy. Eur J Pain. 2009;13:1054–1061. | |
Correa-Illanes G, Roa R, Pineros JL, Calderón W. Use of 5% lidocaine medicated plaster to treat localized neuropathic pain secondary to traumatic injury of peripheral nerves. Loc Reg Anesth. 2012;5:47–53. | |
Casale R, Alaa L, Mallick M, Ring H. Phantom limb related phenomena and their rehabilitation after lower limb amputation. Eur J Phys Rehabil Med. 2009;45(4):559–566. | |
Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–1625. | |
Hans G, Joukes E, Verhulst J, Vercauteren M. Management of neuropathic pain after surgical and non-surgical trauma with lidocaine 5% patches: study of 40 consecutive cases. Curr Med Res Opin. 2009;25:2737–2743. | |
Delorme C, Navez ML, Legout V, Deleens R, Moyse D. Treatment of neuropathic pain with 5% lidocaine-medicated plaster: five years of clinical experience. Pain Res Manag. 2011;16(4):259–263. | |
Ganapathy S, Brookes J, Bourne R. Local infiltration analgesia. Anesthesiol Clin. 2011;29:329–342. | |
Gupta A. Wound infiltration with local anaesthetics in ambulatory surgery. Curr Opin Anesthesiol. 2010;23:708–713. | |
Vlassakov KV, Narang S, Kissin I. Local anesthetic blockade of peripheral nerves for treatment of neuralgias: systematic analysis. Anesth Analg. 2011;112(6):1487–1493. | |
Madsen CS, Johnsen B, Fuglsang-Frederiksen A, Jensen TS, Finnerup NB. Differential effects of a 5% lidocaine medicated patch in peripheral nerve injury. Muscle Nerve. 2013;48(2):265–271. | |
Wall PD. Neuropathic pain and injured nerve: central mechanisms. Br Med Bull. 1991;47(3):631–643. | |
Zanette G, Cacciatori C, Tamburin S. Central sensitization in carpal tunnel syndrome with extraterritorial spread of sensory symptoms. Pain. 2010;148(2):227–236. | |
Correa-Illanes G, Calderón W, Roa R, Piñeros JL, Dote J, Medina D. Treatment of localized post-traumatic neuropathic pain in scars with 5% lidocaine medicated plaster. Local Reg Anesth. 2010;3:77–83. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.