Back to Journals » ClinicoEconomics and Outcomes Research » Volume 9
Budget impact analysis of the simplification to atazanavir + ritonavir + lamivudine dual therapy of HIV-positive patients receiving atazanavir-based triple therapies in Italy starting from data of the Atlas-M trial
Authors Restelli U , Fabbiani M , Di Giambenedetto S, Nappi C , Croce D
Received 8 November 2016
Accepted for publication 21 January 2017
Published 1 March 2017 Volume 2017:9 Pages 173—179
DOI https://doi.org/10.2147/CEOR.S127097
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Giorgio Colombo
Umberto Restelli,1,2 Massimiliano Fabbiani,3 Simona Di Giambenedetto,3 Carmela Nappi,4 Davide Croce,1,2
1Centre for Research on Health Economics, Social and Health Care Management (CREMS), LIUC – Università Cattaneo, Castellanza, Italy; 2School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa; 3Institute of Clinical Infectious Diseases, Catholic University of Sacred Heart, 4Health Economics, Bristol-Myers Squibb S.r.l., Rome, Italy
Background: This analysis aimed at evaluating the impact of a therapeutic strategy of treatment simplification of atazanavir (ATV)+ ritonavir (r) + lamivudine (3TC) in virologically suppressed patients receiving ATV+r+2 nucleoside reverse transcriptase inhibitors (NRTIs) on the budget of the Italian National Health Service (NHS).
Methods: A budget impact model with a 5-year time horizon was developed based on the clinical data of Atlas-M trial at 48 weeks (in terms of percentage of patients experiencing virologic failure and adverse events), from the Italian NHS perspective. A scenario in which the simplification strategy was not considered was compared with three scenarios in which, among a target population of 1,892 patients, different simplification strategies were taken into consideration in terms of percentage of patients simplified on a yearly basis among those eligible for simplification. The costs considered were direct medical costs related to antiretroviral drugs, adverse events management, and monitoring activities.
Results: The percentage of patients of the target population receiving ATV+r+3TC varies among the scenarios and is between 18.7% and 46.9% in year 1, increasing up to 56.3% and 84.4% in year 5. The antiretroviral treatment simplification strategy considered would lead to lower costs for the Italian NHS in a 5-year time horizon between –28.7 million € and –16.0 million €, with a reduction of costs between –22.1% (–3.6 million €) and –8.8% (–1.4 million €) in year 1 and up to –39.9% (–6.9 million €) and –26.6% (–4.6 million €) in year 5.
Conclusion: The therapy simplification for patients receiving ATV+r+2 NRTIs to ATV+r+3TC at a national level would lead to a reduction of direct medical costs over a 5-year period for the Italian NHS.
Keywords: protease inhibitor, economic evaluation, cost, de-intensification, antiretroviral therapy, Italian National Health Service
Corrigendum for this paper has been published.
Background
Strategies of antiretroviral treatment (ART) de-intensification for HIV-infected patients are debated in literature and clinical guidelines since years.1–7 ART simplification aims at decreasing toxicities and drugs resistances, increasing patients’ compliance and quality of life, and often leading to a reduction of therapy costs.8–10 This topic is particularly relevant considering the annual economic burden of ART for the Italian National Health Service (NHS): the most recent data available (referred to 2015) report a total cost of 627.7 million € at a national level, of which 336.9 million € for fixed-dose combinations, 161.8 million € for protease inhibitors (PIs, alone or combined), 52.4 million € for nucleoside/nucleotide reverse transcriptase inhibitors, 33.3 million € for non-nucleoside reverse transcriptase inhibitors (non-NRTIs), and 88.3 million € for other antiretroviral drugs.11
To date, the effectiveness of ART simplification among virologically suppressed patients has been mainly investigated considering PI-based therapies, analyzing the possibility to switch patients to dual therapies or monotherapies removing one or both NRTIs from triple therapies.12–16
As reported earlier, one of the potential advantages of ART simplification is related to a reduction of costs for NHSs. In a context with limited resources as health care, it is essential to identify cost containment strategies that do not affect the effectiveness of treatments. De-intensification of ART in virologically suppressed patients, simplification to single-tablet regimens, use of generic drugs, and use of least expensive therapies in case of non-inferiority are the strategies identified in literature to reduce costs without affecting the quality of care.17–19
Among the studies conducted to investigate the effect of de-intensification, the Atlas-M trial assessed the effectiveness at 48 weeks of treatment simplification to atazanavir (ATV) + ritonavir (r) + lamivudine (3TC) in patients receiving ATV+r+2 NRTIs versus maintaining ATV+r+2 NRTIs.20 The study enrolled HIV-infected adults on ATV+r plus two NRTIs, with stable HIV-RNA <50 copies/mL, and CD4+ >200 cells/mm3. Main exclusion criteria were HBV-coinfection, past virological failure on or resistance to study drugs, recent AIDS, and pregnancy. Patients were randomly assigned 1:1 to either switch to ATV+r+3TC or to continue the same previous regimen (ATV+r+2 NRTIs). At the primary 48-week analysis, treatment simplification to ATV+r+3TC showed non-inferior efficacy (even superiority on a post hoc analysis) and a comparable safety profile over continuing ATV+r+2 NRTIs.
This study presents an analysis aimed at evaluating the impact on the budget of the Italian NHS of a therapeutic strategy of treatment simplification to ATV+r+3TC in virologically suppressed patients receiving ATV+r+2 NRTI.
Methods
A budget impact model with a 5-year time horizon21 was developed based on the clinical data of Atlas-M trial at 48 weeks,20 from the Italian NHS perspective. A deterministic model with annual cycles was developed considering the target population to receive ATV+r+2 NRTI at the baseline and the possibility to be simplified to ATV+r+3TC. The percentage of patients eligible for dual therapy (ATV+r+3TC) was derived from the Atlas-M trial at 48 weeks,20 in which patients were eligible to simplification if aged >17 years, receiving for at least 6 months ATV+r+2 NRTI, with “at least two HIV-RNA levels <50 copies/mL on two consecutive determinations at least 3 months apart,” for at least 6 months with a CD4 cell count >200 cells/mm3, no history of AIDS-related events in the year before enrollment.
The percentage of patients eligible for dual therapy (ATV+r+3TC) was varied to structure three different scenarios. These scenarios were compared with a base case scenario in which patients are not switched to ATV+r+3TC, to estimate the impact of ART simplification.
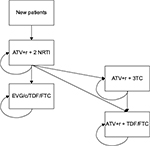
In each cycle, new patients enter the model being assigned to ATV+r+2 NRTI. In the base case scenario, in every cycle, patients with virologic control receiving ATV+r+2 NRTI remain assigned to the same ART. Patients experiencing virologic failure are equally distributed to receive either elvitegravir/cobicistat/tenofovir/emtricitabine (EVG/c/TDF/FTC) or TDF/FTC+ATV+r, as observed in the Atlas-M trial. Patients assigned to EVG/c/TDF/FTC or to TDF/FTC+ATV+r will receive the same ART for the whole period of the analysis.
In the scenarios that consider the possibility to simplify ATV+r+2 NRTI therapy, patients receiving the aforementioned ART may be assigned to EVG/c/TDF/FTC or to TDF/FTC+ATV+r as described earlier, be assigned to ATV+r+3TC (considering the simplification strategies described later), or continue to receive ATV+r+2 NRTI. Patients assigned to EVG/c/TDF/FTC or to TDF/FTC+ATV+r will receive the same ART for the whole period of the analysis, as in the base case scenario. Patients assigned to ATV+r+3TC will continue to use the same ART, with an annual probability equal to the effectiveness of the treatment (defined in terms of patients not experiencing a virologic failure), or will be assigned to TDF/FTC+ATV+r in case of virologic failure.
The structure of the stationary Markov Model implemented to simulate all the scenarios considered (base case and scenarios 1–3) is presented in Figure 1. The percentages of patients experiencing virologic failure, derived from the Atlas-M trial, are 4.51% per year for ATV+r+2 NRTI and 0.75% per year for ATV+r+3TC.
The target population considered in the analysis was identified starting from the number of HIV-positive patients treated in the Italian context, being 82,472 in 2012 (obtained reprocessing data of the Italian National Institute of Health).22 Among the 9,028 HIV-positive patients treated within the larger 10 infectious diseases wards participating in the Atlas-M trial, 2.28% were eligible for simplification to ATV+r+3TC, considering the criteria requested by the trial. The number of patients eligible for simplification at a national level was then considered to be 1,892 (2.28% of 82,472 HIV-positive patients), with an annual incidence of 1.75%, based on expert opinion.
Therefore, 1,892 patients were considered as the target population of the budget impact analysis in year 0, assuming that the simplification strategy investigated would not affect the prescription behavior related to the rest of the HIV-positive population.
In year 1 (the first year of the analysis), the target population eligible for simplification is equal to the number of patients eligible in year 0 (1,892) multiplied by the effectiveness of ATV+r+2 NRTI presented upon (95.5%), being 1,807 patients.
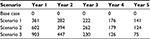
The proportion of patients eligible for ART simplification (being 1,807 in year 1) assigned to ATV+r+3TC in each scenario is: one fifth of eligible patients in scenario 1, one third of eligible patients in scenario 2, and one half of eligible patients in scenario 3, as reported in Table 1.
The costs considered in the analysis refer to 2015 and are direct medical costs related to ART, hospitalizations, outpatient activities, and other drugs consumption due to the management of adverse events and monitoring activities. A bottom up approach was used.
ART cost was derived from data reported within the Italian Guidelines for the use of antiretroviral drugs, 20158 and weighted by the mean 48 weeks adherence observed in the Atlas-M trial. The costs of management of adverse events and monitoring activities were referred to specialist visits, HIV-RNA tests, CD4+ count, creatinine test, lipid profile, bilirubin level, transaminases test, dual X-ray absorptiometry, neurocognitive test, as emerged by the data of Atlas-M trial and are based on national tariffs.
In detail, the costs per patient considered in the model are reported in Table 2. Clinical data were derived from the article published by Di Giambenedetto et al.20 The protocol of Atlas-M trial was approved by the Ethics Committees of each participating center (21 hospitals in Italy), and all procedures were performed in accordance with the Declaration of Helsinki. Patients provided written informed consent to study participation before enrollment. The Atlas study was registered with ClinicalTrials.gov, number NCT01599364. No further approval from Ethics Committees was required for the analysis performed. Data related to the resources used for the management of adverse events were provided anonymously, in accordance with the guidelines for the treatment of personal data of clinical studies.
  | Table 2 Per capita annual costs considered in the model Notes: ^NRTI costs consider a weighted mean of the cost of the backbones used within the Atlas-M trial,20 that is, TDF/FTC; TDF+3TC; ABC+3TC; AZT+3TC; ddl+3TC; TDF+ABC. *Because of lack of data, the same cost of ATV+r+2 NRTI was considered. Abbreviations: ART, antiretroviral treatment; ATV, atazanavir; NRTI, nucleoside reverse transcriptase inhibitor; r, ritonavir; EVG, elvitegravir; c, cobicistat; TDF, tenofovir; FTC, emtricitabine; 3TC, lamivudine; ABC, abacavir; ddl, didanosine; AZT, zidovudine. |
Results
The percentage of patients of the target population receiving ATV+r+3TC varies among the scenarios considered and is between 18.7% and 46.9% in year 1, increasing up to 56.3% and 84.4% in year 5. The percentage of patient distribution among therapies during the 5 years considered in each scenario, emerged by the simulation and based on the effectiveness of each ART and on the simplification strategy adopted, is presented in Table 3.
The annual cost for the Italian NHS to treat the target population in the base case analysis is 16.1 million € in year 1, 16.5 million € in year 2, 16.8 million € in year 3, 17.0 million € in year 4, and 17.3 million € in year 5, with a cumulative 5-year cost of 83.7 million €. The ART simplification strategy described in the “Methods” section (simplification to ATV+r+3TC of patients receiving ATV+r+2 NRTI eligible considering the Atlas-M trial criteria) would lead to lower costs for the Italian NHS in a 5-year time horizon between −28.7 million € and −16.0 million €.
The results of the analysis for each comparative scenario are presented in Table 4. The simplification strategy investigated would lead to a reduction of costs between −22.1% (–3.6 million €) and −8.8% (–1.4 million €) in year 1, up to −39.9% (–6.9 million €) and −26.6% (–4.6 million €) in year 5. The decrease in the 5-year percentage cost would be between 34.2% and −19.2%. The annual per capita direct medical costs for the Italian NHS for the management of the patients considered in the analysis (obtained dividing the yearly management costs by the target population) increase over the years in the base case scenario (from 8,379 € in year 1 up to 8,437 € in year 5) and decrease steadily in the simplification scenarios, from 7,639 € in year 1 to 6,196 € in year 5 in scenario 1; from 7,145 € in year 1 to 5,479 € in year 5 in scenario 2; and from 6,528 € in year 1 to 5,069 € in year 5 in scenario 3.
Discussion
The results of the study presented show how, based on the results of the Atlas-M trial at 48 weeks, the simplification of ATV+r+2 NRTI therapy to ATV+r+3TC would free resources for the Italian NHS, leading to a reduction of direct medical costs between 16.0 million € and 28.7 million € in a 5-year time period, not compromising the efficacy of the antiretroviral therapy. As expected, the scenario that leads to the highest costs reduction is the one in which the highest number of patients eligible for ART simplification considered (one half) is assigned to ATV+r+3TC dual therapy (scenario 3).
As reported in the “Results” section, the 5-year budget impact trend shows a constant reduction of the direct medical cost for the treatment of HIV-positive patients following ART simplification. This is due to the cumulative impact of lower direct medical costs of the new patients receiving ATV+r+3TC in the simplifications scenarios, compared with the base case scenario. Within a sector characterized by scarce resources, as health care, the lower cost of patient management due to ART simplification could allow the access to new expensive treatments for patients with few therapeutic options. In a field, as that of HIV, in which in the last years several new antiretroviral drugs received a marketing authorization by the European Medicines Agency, this wider access to new drugs would result in an increase of the therapeutic options for patients.
In literature, the assessment of cost containment strategies in Italy in the HIV field through budget impact analyses has been investigated considering different approaches, that is, use of generic drugs,17,23 switch from triple therapies to NNRTI-based single-tablet regimens,17 simplification of triple therapies to dual therapies and monotherapies (not separating the results among these last two options).17,18 Therefore, the results of the analysis presented are difficult to compare with those of already published studies, also because of the different costs considered (ie, ART costs vs cost of ART plus hospitalization, outpatient activities, and other drugs for adverse events’ management).
In the two articles that considered de-intensification strategies, Angeletti et al performed an analysis within Lazio Region,17 considering direct medical costs (referred to 2011) related to ART, hospitalization, outpatient activities, and other drugs over a 5-year time horizon (2012–2016). The average annual direct medical costs for the management of HIV patients at a regional level resulted to be 147 million €. Among the cost containment strategies assessed, the “switch of 30% of suppressed subjects on first and second lines of treatment with PI/r-based triple regimen to single-tablet regimens” would lead to a cost decrease of −0.8 million €, and the “switch of 30% of suppressed subjects on first and second lines of treatment with PI/r-based triple regimen to PI/r monotherapy” would lead to a cost decrease of −1.5 million €. The analysis shows how the most cost containing strategy would be the use of generic drugs, followed by simplification to monotherapy. Unfortunately, the authors does not present subgroup analyses to estimate the percentage cost reduction among patients who switched to simplified regimens.
In 2014, a further analysis investigated the budget impact of ART simplification to less drug regimens over a 3-year horizon,18 assuming the Italian NHS point of view and considering ART costs referred to 2013. The base case scenario was derived collecting data within four Italian hospitals, cumulatively providing ART to 11,269 HIV-positive patients (~13.7% of the total number of treated patients in Italy at the time). Four simplification scenarios were considered (de-intensifying only PI-based triple therapies over 1 year or over a 3-year period, and de-intensifying PI-based triple therapies and NRTIs + Efavirenz over 1 year or over a 3-year period). Results showed that over a 3 year period, ART cost decreased between 23.1 million € and 44.3 million € considering different scenarios. The percentage reduction of 3-year costs varied between −6.7% and −12.8%. These reduction is less than the one observed in the study presented. This is mainly because of the target population considered in the two analyses: being only patients potentially eligible for simplification in the study presented here and the total number of HIV-positive patients treated with ART within the infectious diseases wards of four Italian hospitals, therefore considering also patients not eligible for simplification, in the previous analysis.
At an international level, two articles proposed economic analysis of cost containment strategies in the field of HIV in Spain24 and in the UK.25 Llibre et al investigated the potential decrease in ART costs because of different therapy switch strategies within a hospital in Catalonia.24 The three main cost reduction strategies were switches to PI monotherapies, withdrawal of high cost molecules, and switch to less expensive backbones. Gazzard et al investigated three possible cost containment strategies for the British NHS: stopping CD4 counts testing for patients with recent CD4+ counts >350 cells/mm3 and full HIV RNA suppression, wider use of generic antiretrovirals, and use of DRV+r monotherapy.25 The latter leading to a reduction of antiretroviral costs for patients eligible to simplification of 45%.
The results presented, related to a projection based on real data from the Atlas-M trial, suggest that the simplification strategy investigated would grant a containment of costs, both related to antiretrovirals and patient management, without affecting the effectiveness of ART. Furthermore, the reduction of drugs received by patients is likely to reduce the toxicity burden of the therapy in a medium-term horizon.
However, among the strategies investigated within literature, the use of generic drugs seems to be the one able to grant a higher cost reduction, because of the wider number of patients potentially interested in this switch.
The main strength of the analysis is the use of the Atlas-M trial, which provides data on the effectiveness of treatment simplification to ATV+r+3TC in patients receiving ATV+r+2 NRTIs compared with maintaining ATV+r+2 NRTIs and the health care activities performed to monitor patients and to manage adverse events. The aforementioned data refer to a sample of patients consistent with HIV-positive patients treated within Italian infectious diseases wards and reflect the real clinical practice of the context that was investigated. However, a limitation of using this study was that it only presents data up to 48 weeks of follow-up. Because of the lack of long-term effectiveness data, the same percentage of virological failures was considered for each year of the analysis. Future publication of the Atlas-M trial at 96 weeks could provide an update of the results presented and validate the assumptions in the current analysis on the long-term efficacy.
Conclusion
The therapy simplification of patients receiving ATV+r+2 NRTI to ATV+r+3TC at a national level would lead to a reduction of direct medical costs over a 5-year period for the Italian NHS between −28.7 million € and −16.0 million €, without affecting therapeutic efficacy. The results observed could be used by decision makers to support therapeutic decisions as well as economic resource allocation programs.
Acknowledgments
The authors would like to thank the Atlas-M Study Group for their contribution to the sharing of the clinical Atlas-M data. The analysis was supported by an unconditional grant from Bristol-Myers Squibb.
Disclosure
UR declares speaker fees (Janssen Cilag, Abbvie). MF received speakers’ honoraria and support for travel to meetings from Bristol-Myers Squibb, Gilead, Merck Sharp & Dohme, ViiV Healthcare, and Janssen-Cilag. SDG declares speakers’ honoraria and support for travel to meetings from Bristol-Myers Squibb, Gilead, Merck Sharp & Dohme, ViiV Healthcare, and Janssen-Cilag. CN is employed by Bristol-Myers Squibb S.r.l. DC declares advisory board fees (Merck Sharp & Dohme, Abbvie). The authors report no other conflicts of interest in this work.
References
Swindells S, DiRienzo AG, Wilkin T, et al. Regimen simplification to atazanavir-ritonavir alone as maintenance antiretroviral therapy after sustained virologic suppression. JAMA. 2006;296(7):806–814. | ||
Martínez E, Arnaiz JA, Podzamczer D, et al. Three-year follow-up of protease inhibitor-based regimen simplification in HIV-infected patients. AIDS. 2007;21(3):367–369. | ||
Dejesus E, Young B, Morales-Ramirez JO, et al. Simplification of antiretroviral therapy to a single-tablet regimen consisting of efavirenz, emtricitabine, and tenofovir disoproxil fumarate versus unmodified antiretroviral therapy in virologically suppressed HIV-1-infected patients. J Acquir Immune Defic Syndr. 2009;51(2):163–174. | ||
Martin A, Bloch M, Amin J, et al. Simplification of antiretroviral therapy with tenofoviremtricitabine or abacavirlamivudine: a randomized, 96-week trial. Clin Infect Dis. 2009;49:1591–1601. | ||
Wohl DA, Bhatti L, Small CB, et al. Simplification to abacavir/lamivudine+atazanavir maintains viral suppression and improves bone and renal biomarkers in ASSURE, a randomized, open label, noninferiority trial. PLoS One. 2014;9:e96187. | ||
Di Giambenedetto S, Fabbiani M, Colafigli M, et al. A Safety and feasibility of treatment simplification to atazanavir/ritonavir + lamivudine in HIV-infected patients on stable treatment with two nucleos(t)ide reverse transcriptase inhibitors + atazanavir/ritonavir with virological suppression (Atazanavir and Lamivudine for treatment Simplification, AtLaS pilot study). J Antimicrob Chemother. 2013;68(6):1364–1372. | ||
Burgos J, Crespo M, Falcó V, et al. Simplification to dual antiretroviral therapy including a ritonavir-boosted protease inhibitor in treatment-experienced HIV-1-infected patients. J Antimicrob Chemother. 2012;67(10):2479–2486. | ||
HIV/AIDS Italian Expert Panel. Linee Guida Italiane sull’utilizzo dei farmaci antiretrovirali e sulla gestione diagnostico-clinica delle persone con infezione da HIV-1; December 17, 2015. Available from: http://www.salute.gov.it/imgs/C_17_pubblicazioni_2442_allegato.pdf. Accessed November 4, 2016. | ||
Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Last updated January 28, 2016; last reviewed January 28, 2016. Available from: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed November 4, 2016. | ||
Churchill D, Waters L, Ahmed N, et al. British HIV Association guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy 2015. Available from: http://www.bhiva.org/documents/Guidelines/Treatment/2015/2015-treatment-guidelines.pdf. Accessed November 4, 2016. | ||
The Medicines Utilisation Monitoring Centre. National Report on Medicines use in Italy. Year 2015. Rome: Italian Medicines Agency; 2016. Available from: http://www.agenziafarmaco.gov.it/sites/default/files/Rapporto_OsMed_2015__AIFA.pdf. | ||
Cossarini F, Salpietro S, Galli L, et al. Monotherapy with atazanavir as a simplification strategy: results from an observational study. J Acquir Immune Defic Syndr. 2012;60(3):e101–e103. | ||
Pulido F, Arribas JR, Delgado R, et al. Lopinavir-ritonavir monotherapy versus lopinavir-ritonavir and two nucleosides for maintenance therapy of HIV. AIDS. 2008;22(2):F1–F9. | ||
Arribas JR, Clumeck N, Nelson M, et al. The MONET trial: week 144 analysis of the efficacy of darunavir/ritonavir (DRV/r) monotherapy versus DRV/r plus two nucleoside reverse transcriptase inhibitors, for patients with viral load, 50 HIV-1 RNA copies/mL at baseline. HIV Med. 2012;13(7):398–405. | ||
Valantin MA, Lambert-Niclot S, Flandre P, et al. Long-term efficacy of darunavir/ritonavir monotherapy in patients with HIV-1 viral suppression: week 96 results from the MONOI ANRS 136 study. J Antimicrob Chemother. 2012;67(3):691–695. | ||
Bierman WF, van Agtmael MA, Nijhuis M, et al. HIV monotherapy with ritonavir-boosted protease inhibitors: a systematic review. AIDS. 2009;23(3):279–291. | ||
Angeletti C, Pezzotti P, Antinori A, et al. Antiretroviral treatment-based cost saving interventions may offset expenses for new patients and earlier treatment start. HIV Med. 2014;15(3):165–174. | ||
Restelli U, Andreoni M, Antinori A, et al. Budget impact analysis of antiretroviral less drug regimen simplification in HIV-positive patients on the Italian National Health Service. Clinicoecon Outcomes Res. 2014;6:409–414. | ||
Hill A, Hill T, Jose S, Pozniak A. Predicted savings to the UK National Health Service from switching to generic antiretrovirals, 2014-2018. J Int AIDS Soc. 2014;17(4 Suppl 3):19497. | ||
Di Giambenedetto S, Fabbiani M, Quiros Roldan E, et al. Treatment simplification to atazanavir/ritonavir plus lamivudine versus maintenance of atazanavir/ritonavir plus two nucleoside reverse transcriptase inhibitors in virologically-suppressed HIV-1-infected patients: 48-weeks results from a randomized trial (ATLAS-M). J Antimicrob Chemother. In press 2017. | ||
Sullivan SD, Mauskopf JA, Augustovski F, et al. Budget impact analysis-principles of good practice: report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health. 2014;17(1):5–14. | ||
Istituto Superiore di Sanità, Centro Operativo AIDS. Aggiornamento delle nuove diagnosi di infezione da HIV e dei casi di AIDS in Italia al 31 dicembre 2012. [Characteristics of persons with HIV and AIDS in Italy – 31 December 2012]. Not Ist Super Sanità. 2013;26(7–8). Italian. | ||
Restelli U, Scolari F, Bonfanti P, et al. New highly active antiretroviral drugs and generic drugs for the treatment of HIV infection: a budget impact analysis on the Italian National Health Service (Lombardy Region, Northern Italy). BMC Infect Dis. 2015;15:323. | ||
Llibre JM, Cardona G, Santos JR, et al. Antiretroviral treatment switch strategies for lowering the costs of antiretroviral therapy in subjects with suppressed HIV-1 viremia in Spain. Clinicoecon Outcomes Res. 2013;5:215–221. | ||
Gazzard B, Moecklinghoff C, Hill A. New strategies for lowering the costs of antiretroviral treatment and care for people with HIV/AIDS in the United Kingdom. Clinicoecon Outcomes Res. 2012;4:193–200. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.